Multiple sclerosis (MS) is a autoimmune disorder which preferentially affects young women of childbearing age. During pregnancy, the annualised relapse rate (AAR) is modified, but pregnancy has no harm effect on the long-term course of the disease. We aimed to study the clinical course of our MS patients during pregnancy, and compare their obstetrics outcomes with a control group of non-MS patients.
MethodsA single centre prospective observational study was conducted. We assessed the reproductive history, MS history, pregnancy course and new-born outcome of a cohort of MS patients who had had a pregnancy between January 2007 and July 2012. We compared the global outcomes with a control cohort of 58 age-matched healthy pregnancies.
ResultsComplete data from 35 consecutive women were analysed, 40 deliveries. Control groups: 58 patients, 60 deliveries. EDSS at pregnancy 0.7. ARR before pregnancy 0.5. During pregnancy 0.3, after pregnancy 0.4. Twelve patients were on disease-modifying drugs (DMD) before pregnancy, 4 prenatal exposure occurs. The comparison between relapse rate and EDSS before, during and after delivery showed no statistically significant difference. In addition, compared to control group, there were also no differences in the obstetric outcomes. In MS cohort, we found a higher incidence of assisted reproductive treatments and lower breastfeeding rate, both statistically significant.
ConclusionsOur series confirms that pregnancy has no negative long term impact on the progression of MS and also suggest that there is no additional morbidity in the pregnancy, comparing to the rest of the population.
La esclerosis múltiple (EM) es una enfermedad autoinmune que afecta preferentemente a mujeres en edad fértil. Durante el embarazo y puerperio, cambia la tasa anual de brotes (TAB) de EM, sin modificar la evolución a largo plazo. Analizamos la repercusión del embarazo en pacientes con EM, y comparamos sus resultados obstétricos con embarazos de mujeres sanas.
MétodosEstudio unicéntrico, observacional descriptivo, de diseño longitudinal prospectivo. Se analizan los datos globales de una cohorte de pacientes con EM que han dado a luz entre enero de 2007 y julio de 2012, con un seguimiento de 2 años posparto. Los resultados obstétricos se compararon con un grupo control de 58 embarazadas sanas, elegidas al azar de nuestro centro durante el mismo período de tiempo.
ResultadosUn total de 35 pacientes con EM, 40 partos. Grupo control: 58 mujeres, 60 partos. EDSS preembarazo: 0,7. TAB 2 años preembarazo: 0,5. Durante el embarazo: 0,3, a los 2 años posparto: 0,4. Doce pacientes recibían FME previo al embarazo, 4 iniciaron la gestación con FME. No hubo diferencias estadísticamente significativas en la TAB ni en la EDSS entre períodos preembarazo, embarazo y posparto. Al comparar con grupo control, no hubo diferencias en edad materna, semanas de gestación, peso al nacer, porcentaje de cesáreas, ni complicaciones obstétricas. En pacientes con EM hubo mayor porcentaje de tratamientos por infertilidad y menor porcentaje de lactancia, ambos estadísticamente significativos.
ConclusionesNuestro trabajo confirma que el embarazo no repercute negativamente en el curso de la EM y que no existe mayor morbilidad obstétrica comparado con mujeres sanas.
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system that predominantly affects women of childbearing potential.1 Susceptibility to MS is thought to depend on environmental factors in individuals with a genetic risk profile.2–6
Pregnancy in a woman with MS will change her annual relapse rate (ARR), with relapses becoming less frequent during the third trimester and subsequently increasing during the first 3 months postpartum.7 In the long term, the ARR and global disability do not seem to be affected by the changes in relapse frequency occurring in pregnancy. Regarding other parameters frequently analysed in pregnant MS patients or newborns of women with MS (duration of the gestational period, percentage of caesarean sections, birth weight, incidence of obstetric complications, etc.), results in the literature are not always comparable and may even be contradictory.8
There is currently no international consensus on how to manage MS patients planning to conceive; clinical recommendations vary between countries. Pregnancy planning and satisfactory management of MS are essential for these patients. Disease-modifying drugs (DMD) are not recommended during pregnancy since there is little knowledge of the effects they may have on the fetus.1 However, involuntary prenatal exposure to DMDs is relatively frequent, especially in cases of unplanned pregnancy, meaning that the body of evidence is growing.9,10
Our purpose was to analyse clinical experiences with managing female patients with MS during pregnancy and compare gynaecological and obstetric outcomes in these patients to those of healthy pregnant women at our hospital.
Material and methodsWe conducted a prospective descriptive longitudinal study at Hospital General Universitario Gregorio Marañón (HGUGM). HGUGM is a public hospital in the Madrid healthcare area; it has 1671 beds and serves a population of 317940 inhabitants.11 We analysed global data from a cohort of female patients diagnosed with MS who gave birth between January 2007 and July 2012. MS diagnosis was based on the McDonald criteria and the 2010 revisions to these criteria, depending on the date of diagnosis.12
All patients were prospectively evaluated at our department once pregnancy was confirmed and patients had agreed to participate in the study. We analysed the different clinical variables according to standard procedures. Assessments were conducted every 3 months during pregnancy and every 6 months after labour until the 2-year mark.
The study of MS patients gathered the following data: age, reproductive history, clinical form of MS, disease progression in years, ARR from disease onset, degree of disability according to the Kurtzke EDSS, and history of pharmacological treatment.13 We used baseline data from our cohort of patients with MS to compare the number of relapses and level of disability before, during, and after pregnancy. The appearance, reappearance, or worsening of focal neurological signs lasting more than 24 hours in the absence of fever was regarded as a relapse.
Pregnancy and childbirth data from our patients with MS were compared to those from a control group comprising 58 healthy pregnant woman who were randomly selected from among all pregnant women attended at our hospital during the study period. All our patients were attended by the hospital's gynaecology department and received prenatal and postnatal care.
We conducted personalised interviews with our patients with MS using semi-structured questionnaires to gather any data that might have been lacking.
Statistical analysisContinuous variables were expressed as either means±SD or means and ranges. Normal distribution was assessed with the Kolmogorov–Smirnov test. To compare means between 2 groups, we used parametric (t test) and non-parametric tests (Mann–Whitney U test), depending on whether or not data was normally distributed and on the total number of patients of each group. We used the Wilcoxon test, the McNemar test, and the t test for repeated measures to study the ARR over time (before, during, and after pregnancy). The association between qualitative variables was studied with the chi-square test or the Fisher exact test. Statistical analysis was conducted using SPSS version 21.0 and statistical significance was set at P<.05.
ResultsWe recorded 39 women with MS who were pregnant during the study period; the last delivery occurred in May 2012. Four patients were lost to follow-up. The 35 women who were analysed had given birth to 40 infants: 4 patients (11.4%) gave birth on 2 occasions during the study period and one patient (2.8%) had twins. The control group (58 patients) had given birth to 60 infants (2 women had twins).
Epidemiological characteristics of MS patientsAll our patients with MS had experienced relapses: 32 patients had relapsing-recurrent MS (91.4%), 2 had primary progressive MS with relapses (5.7%), and one had secondary progressive MS (2.8%). Mean progression time was 8.55 years (range, 2-22). The mean level of disability for our sample during pregnancy was 0.7 on the EDSS (range, 0-5): 23 patients (65.8%) scored 0; 6 (17.1%) scored 1; and the remaining 6 (17.1%) scored 2 to 5. The mean ARR since disease onset was 0.7 (range, 0.1-2.5); mean ARR during the 2 years prior to pregnancy was 0.5 (range, 0.3-0.6). Ten patients (28.5%) had experienced no relapses in the 2 years preceding pregnancy.
At onset of pregnancy, 23 patients (65.8%) were not receiving immunomodulatory therapy for MS. The remaining 12 (34.2%) were treated with DMDs: 4 with Rebif® (interferon beta 1a), 2 with Avonex® (interferon beta 1a), 4 with Betaferon® (interferon beta 1b), and 2 with Copaxone® (glatiramer acetate). Mean duration of treatment with DMDs was 43.4 months (range, 1-120). Eight patients discontinued treatment with DMDs prior to a planned pregnancy and had received no medication for a mean of 25.5 weeks (range, 8-64) before the first day of their last menstrual period. Four patients became pregnant while receiving DMDs: Avonex® in one case, Copaxone® in one case, one with Rebif®, and one with Betaferon®. In these 4 patients, DMDs were discontinued immediately after pregnancy was diagnosed; in all cases, discontinuation took place in the first trimester, a mean of 6.5 weeks (range, 3-10) after the first day of the last menstrual period. Five patients with MS (14.2%) had undergone fertility treatments.
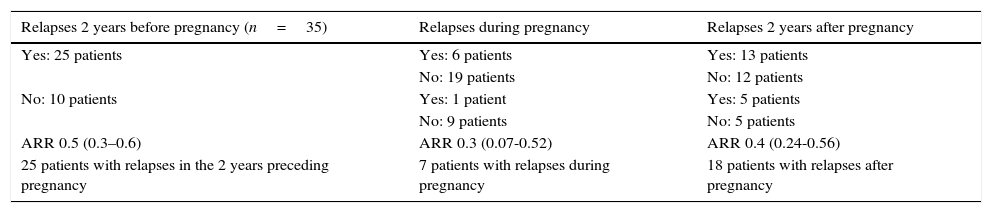
Functional status of MS patients during pregnancyThe mean ARR during the 2 years preceding pregnancy was 0.5 (range, 0.3-0.6), 0.3 during pregnancy (range, 0.07-0.52), and 0.4 in the first 2 years after pregnancy (range, 0.24-0.56). The Wilcoxon test found no statistically significant differences in ARRs between periods before, during, and after pregnancy. In the comparison of ARRs before and during pregnancy, the t test revealed a decrease in the ARR during pregnancy, although this change was not statistically significant (P=.23). No significant differences were found between ARRs before and after pregnancy (Table 1).
Incidence of MS relapses before, during, and after pregnancy.
| Relapses 2 years before pregnancy (n=35) | Relapses during pregnancy | Relapses 2 years after pregnancy |
|---|---|---|
| Yes: 25 patients | Yes: 6 patients | Yes: 13 patients |
| No: 19 patients | No: 12 patients | |
| No: 10 patients | Yes: 1 patient | Yes: 5 patients |
| No: 9 patients | No: 5 patients | |
| ARR 0.5 (0.3–0.6) | ARR 0.3 (0.07-0.52) | ARR 0.4 (0.24-0.56) |
| 25 patients with relapses in the 2 years preceding pregnancy | 7 patients with relapses during pregnancy | 18 patients with relapses after pregnancy |
Of the total sample, 25 patients (71.4%) experienced relapses in the 2 years preceding pregnancy, 7 (20%) experienced relapses during pregnancy, and 18 (51.4%) had at least one relapse during the 2-year follow-up period after pregnancy. Of the patients with postpartum relapses, 94.4% experienced the first relapse in the first 3 months postpartum.
We analysed the likelihood of postpartum relapses based on data on the incidence of relapses before pregnancy. The McNemar test showed no statistically significant differences in the incidence of relapses before and after pregnancy (P=.14) for periods of 2 years. Differences between EDSS scores before and after pregnancy were not statistically significant.
Characteristics of labour in MS patientsOf the total of 40 deliveries (40), 35 (87.5%) were vaginal and 5 (12.5%) were caesarean. As for the patient who had twins, one infant was born vaginally and the other by caesarean delivery. Twenty-three of the newborns (57.5%) were boys. Mean birth weight was 3012.8g (range, 1200-3800). Only one infant was born preterm and required resuscitation. We recorded the following medical complications: oligohydramnios (1), gestational anaemia (2), gestational diabetes (1), placenta praevia (1), and XXY syndrome (1). The mother whose infant had oligohydramnios received Betaferon® until the seventh week of pregnancy. None of the mothers had received DMDs before pregnancy in the cases of the remaining complications.
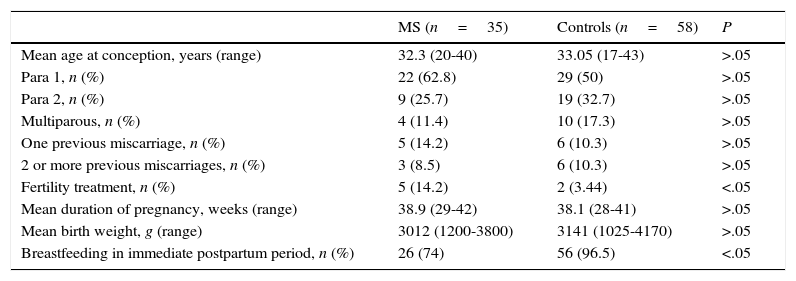
Comparison of pregnancy characteristics between women with MS and healthy womenWe compared pregnancy and childbirth characteristics between patients with MS and healthy controls. There were no statistically significant differences in maternal age at conception, reproductive history, history of miscarriage, weeks of gestation, newborn birth weight, percentage of caesarean deliveries, or obstetric complications (Table 2).
General and obstetric data in our sample of patients with MS and the control group of healthy women.
| MS (n=35) | Controls (n=58) | P | |
|---|---|---|---|
| Mean age at conception, years (range) | 32.3 (20-40) | 33.05 (17-43) | >.05 |
| Para 1, n (%) | 22 (62.8) | 29 (50) | >.05 |
| Para 2, n (%) | 9 (25.7) | 19 (32.7) | >.05 |
| Multiparous, n (%) | 4 (11.4) | 10 (17.3) | >.05 |
| One previous miscarriage, n (%) | 5 (14.2) | 6 (10.3) | >.05 |
| 2 or more previous miscarriages, n (%) | 3 (8.5) | 6 (10.3) | >.05 |
| Fertility treatment, n (%) | 5 (14.2) | 2 (3.44) | <.05 |
| Mean duration of pregnancy, weeks (range) | 38.9 (29-42) | 38.1 (28-41) | >.05 |
| Mean birth weight, g (range) | 3012 (1200-3800) | 3141 (1025-4170) | >.05 |
| Breastfeeding in immediate postpartum period, n (%) | 26 (74) | 56 (96.5) | <.05 |
We did find statistically significant differences in the percentage of women undergoing fertility treatment (P<.05). In vitro fertilisation was used in 4 of the 5 women with MS who underwent fertility treatment (13.5%) and artificial insemination in the remaining patient. In the control group, 2 patients (3.3%) underwent fertility treatment (in vitro fertilisation in both cases).
Significant differences were also found in breastfeeding rates. In the immediate postnatal period, 26 women with MS (74%) began breastfeeding. In 5 of the 9 patients with MS who did not start breastfeeding, the reason not to breastfeed was resuming DMD treatment immediately after childbirth. In the control group, 56 women (96.5%) began breastfeeding in the immediate postpartum period.
DiscussionAutoimmune diseases are more prevalent among women; the reasons for these inter-sex differences are still to be established.14 Female sex hormones and the expression of various genes linked to these hormones may be responsible for increased susceptibility to autoimmune diseases. Hormonal changes during pregnancy may affect the courses of multiple autoimmune diseases, including MS, rheumatoid arthritis, and psoriasis. In patients with these disorders, symptoms improve during pregnancy and worsen during the first postpartum months.15 From an immunological viewpoint, the fetus acts as an allograft in the uterus since it contains antigens inherited from the father. Therefore, changes in immune response are thought to be necessary to prevent the maternal immune system from rejecting the fetus.16–18 This is achieved through a change in cytokine secretion by activated T cells, down-regulation of Th1 response (cellular immunity), and relative up-regulation of Th2 response (humoural immunity). This change in immune response is thought to be responsible for improvements during pregnancy in patients with MS.
Oral contraceptives and the number of pregnancies have traditionally been thought to play a role in the development of the first clinical demyelinating event; results vary greatly depending on the year of publication and study methodology.19,20 Use of oral contraceptives and pregnancy history have no protective effects against MS.21,22
Fertility in general seems not to be affected by MS, but infertility and MS may co-occur; patients with MS may therefore need to undergo fertility treatments. Patients undergoing assisted reproductive techniques have an increased risk of relapse, which seems to be independent from the hormonal protocol used and time between stimulations.23 The mechanisms involved in increased ARR include temporary discontinuation of DMDs and immunological changes induced by sex hormones used during fertility treatment, which may lead to increased levels of proinflammatory cytokines and increased migration of immune cells through the blood–brain barrier.24,25
In women with MS, the decision to become pregnant is greatly influenced by disease activity and level of disability.7 Full-term pregnancy modifies the risk of relapse but it does not have an impact on disease progression in the long term, and neither does it result in greater disability.7,26–28 Postpartum relapses are independent from breastfeeding, use of epidural anaesthesia, age at disease onset, time of disease progression, total number of relapses before pregnancy, number of pregnancies, or sex of the newborn.26,29 Despite the well-documented fact that relapse incidence increases in the first 3 postpartum months, nearly 72% of the patients in the PRIMS study cohort experienced no relapses during that period.26 The likelihood of experiencing postpartum relapses increases with higher ARRs before pregnancy, presence of relapses during pregnancy, and probably with higher EDSS scores before pregnancy. Although it is true that the relapse rate decreases during pregnancy and subsequently increases during the first 3 months postpartum, the incidence of relapses during that 12-month period did not differ significantly from the ARRs recorded in the years preceding pregnancy.26 Furthermore, one study showed that only 13 out of 33 patients experiencing at least one relapse during the year before pregnancy or during pregnancy experienced a relapse in the first 3 months after childbirth. According to this study, there is no mathematical algorithm able to predict the risk of postpartum relapses in a specific patient, which makes it difficult to select patients for empirical treatment.26 The ARR after the first 3 months postpartum does not differ significantly from those recorded in the years preceding the pregnancy. In our series, a high percentage of patients with MS were either asymptomatic or mildly symptomatic at pregnancy onset: 65.8% had an EDSS score of 0 and 17.1% scored 1. Regarding relapses, the ARR decreased during pregnancy, although the difference was not statistically significant. This finding may be attributed both to EDSS scores and to ARRs before pregnancy, although we cannot rule out a bias due to the small size of our sample. The ARR was observed to increase during puerperium; 94.4% of the patients experienced the first relapse in the first 3 postpartum months. As for the incidence of relapse, our series showed no statistically significant differences in ARRs between the 2 years preceding pregnancy and the 2 years following childbirth. Likewise, no factors able to predict relapses in the postpartum period were identified in our series.
Use of disease-modifying drugs during pregnancyWomen with MS are recommended to discontinue treatment with DMDs before achieving pregnancy to minimise the risk of harm to the fetus. Treatment discontinuation may have an impact on the course of MS and may even alter disease stability. A recent study retrospectively analysed 152 pregnancies in 132 women with MS, including 61 cases (40.1%) of DMD exposure for at least 8 weeks. However, the rates of obstetric and neonatal complications were similar in these patients and in those not receiving DMDs during pregnancy, except for newborn birth weight and length, which were lower in babies exposed to DMDs. On the other hand, both the rate of postpartum relapses and EDSS scores were higher in patients not receiving DMDs before pregnancy.30
The U.S. Food and Drug Administration places DMDs in the following pregnancy safety categories: glatiramer acetate, category B (studies report no risk for animal fetuses, no studies have been conducted in humans); interferon beta, natalizumab, and fingolimod, category C (adverse effects have been observed in animal fetuses, no studies have been conducted in humans); and mitoxantrone, category D (potentially harmful to human fetuses). A recent systematic analysis of the results of 15 published studies addressing DMD use during pregnancy analysed a total of 761 women exposed to interferon beta, 97 to glatiramer acetate, and 35 to natalizumab.10 After assessing results reported in the literature, these authors proposed the following classification: interferon beta and mitoxantrone, class III (evidence from some studies suggests potential harm, specifically lower mean birth weight, shorter mean birth length, and preterm birth); and glatiramer acetate, natalizumab, and fingolimod, indeterminate (further research is needed because results are not compelling). Despite these data, discontinuing DMDs is still recommended for MS patients trying to conceive.10 In our sample, we recommended discontinuing DMDs to patients planning to become pregnant. However, 4 pregnancies in our sample occurred in patients treated with DMDs, and these cases were not associated with increased morbidity.
Prevention of postpartum relapsesIncreased relapse incidence after childbirth is one of the main pregnancy-related risks in women with MS. Determining the most suitable prevention and treatment strategies is therefore essential. De Seze et al.31 analysed the effectiveness of intravenous corticosteroids (CS) for reducing the incidence of relapses in the postpartum period. Twenty women were administered 1g intravenous methylprednisolone monthly for 6 months after delivery and compared to a historical control group of 22 women who had not received CS. While the rate of relapses increased in both groups after delivery, increases were less marked in the group receiving CS. This suggests that monthly empirical treatment with CS may be beneficial for these patients. However, CS have also been associated with increased risk of infections and may have a negative impact on wound granulation.32,33 Intravenous immunoglobulins (ivIG) may be an alternative for preventing postpartum relapses. One retrospective study compared the effectiveness of ivIG for preventing relapses in the postpartum period.34 Patients in group 1 were not treated, patients in group 2 received 0.4g ivIG/kg for 5 consecutive days during the first week after childbirth plus an additional dose at 6 and 12 weeks postpartum, and patients in group 3 received ivIG during pregnancy and the postpartum period (ivIG during the first 8 weeks of pregnancy plus additional doses once every 6 weeks until 12 weeks postpartum). This study observed a significant reduction in relapse rates during pregnancy and after childbirth; ivIG use was not associated with increased risk of comorbidities. Early treatment with DMDs may be another treatment option in patients who choose not to breastfeed.35 In our series, DMD treatment was started immediately after childbirth in 5 women, who were advised to avoid breastfeeding. This may explain the lower breastfeeding rate in the group of patients with MS.
Our study has several limitations, including its single-centre design, the fact that our hospital is a centre of reference for MS, and the small sample size; these factors may have introduced biases and limited the extent to which our results can be generalised to other populations.
ConclusionsWe wish to highlight the high percentage of women with MS who were either asymptomatic or mildly symptomatic at the time pregnancy was planned. Our series confirms that ARRs decrease during pregnancy and increase during the first 3 months of the postpartum period. However, these differences are not significant if we compare relapse rates in this period to those pertaining to the 2 years preceding or following pregnancy. In conclusion, our study showed that women with MS do not experience more obstetric complications than healthy women.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank our colleague H. Goicoechea Briceño for her unwavering assistance in caring for our patients.
Please cite this article as: Cuello JP, Martínez Ginés ML, Martin Barriga ML, de Andrés C. Esclerosis múltiple y embarazo: estudio unicéntrico prospectivo y comparativo. Neurología. 2017;32:92–98.
A short version of this study was presented in poster format at the 64th Annual Meeting of the Spanish Society of Neurology, Barcelona, 2012, and at the 65th Annual Meeting of the American Academy of Neurology, San Diego, 2013.