The definition, associated aetiologies, diagnosis, and treatment of idiopathic intracranial hypertension, or pseudotumour cerebri (PTC), are constantly being revised in the paediatric population.
Objectives and methodsOur study included children younger than 15 years old with PTC and attended at a reference hospital in the past 12 years. We analysed the clinical and epidemiological features of our sample and the diagnostic and treatment approaches. PTC was defined as presence of intracranial hypertension (CSF opening pressure>25cmH2O) and absence of space-occupying lesions in brain MR images.
ResultsA total of 12 children with PTC were included; mean age was 10 years and 90% were girls. Weight was normal in all patients. Eighty-two percent of the patients had symptoms: headache (66%), diplopia (8%), and visual loss (8%). All of them displayed papilloedema (17% unilaterally). Lumbar puncture (LP) provided the diagnosis in all cases and 91% showed no relevant MRI findings. A potential cause of PTC was identified in 5 cases: pharmacological treatment in 2 and infection (Mycoplasma pneumoniae [M. pneumoniae]) in 3. Ninety-one percent of the patients received treatment: 75% underwent several LPs and 42% received acetazolamide and/or prednisone. Outcomes were favourable in all cases.
ConclusionsThe incidence of PTC was estimated at approximately 1 case per 100000 children/years, in line with data reported by previous studies. Overweight was not found to be a risk factor for PTC in this population. M. pneumoniae infection may trigger PTC and cause recurrences at later stages. The absence of symptoms seems to be independent from the degree of intracranial hypertension. Acetazolamide treatment is effective in most cases, and it represents a viable alternative to repeated LP.
El síndrome de hipertensión intracraneal idiopática o seudotumor cerebri (STC) en niños está en constante revisión, respecto a su definición, etiologías asociadas, diagnóstico y terapéutica más apropiada.
Objetivos y métodosSe revisaron los casos de STC < 15 años de edad en un hospital de referencia en los últimos 12 años. Se estudiaron las características clínico-epidemiológicas y el procedimiento diagnóstico-terapéutico empleado. Se definió STC como presión intracraneal > 25cmH2O por punción lumbar (PL), con estudio de resonancia magnética cerebral sin lesión ocupante de espacio.
ResultadosSe registró a 12 niños con STC, media de edad de 10 años, 90% mujeres. Todos presentaban peso normal. El 82% manifestaba síntomas: cefalea (66%), diplopía (8%) o baja visión (8%). Todos asociaban papiledema (17% unilateral). La PL fue diagnóstica en el 100% y la neuroimagen fue normal en el 91%. Se evidenció un posible desencadenante en 5 casos (2 farmacológico y 3 infeccioso por Mycoplasma pneumoniae [M. pneumoniae]). El 91% recibió tratamiento médico: en el 75% consistió en PL repetidas y en el 42% solo acetazolamida y/o prednisona. La evolución fue favorable en todos ellos.
ConclusionesLa incidencia de STC fue de aproximadamente 1/100.000 niños/año, similar a estudios previos. En esta población, el sobrepeso no es un factor de riesgo. La infección por M. pneumoniae podría actuar como desencadenante de STC y favorecer recurrencias tardías. La ausencia de síntomas parece independiente del grado de presión intracraneal. El tratamiento con acetazolamida es eficaz en la mayoría de los casos, desterrando el uso de PL repetidas.
Benign or idiopathic intracranial hypertension (IIH), also known as pseudotumour cerebri (PTC), is defined as the presence of signs and symptoms of increased intracranial pressure, with normal results in the neurological examination (with the exception of sixth cranial nerve palsy) and in cerebrospinal fluid (CSF) analysis and neuroimaging studies.1–3 In recent years, changes have been made to the terminology used in the diagnostic process and the therapeutic approach, which has had an impact on the clinical management of these patients.4–6
The term “benign” is currently subject to controversy as multiple causes of PTC have been identified in paediatric patients, and the condition may be associated with a high morbidity rate, with severely impaired visual function.1–3 For this reason, many authors have recently defended the terms primary and secondary PTC, according to whether or not a cause is identified, and avoid using the terms idiopathic and benign.3–7
At present, there is no clear consensus on the therapeutic approach; the current trend is towards watchful waiting or pharmacological treatment, with invasive techniques used only exclusively in patients with severe or recurrent symptoms.1,4–7
The aim of this study was to review our experience with paediatric patients diagnosed with PTC, focusing on clinical progression and the diagnostic and therapeutic approach taken over the past 12 years.
Patients and methodsWe conducted a retrospective, descriptive study of all patients diagnosed with PTC at the paediatric neurology department from January 2003 to January 2015.
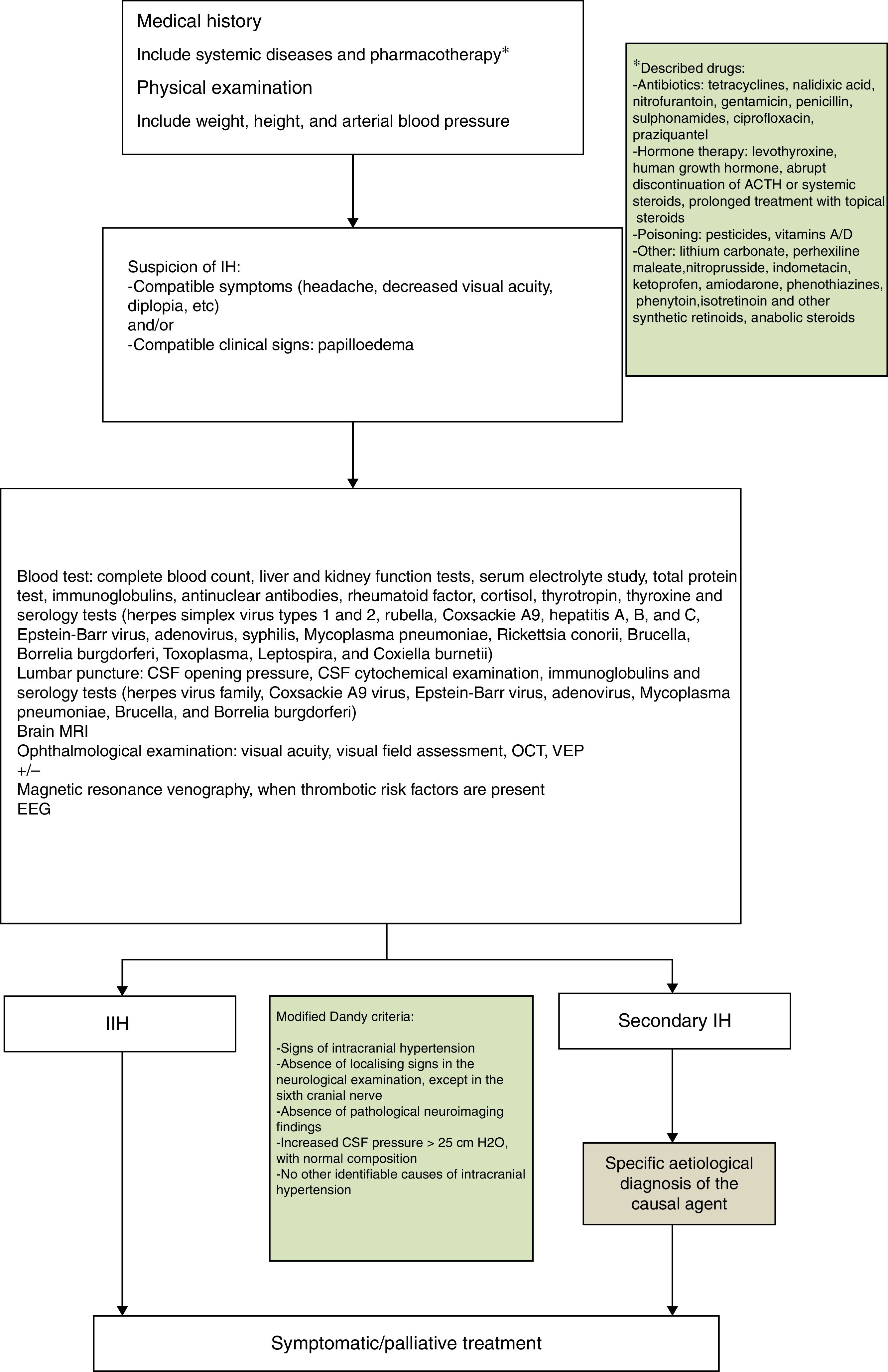
PTC was diagnosed according to the following criteria1,2,4:
- 1.
Papilloedema with/without accompanying symptoms: headache, vomiting, visual alterations, and sixth cranial nerve palsy.
- 2.
CSF pressure higher than 25cmH2O as detected by lumbar puncture and measured with a manometer through a 3-way stopcock.
- 3.
Magnetic resonance imaging (MRI) scans show no signs of space-occupying lesion.
During our review of clinical histories, we recorded the age at onset, sex, personal history, intercurrent diseases, medication, reason for consultation, progression time from symptom onset to the first medical consultation, complementary tests performed, treatment received, length of hospital stays, and time from treatment onset to normalisation of the eye fundus.
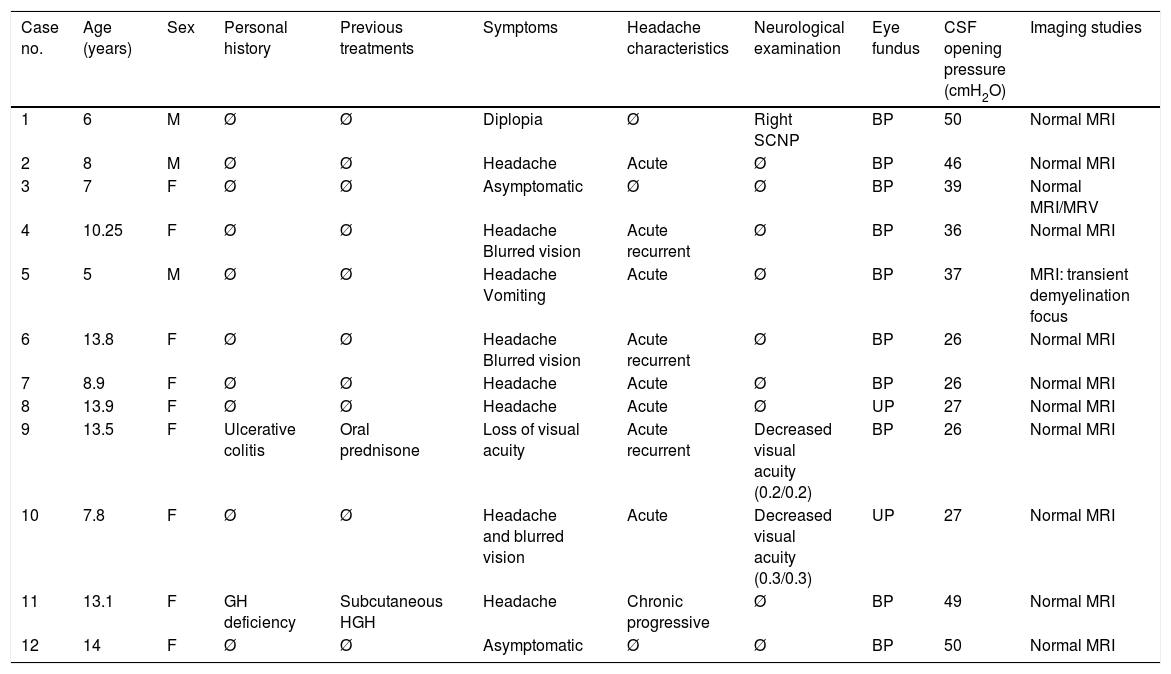
ResultsClinical characteristicsThe sample comprised 12 patients (9 girls and 3 boys) with an age range of 5 to 14 years. Patients’ demographic and clinical characteristics, and the complementary tests performed, are listed in Table 1.
Characteristics of the 12 patients with PTC.
| Case no. | Age (years) | Sex | Personal history | Previous treatments | Symptoms | Headache characteristics | Neurological examination | Eye fundus | CSF opening pressure (cmH2O) | Imaging studies |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | M | Ø | Ø | Diplopia | Ø | Right SCNP | BP | 50 | Normal MRI |
| 2 | 8 | M | Ø | Ø | Headache | Acute | Ø | BP | 46 | Normal MRI |
| 3 | 7 | F | Ø | Ø | Asymptomatic | Ø | Ø | BP | 39 | Normal MRI/MRV |
| 4 | 10.25 | F | Ø | Ø | Headache Blurred vision | Acute recurrent | Ø | BP | 36 | Normal MRI |
| 5 | 5 | M | Ø | Ø | Headache Vomiting | Acute | Ø | BP | 37 | MRI: transient demyelination focus |
| 6 | 13.8 | F | Ø | Ø | Headache Blurred vision | Acute recurrent | Ø | BP | 26 | Normal MRI |
| 7 | 8.9 | F | Ø | Ø | Headache | Acute | Ø | BP | 26 | Normal MRI |
| 8 | 13.9 | F | Ø | Ø | Headache | Acute | Ø | UP | 27 | Normal MRI |
| 9 | 13.5 | F | Ulcerative colitis | Oral prednisone | Loss of visual acuity | Acute recurrent | Decreased visual acuity (0.2/0.2) | BP | 26 | Normal MRI |
| 10 | 7.8 | F | Ø | Ø | Headache and blurred vision | Acute | Decreased visual acuity (0.3/0.3) | UP | 27 | Normal MRI |
| 11 | 13.1 | F | GH deficiency | Subcutaneous HGH | Headache | Chronic progressive | Ø | BP | 49 | Normal MRI |
| 12 | 14 | F | Ø | Ø | Asymptomatic | Ø | Ø | BP | 50 | Normal MRI |
| Case no. | VEP | Aetiology | No. of LPs | Treatment | Total duration of treatment (days) | Papilloedema resolution (months) | Recurrence | Sequelae |
|---|---|---|---|---|---|---|---|---|
| 1 | Not performed | Unknown | 1 | ACT | 21 | 5 | No | No |
| 2 | Increased latency | Unknown | 3 | ACT/oCT | 57 | 13 | No | No |
| 3 | Normal | Unknown | 3 | ACT/oCT | 49 | 12 | No | No |
| 4 | Normal | Unknown | 2 | ACT | 50 | 4 | Yes | No |
| 5 | Increased latency | Mycoplasma pneumoniae | 5 | ATB/oCT | 60 | 14 | Yes | No |
| 6 | Not performed | Mycoplasma pneumoniae, Borrelia burgdorferi | 2 | ATB | 12 | 15 | No | No |
| 7 | Normal | Mycoplasma pneumoniae | 2 | ATB | 10 | 6 | No | No |
| 8 | Normal | Unknown | 1 | Ø | 0 | 5 | No | No |
| 9 | Increased latency | Pharmacological | 2 | Ø | 0 | 5 | No | No |
| 10 | Increased latency | Unknown | 2 | ACT | 49 | 6 | No | No |
| 11 | Normal | Pharmacological | 1 | ACT | 60 | 6 | No | No |
| 12 | Not performed | Unknown | 5 | ACT | 15 | 24 | No | No |
ACT: acetazolamide; ATB: antibiotics; BP: bilateral papilloedema; F: female; GH: growth hormone; HGH: treatment with human growth hormone; M: male; MRI: magnetic resonance imaging; MRV: magnetic resonance venography; oCT: oral corticosteroids; SCNP: sixth cranial nerve palsy; UP: unilateral papilloedema; VEP: visual evoked potentials.
No patient presented relevant personal or perinatal history; psychomotor development was normal in all cases. We should mention that we observed a weight below percentile 85 for age and sex in all patients, with arterial blood pressure values below percentile 90 for age.
The predominant symptom was headache in 66% of patients, with half of these cases presenting additional symptoms: 3 cases with blurred vision, (cases 4, 6, and 10 in Table 1) and one with vomiting (case 5). Headache was acute in 62% of cases, acute and recurrent in 37%, and chronic and progressive in 12%. It did not interrupt sleep in any case. One patient (case 9) reported significantly decreased visual acuity with no headache; another patient displayed binocular diplopia due to right sixth cranial nerve palsy (case 1). Two patients presented no symptoms but were referred to the paediatric neurology department due to detection of bilateral papilloedema in a routine ophthalmological study (cases 3 and 12).
Time from symptom onset to diagnosis ranged between 24hours and 12 months, depending on the increasing or incapacitating severity of symptoms.
The neurological examination revealed the presence of papilloedema in all cases: bilateral in 83% of patients (n=10) and unilateral in 17% (n=2). Papilloedema manifested as optic disc elevation, with blurred disc margins and darkened retinal vessels crossing the margins of the disc.
Complementary testsAll patients underwent an MRI study, a complete blood study, and a lumbar puncture. The neurophysiological study showed altered visual-evoked potentials in 44% of the patients examined (n=4/9), with 2 (cases 9 and 10) also presenting decreased visual acuity.
Given the presence of papilloedema, all patients underwent a computed tomography study before lumbar puncture; this obtained normal results. In the brain MRI study, only one patient showed alterations (case 5): a right parietal demyelinating lesion which was observed to have resolved after one month of corticosteroid treatment in a follow-up study. In this case, it was associated with positive results for Mycoplasma pneumoniae.
According to our protocol, all patients underwent a complete blood count; liver and kidney function studies; serum electrolyte study; total protein test; tests for blood and CSF immunoglobulins, antinuclear antibodies, rheumatoid factor, cortisol, thyroid-stimulating hormone, and thyroxine; and serology blood tests for herpes simplex virus (types 1 and 2), rubella, Coxsackie A9 virus, hepatitis A, B, and C virus, Epstein-Barr virus, adenovirus, syphilis, M. pneumoniae, Rickettsia conorii, Brucella, Borrelia burgdorferi, Toxoplasma, Leptospira, and Coxiella burnetii. CSF serology tests were performed for herpes viruses, Coxsackie A9 virus, Epstein-Barr virus, adenovirus, M. pneumoniae, Brucella, and B. burgdorferi. Results were positive in 3 cases (25%): serology tests were positive for M. pneumoniae in 2 patients (cases 5 and 7); a third patient was positive for M. pneumoniae and B. burgdorferi (case 6), although these results were not confirmed in the CSF study.
AetiologyNo patient presented overweight or obesity and no associated factors were found in 7 patients (67%). We detected possible trigger factors in 5 patients: 2 of pharmacological origin (one treated with growth hormone due to small stature and the other with oral prednisone due to history of ulcerative colitis) and 3 due to the above-mentioned infectious processes (cases 5, 6, and 7).
Treatment and outcomeAll patients underwent at least one diagnostic lumbar puncture, with a mean opening pressure of 37cmH2O (range, 26-50). After this first puncture, early clinical improvement was observed in 66% of patients in our series (n=8). Lumbar puncture was performed once in 3 patients (25%), twice in 5 (42%), 3 times in 2 (17%), and 5 times in another 2 patients (17%). Oral acetazolamide dosed at 30mg/kg/day was added in 5 patients (42%); prednisone was administered to 2 of these due to lack of response. Papilloedema progressively resolved in all cases. Patients with infectious processes received the standard antibiotic treatment according to the pathogen and the type of infection.
After discharge, we performed follow-up on an outpatient basis, which included periodic eye fundus examinations; we observed that papilloedema had resolved completely at 6 months in 8 patients (67%) and at 12 months in the remaining 4 (33%).
DiscussionPTC is usually characterised by papilloedema, signs and symptoms of increased intracranial pressure, normal results in neuroimaging studies, and absence of an underlying cause.1–6 However, atypical cases are frequently described, calling into question the clinical criteria established by several authors.1,2,4 In our series of 12 patients, papilloedema was an incidental finding in 2 patients (17%), who were asymptomatic, despite CSF pressure being above the mean for the sample. No aetiology was found in either patient. Furthermore, the youngest patient in our series (a 5-year-old boy) presented the typical symptoms of PTC as well as a transient supratentorial demyelinating lesion and positive serology results for M. pneumoniae, which resolved with corticotherapy. One year later, the same patient presented recurrence of the typical symptoms, associated with a new M. pneumoniae infection, this time with normal neuroimaging results. The patient did not meet criteria for encephalitis or acute disseminated encephalitis in either episode.
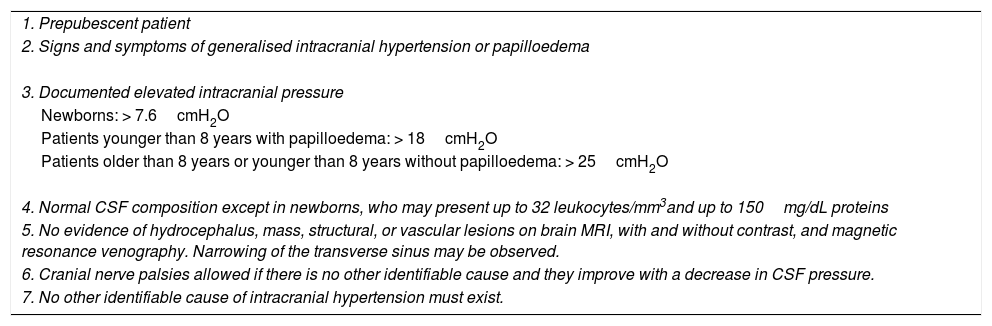
The first description of PTC was published by Quincke8 in 1893. The first diagnostic criteria were established by Dandy9 in 1937 and subsequently revised by Smith in 198510 to include radiological imaging criteria, and are currently known as the modified Dandy criteria. Scientific progress, further knowledge on this field, and the development of neuroimaging techniques have led to several proposed modifications, such as the one by Rangwala and Liu,10 adapted to paediatric patients (Tables 2 and 3).
Modified Dandy criteria for the diagnosis of PTC.
| Signs of intracranial hypertension |
| Absence of localising signs in the neurological examination, with the exception of the sixth cranial nerve |
| Absence of pathological neuroimaging findings |
| Increased CSF pressure>25cmH2O, with normal composition |
| No other identifiable causes of intracranial hypertension |
PTC criteria modified by Rangwala.
| 1. Prepubescent patient |
| 2. Signs and symptoms of generalised intracranial hypertension or papilloedema |
| 3. Documented elevated intracranial pressure |
| Newborns: > 7.6cmH2O |
| Patients younger than 8 years with papilloedema: > 18cmH2O |
| Patients older than 8 years or younger than 8 years without papilloedema: > 25cmH2O |
| 4. Normal CSF composition except in newborns, who may present up to 32 leukocytes/mm3and up to 150mg/dL proteins |
| 5. No evidence of hydrocephalus, mass, structural, or vascular lesions on brain MRI, with and without contrast, and magnetic resonance venography. Narrowing of the transverse sinus may be observed. |
| 6. Cranial nerve palsies allowed if there is no other identifiable cause and they improve with a decrease in CSF pressure. |
| 7. No other identifiable cause of intracranial hypertension must exist. |
The correct nomenclature and terminology for PTC is still subject to debate in the literature. Thus, several authors refuse to accept PTC as an equivalent of the term “IIH,” as multiple causes have been identified in recent years; although these are less frequent in adults, they represent up to 50% of paediatric cases in several series.1 In 2013, the American Academy of Neurology published a review proposing the exclusive use of the term PTC to refer to that syndrome, whether primary or secondary, and relegating the term IIH to a category of exclusively primary PTC.4
Recently published studies have added to the list of possible causes of PTC, including a large number of cerebral venous anomalies, drugs, infections, and associated endocrine diseases.1–3,5–7 We detected possible precipitating causes in 5 of our patients: 2 pharmacological and 3 infectious, with detection of M. pneumoniae being particularly significant as a possible trigger factor. This pathogen is included in the spectrum of neurological diseases to be studied, as it is present in such diverse conditions as aseptic meningitis, cerebellar ataxia, and transverse myelitis, among others.11 The pathogenic mechanism, which is thought to be immunological, is yet to be determined. Furthermore, after studying these patients, it seems to be advisable to include a serology test for M. pneumoniae and to test for this pathogen in the nasal discharge in all cases of PTC.12
Another area of discussion is what upper limit of CSF opening pressure should be considered diagnostic in paediatric patients. All our patients underwent a first lumbar puncture under sedation, in a lateral decubitus position with the legs bent, and had opening pressures of 26 to 50cmH2O. Measuring CSF pressure in children is not simple; changes in opening pressure are reported to be influenced by several factors, including age (values in children older than 8 are reported to be similar to those of adults5), Valsalva manoeuvres, or the patient's position.4 A recent prospective study conducted to establish opening pressure in paediatric patients found increased pressure to be significantly associated with sedation and higher body mass index (BMI). These authors propose that an opening pressure≥28cmH2O should be considered abnormally elevated (25cmH2O if the patient is not obese or sedated6).
Our tertiary care hospital is a reference centre for paediatric neurological disorders in the region of Navarre, with a population of approximately 100000 children. In Navarre, incidence of PTC is approximately 1/100000 children/year, similar to that of the general population (0.9/100000 children/year).5 However, the different series show a clear predominance of obese women, whereas in our series, despite the predominance of adolescent girls, none presented a BMI above percentile 85 for their age. Both the actual incidence in children and its association with sex and BMI are yet to be established, with this association showing large differences, depending on the series. Thus, the classic studies by Babikian et al.13 found that 60% of patients with PTC were older than 10, and the study by Balcer et al.14 discovered a clear association between obesity, sex, and PTC, in contrast with such series as those by Cinciripini et al.,15 who did not find statistically significant differences.
The ophthalmological study, including eye fundus examination and visual field assessment, is essential for diagnosis, follow-up, and deciding treatment aggressiveness. The first diagnostic approach should differentiate between papilloedema and pseudopapilloedema and rule out other cases of papilloedema, such as optic neuritis, ischaemic optic neuropathy, or neuroretinitis. Eye fundus imaging and clinical symptoms may be sufficient to establish a correct differential diagnosis, although diagnosis occasionally requires an ocular ultrasound or a fluorescein angiography to confirm the presence of papilloedema and rule out causes of pseudopapilloedema such as optic nerve drusen.16 Optical coherence tomography, a non-invasive, reproducible, objective, high-resolution technique, is used to correlate the thickness of the retinal nerve fibre layer with its corresponding eye fundus image, using the modified Frisén scale.17 It is useful not only as a complementary test, but also in the subsequent clinical and therapeutic follow-up.16–18 The need to perform a magnetic resonance venography to rule out venous sinus thrombosis is questioned, since partial occlusions may go unnoticed. The technique's usefulness in paediatric patients is yet to be determined, as no randomised studies in children have been published. Therefore, certain authors suggest considering this technique only in patients with thrombosis risk factors, such as recent history of otitis media or sinusitis.5 In our series, we performed a magnetic resonance venography in only one patient due to the presence of prothrombotic factors; the study yielded normal results. There are limited references on the usefulness of neurophysiological studies in PTC. Alterations in the electroencephalography during the acute phase may be explained by the stress on the cerebral cortex during the study. Furthermore, visual evoked potentials display low sensitivity as a prognostic factor of visual function.19
There is no consensus algorithm for the treatment of this condition, since there are no randomised studies to inform evidence-based treatment.1–3,20 The current trend is towards watchful waiting, limiting the use of pharmacological or surgical treatment according to clinical severity, the degree of visual involvement, or eye fundus findings. Therefore, a comprehensive ophthalmological examination is essential after diagnosis of PTC in order to decide whether to start early treatment to reduce clinical symptoms and the risk of loss of visual function due to atrophy of the optic nerve. This effect of the ophthalmological study is shown in our series: we decided to start treatment in the 2 asymptomatic cases due to detection of moderate bilateral papilloedema through eye fundus examination and optical coherence tomography. Treatment has classically been based on lumbar CSF drain, medical treatment, and various surgical techniques. The use of lumbar CSF drain is becoming less prevalent due to its questioned effectiveness in paediatric patients and its invasiveness and associated complications.5
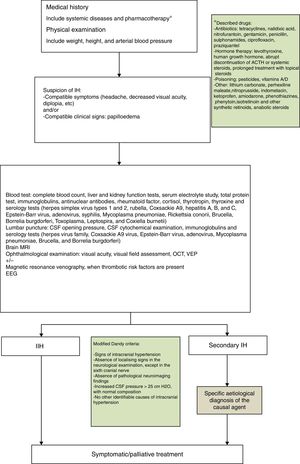
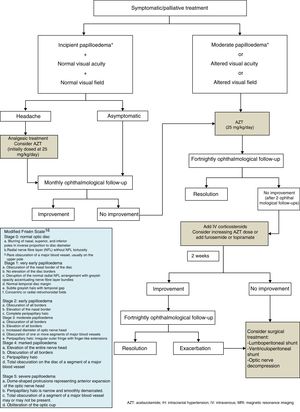
Regarding medical treatment, the first-line drug for children is acetazolamide, a carbonic anhydrase inhibitor. Another alternative is the administration of furosemide, corticosteroids, or topimarate to complement or substitute treatment.1–3,5,7,20,21 Beta-blockers and tricyclic antidepressants have been reported to achieve good control of refractory headache in some series.22 In this context, treatment of the underlying pathology is essential for correct clinical management in cases of secondary PTC. In our series, the drug of first choice was acetazolamide at 25-30mg/kg/day in 2 daily doses, with prednisone to be added depending on the response to acetazolamide. Fig. 1 shows the therapeutic algorithm followed in our department.
In general, medical treatment of PTC is satisfactory in paediatric patients, but treatment duration is not standardised; it is therefore adapted according to clinical symptoms and the progression of visual acuity, with a mean range of 2 to 6 months. Surgical techniques are reserved for those patients with progressive impairment of visual acuity and/or refractory cases. These techniques include lumboperitoneal or ventriculoperitoneal shunt, or even optic nerve decompression.1–3,5,7,20–22
Lastly, we should mention the risk of recurrence. Patients should be strictly monitored until symptoms resolve completely, and undergo periodic ophthalmological examinations, which may reveal both recurrence and treatment failure at an early juncture.
In conclusion, while PTC is an infrequent clinical entity in children, diagnosis and early treatment are essential to prevent potentially irreversible sequelae. Further multicentre clinical trials are needed to establish the correct diagnosis and treatment for PTC.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Mosquera Gorostidi A, Iridoy Zulet M, Azcona Ganuza G, Gembero Esarte E, Yoldi Petri ME, Aguilera Albesa S. Seudotumor cerebri en niños: etiología, características clínicas y evolución. Neurología. 2019;34:89–97.