The 22q11.2 deletion syndrome is a genetic disorder with variable clinical manifestations. It affects one out of 5950 neonates and has an autosomal dominant inheritance pattern. The aim of this article is to review its psychiatric manifestations and any underlying genetic alterations.
MethodsWe reviewed the scientific literature available as of October 2014 in the LILACS and Medline databases.
ResultsSixty per cent of these patients fulfilled diagnostic criteria for a mental disorder at some point in their lives, referring to psychotic disorders, attention deficit hyperactivity disorder, mood disorders, anxiety disorders, and autism spectrum disorders. Specific genes, such as COMT and PRODH, have been linked to these psychiatric manifestations.
ConclusionsIt is necessary to raise awareness among all health care professionals so that they understand the relevance of these manifestations, are able to anticipate them, and can provide appropriate information to patients and family members.
El síndrome de deleción 22q11.2 es un trastorno genético con manifestaciones clínicas variables. Afecta a 1 de cada 5.950 recién nacidos y tiene un patrón de herencia autosómico dominante. El objetivo de este artículo es realizar una revisión de las manifestaciones psiquiátricas y de las bases genéticas asociadas.
MétodosSe realizó revisión bibliográfica de la literatura científica disponible hasta octubre de 2014 en bases de datos LILACS y Medline.
ResultadosEl 60% de estos pacientes en algún momento de la vida cumple criterios diagnósticos de psicopatología, incluyendo trastornos psicóticos, trastorno por déficit atencional con hiperactividad, trastornos del ánimo, trastornos de ansiedad y trastornos del espectro autista. Se han identificado genes, como COMT y PRODH, que estarían relacionados con las manifestaciones psiquiátricas del síndrome.
ConclusionesLa sensibilización de los equipos de salud acerca de estas manifestaciones, permitiría su búsqueda dirigida y la información adecuada para el paciente y su familia.
The 22q11.2 deletion syndrome (22q11.2DS) is a genetic disorder with highly variable clinical manifestations. Due to its phenotypic heterogeneity, the syndrome was initially known by different names, including DiGeorge syndrome, velocardiofacial syndrome, CHARGE syndrome, and conotruncal anomaly face syndrome, depending on the clinical manifestation. It is now known that the vast majority of these patients present the same deletion on the long arm of chromosome 22; they are therefore grouped under a single entity, named for the genetic alteration.1
This condition has an estimated incidence of 1 case per 5950 live births and is more frequent in Hispanic populations.2 The most frequent clinical manifestations include heart disease; hypocalcaemia; velopharyngeal abnormalities; thymic hypoplasia associated with immunodeficiency; and renal, ophthalmological, and dental alterations.1
Diagnosis is performed by fluorescence in situ hybridisation (FISH). Although de novo presentation accounts for the majority of cases, the disease has an autosomal dominant inheritance pattern, meaning that 50% of the offspring of affected individuals will inherit the mutation.
This article reviews the psychiatric symptoms affecting patients with 22q11.2DS, and the genetic basis of these manifestations.
MethodsWe conducted a literature review in the LILACS and Medline databases using the MeSH term “22q11 deletion syndrome” to gather all articles written in either English or Spanish and published until October 2014. Our search yielded a total of 1108 articles. The articles in our final sample (n=76) included at least one of the following keywords in the title: psychiatric, neuropsychiatric, psychosis, schizophrenia, ADHD, bipolar, depression, anxiety, or autism.
Psychiatric manifestations of 22q11.2 deletion syndromeAlthough schizophrenia is the neuropsychiatric phenotype most frequently associated with the 22q11.2DS, around 60% of patients with the syndrome are estimated to meet diagnostic criteria at some point in their lives for some type of psychiatric disorder, including psychotic disorders, attention-deficit/hyperactivity disorder (ADHD), mood disorders, anxiety, and autism spectrum disorders (ASD), among others.3 Furthermore, 50% of these patients have some level of cognitive impairment, with mean IQ scores ranging from 71 to 73.4,5
Despite the high frequency of psychiatric manifestations in patients with 22q11.2DS, geneticists in the United States and Canada provide families with much less information on these manifestations than on any other symptom of the disease, especially in the case of preschool and school-age children.6 This results in a majority of parents receiving more information on psychiatric manifestations from the Internet than through their healthcare providers.7,8
Psychotic disordersThe first description of psychotic disorders in adolescents and adults with 22q11.2DS was made by Shprintzen et al.9 in 1992, nearly 15 years after the same research group described velocardiofacial syndrome. Since then, there has been a growing body of scientific evidence on the association between psychotic symptoms and this syndrome. Today, deletion of 22q11.2 is regarded as a major risk factor for psychosis and considered to be responsible for 1% to 2% of all cases of schizophrenia.3
In 1999, Murphy et al.10 gathered a total of 50 patients with the condition, of whom 30% had a history of psychosis and 24% met DSM-IV diagnostic criteria for schizophrenia. In a recently published series of 1402 patients with 22q11.2DS, the prevalence of psychotic disorders in adolescents was 10.12% and increased with age (over 40% of adults were affected). In the same series, prevalence of schizophrenia was 3.8% in adolescents (13 to 17 years) and around 30% in patients over 36. Schizoaffective disorders were observed to be more frequent in patients over 25, with a prevalence of 7.58%; they were infrequent in adolescents and extremely rare in children.11
A number of studies following up series of patients with 22q11.2DS have shown that presence of anxiety disorders, a lower overall IQ, and particularly lower verbal IQ scores are predictors of psychotic disorder.12,13 These risk factors have been found to be positively correlated with severity of positive symptoms of psychosis (Table 1).
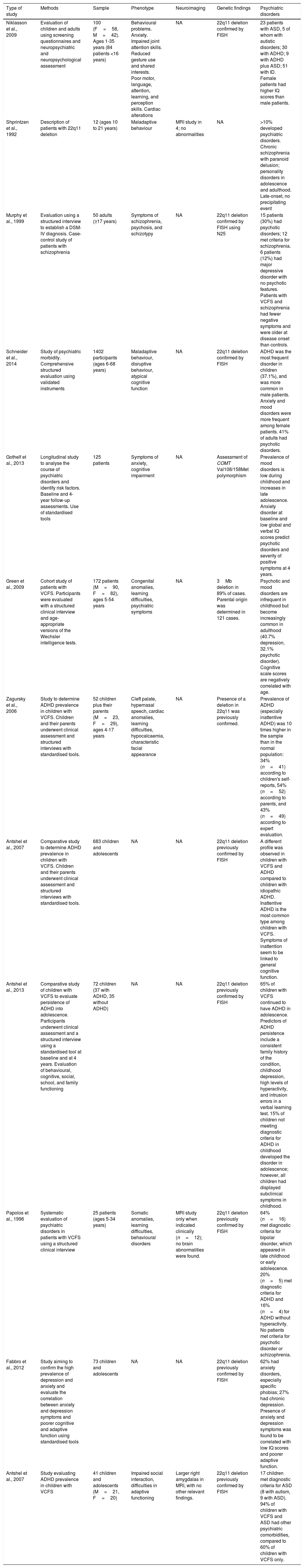
Description of the studies included in our literature review.
| Type of study | Methods | Sample | Phenotype | Neuroimaging | Genetic findings | Psychiatric disorders |
|---|---|---|---|---|---|---|
| Niklasson et al., 2009 | Evaluation of children and adults using screening questionnaires and neuropsychiatric and neuropsychological assessment | 100 (F=58, M=42). Ages 1-35 years (84 patients <16 years) | Behavioural problems. Anxiety. Impaired joint attention skills. Reduced gesture use and shared interests. Poor motor, language, attention, learning, and perception skills. Cardiac alterations | NA | 22q11 deletion confirmed by FISH | 23 patients with ASD, 5 of whom with autistic disorders; 30 with ADHD; 9 with ADHD plus ASD; 51 with ID. Female patients had higher IQ scores than male patients. |
| Shprintzen et al., 1992 | Description of patients with 22q11 deletion | 12 (ages 10 to 21 years) | Maladaptive behaviour | MRI study in 4; no abnormalities | NA | >10% developed psychiatric disorders. Chronic schizophrenia with paranoid delusion; personality disorders in adolescence and adulthood. Late-onset, no precipitating event |
| Murphy et al., 1999 | Evaluation using a structured interview to establish a DSM-IV diagnosis. Case-control study of patients with schizophrenia | 50 adults (≥17 years) | Symptoms of schizophrenia, psychosis, and schizotypy | NA | 22q11 deletion confirmed by FISH using N25 | 15 patients (30%) had psychotic disorders; 12 met criteria for schizophrenia. 6 patients (12%) had major depressive disorder with no psychotic features. Patients with VCFS and schizophrenia had fewer negative symptoms and were older at disease onset than controls. |
| Schneider et al., 2014 | Study of psychiatric morbidity. Comprehensive structured evaluation using validated instruments | 1402 participants (ages 6-68 years) | Maladaptive behaviour, disruptive behaviour, atypical cognitive function | NA | 22q11 deletion confirmed by FISH | ADHD was the most frequent disorder in children (37.1%), and was more common in male patients. Anxiety and mood disorders were more frequent among female patients. 41% of adults had psychotic disorders. |
| Gothelf et al., 2013 | Longitudinal study to analyse the course of psychiatric disorders and identify risk factors. Baseline and 4-year follow-up assessments. Use of standardised tools | 125 patients | Symptoms of anxiety, cognitive impairment | NA | Assessment of COMT Val108/158Met polymorphism | Prevalence of mood disorders is low during childhood and increases in late adolescence. Anxiety disorder at baseline and low global and verbal IQ scores predict psychotic disorders and severity of positive symptoms at 4 years. |
| Green et al., 2009 | Cohort study of patients with VCFS. Participants were evaluated with a structured clinical interview and age-appropriate versions of the Wechsler intelligence tests. | 172 patients (M=90, F=82), ages 5-54 years | Congenital anomalies, learning difficulties, psychiatric symptoms | NA | 3Mb deletion in 89% of cases. Parental origin was determined in 121 cases. | Psychotic and mood disorders are infrequent in childhood but become increasingly common in adulthood (40.7% depression, 32.1% psychotic disorder). Cognitive scale scores are negatively correlated with age. |
| Zagursky et al., 2006 | Study to determine ADHD prevalence in children with VCFS. Children and their parents underwent clinical assessment and structured interviews with standardised tools. | 52 children plus their parents (M=23, F=29), ages 4-17 years | Cleft palate, hypernasal speech, cardiac anomalies, learning difficulties, hypocalcaemia, characteristic facial appearance | NA | Presence of a deletion in 22q11 was previously confirmed. | Prevalence of ADHD (especially inattentive ADHD) was 10 times higher in the sample than in the normal population: 34% (n=41) according to children's self-reports, 54% (n=52) according to parents, and 43% (n=49) according to expert evaluation. |
| Antshel et al., 2007 | Comparative study to determine ADHD prevalence in children with VCFS. Children and their parents underwent clinical assessment and structured interviews with standardised tools. | 683 children and adolescents | NA | NA | 22q11 deletion previously confirmed by FISH | A different profile was observed in children with VCFS and ADHD compared to children with idiopathic ADHD. Inattentive ADHD is the most common type among children with VCFS. Symptoms of inattention seem to be linked to general cognitive function. |
| Antshel et al., 2013 | Comparative study of children with VCFS to evaluate persistence of ADHD into adolescence. Participants underwent clinical assessment and a structured interview using a standardised tool at baseline and at 4 years. Evaluation of behavioural, cognitive, social, school, and family functioning | 72 children (37 with ADHD, 35 without ADHD) | NA | NA | 22q11 deletion previously confirmed by FISH | 65% of children with VCFS continued to have ADHD in adolescence. Predictors of ADHD persistence include a consistent family history of the condition, childhood depression, high levels of hyperactivity, and intrusion errors in a verbal learning test. 15% of children not meeting diagnostic criteria for ADHD in childhood developed the disorder in adolescence; however, all children had displayed subclinical symptoms in childhood. |
| Papolos et al., 1996 | Systematic evaluation of psychiatric disorders in patients with VCFS using a structured clinical interview | 25 patients (ages 5-34 years) | Somatic anomalies, learning difficulties, behavioural disorders | MRI study only when indicated clinically (n=12); no brain abnormalities were found. | 22q11 deletion previously confirmed by FISH | 64% (n=16) met diagnostic criteria for bipolar disorder, which appeared in late childhood or early adolescence. 20% (n=5) met diagnostic criteria for ADHD and 16% (n=4) for ADHD without hyperactivity. No patients met criteria for psychotic disorder or schizophrenia. |
| Fabbro et al., 2012 | Study aiming to confirm the high prevalence of depression and anxiety and evaluate the correlation between anxiety and depression symptoms and poorer cognitive and adaptive function using standardised tools | 73 children and adolescents | NA | NA | 22q11 deletion previously confirmed by FISH | 62% had anxiety disorders, especially specific phobias; 27% had chronic depression. Presence of anxiety and depression symptoms was found to be correlated with low IQ scores and poorer adaptive function. |
| Antshel et al., 2007 | Study evaluating ADHD prevalence in children with VCFS | 41 children and adolescents (M=21, F=20) | Impaired social interaction, difficulties in adaptive functioning | Larger right amygdalas in MRI, with no other relevant findings. | 22q11 deletion previously confirmed by FISH | 17 children met diagnostic criteria for ASD (8 with autism, 9 with ASD). 94% of children with VCFS and ASD had other psychiatric comorbidities, compared to 60% of children with VCFS only. |
IQ: intelligence quotient; ID: intellectual disability; DSM: Diagnostic and Statistical Manual of Mental Disorders; FISH: fluorescence in situ hybridisation; NA: not applicable or not included in the study; MRI: magnetic resonance imaging; VCFS: velocardiofacial syndrome; ADHD: attention-deficit/hyperactivity disorder; ASD: autism spectrum disorders.
Furthermore, the risk of schizophrenia is twice as high in patients with mood disorders and 6 times higher in those with anxiety.11
Attention-deficit/hyperactivity disorderIn 2006, Zagursky et al.14 published a study of a series of 52 children with 22q11.2DS. Prevalence of ADHD in their sample was estimated using the child and parent versions of the Children's Interview for Psychiatric Syndromes (ChIPS and P-ChIPS) and was found to reach 65%, nearly 10 times higher than in the general population. More recent series have reported a prevalence of ADHD of 37.1% during childhood, progressively decreasing to 15.59% in patients older than 18.11 ADHD is currently the most frequently reported comorbidity in patients with 22q11.2DS.
Unlike with idiopathic ADHD, patients with 22q11.2DS more frequently display inattentive ADHD than hyperactive-impulsive or combined-type ADHD.11,14,15 Evidence regarding the frequency of ADHD subtypes by sex is heterogeneous. Predictors of ADHD persistence into adolescence in patients with 22q11.2DS were similar to those of children without the disease, and included a family history of ADHD and a personal history of childhood depression.16
ADHD is believed to be associated with a higher risk of presenting other psychiatric comorbidities in these patients. However, according to a study by Antshel et al.,15 patients with idiopathic ADHD are significantly more likely to present major depressive disorder, behavioural disorders, or oppositional defiant disorder at some point in their lives than patients with ADHD plus 22q11.2DS. Other psychiatric disorders, particularly anxiety disorders (social phobia, simple phobia, separation anxiety disorder, generalised anxiety disorder), affect both groups similarly.15
Depressive disorder and bipolar-spectrum disordersAccording to current evidence, bipolar-spectrum disorders are also more prevalent among patients with 22q11.2DS than in the general population. In 1996, Papolos et al.17 published a study of a series of 25 patients with 22q11.2DS; 64% met the DSM-III-R criteria for bipolar-spectrum disorders, including bipolar I disorder, bipolar II disorder, cyclothymia, and schizoaffective disorder (mania). In this series, mean age at symptom onset was 12 years, which suggests that 22q11.2DS promotes early onset of bipolar disorder. In a study including 1420 patients, Schneider et al.11 reported that the prevalence of bipolar-spectrum disorders increases with age, reaching nearly 4% in adulthood.
Depression is present in 12% to 29% of patients with 22q11.2DS; this psychiatric disorder is more frequent in early adolescence (ages 12-15).4,18 Major depressive disorder has a prevalence of 8%, and is more frequent among female patients.11
Anxiety disordersAnxiety disorders have been found to be as frequent as ADHD in some series, with an estimated mean incidence of 39%. The most frequent types are specific phobias (fear of the dark and fear of animals), followed by generalised anxiety disorder, separation anxiety disorder, social phobia, and obsessive-compulsive disorder.4,18 Prevalence of anxiety disorders decreases with age. These disorders are more frequent among female patients.11
Patients with cognitive impairment are 2.5 times more likely to present anxiety. There is low-quality evidence suggesting that children with 22q11.2DS and anxiety during childhood have a higher risk of presenting bipolar disorder at an early age.4
Autism spectrum disordersAlterations in social interaction, patterns of restricted interests, and alterations in communication are frequent in patients with 22q11.2DS. According to the literature, 20% to 50% of patients with 22q11.2DS meet diagnostic criteria for ASD. In a study by Niklasson et al.,5 23% of the 100 patients included met DSM-IV diagnostic criteria for ASD, although only 5% of these had autistic disorder. No association was found between presence of ASD and cardiac and auditory manifestations or velopharyngeal insufficiency in these patients.
We mentioned previously that around 60% of patients with 22q11.2DS have some type of psychiatric disorder. However, this percentage increases to 94% in patients with 22q11.2DS plus ASD.19 ADHD is the psychiatric disorder most frequently associated with ASD. Combined-type ADHD is more frequently associated with ASD than inattentive ADHD.5
From a neuroanatomical viewpoint, patients with 22q11.2DS display reduced cerebellar volume and increased amygdala volume in imaging studies; the same is true of patients with autistic disorder. It is not clear whether these anatomical alterations bear an association with increased risk of autistic disorder. Comparison of images from children with 22q11.2DS and children with 22q11.2DS plus ASD reveals very few differences, the only significant finding being increased volume of the right amygdala in the second group.19
Genetics and psychiatric manifestationsWhile 80% to 95% of the cases are due to de novo mutations, there is a small percentage of patients who inherit the mutation from a parent, more frequently from the mother, following an autosomal dominant pattern. This vulnerability of the 11.2 region of the long arm of chromosome 22 is explained by the presence of several low copy repeats (LCR). LCRs are highly homologous genetic elements; this promotes improper alignment during recombination in gametogenesis, resulting in a deletion on one recombinant chromosome and a duplication on the other.20
The size of the deletion is variable. The 2 most frequent deletions are 1.5 and 3 Mb in size. However, no association has been found between deletion size and clinical phenotype.3 Research into the genes of this region of chromosome 22 has found a strong association between the absence of certain genes and clinical phenotype, and particularly psychiatric manifestations of the syndrome. The COMT and PRODH genes are of particular interest.21,22
COMT codes for a postsynaptic enzyme involved in dopamine degradation, especially in the prefrontal cortex. This gene is highly polymorphic due to a guanine-to-adenine substitution at codon 158, which leads to a valine-to-methionine substitution in this position. The isoform with the methionine substitution results in a 30% decrease in enzyme activity and, consequently, dopamine accumulation in the prefrontal cortex. In patients with 22q11.2DS, this polymorphism combined with a deletion in the other allele has been associated with schizophrenia and other psychiatric disorders.20
PRODH codes for proline dehydrogenase, an enzyme participating in the degradation of proline, a glutamatergic receptor agonist and promoter of excitatory neurotransmission. Half of patients with 22q11.2DS have hyperprolinaemia, which has been associated with lower intellectual level, epilepsy, and psychosis.20
Furthermore, these 2 genes have an epistatic relationship by which PRODH deficiency leads to increased expression of COMT in the prefrontal cortex. This compensatory mechanism would be reduced in patients with 22q11.2DS, altering dopamine regulation and increasing the risk of psychotic symptoms.3
The TBX1 gene, a transcription factor of the T-Box family, plays a crucial role in organogenesis and has extensively been linked to multiple manifestations of 22q11.2DS. However, no association has been found with psychosis or other psychiatric symptoms.20
A recent study suggested that 22q11.2 duplications may have a protective effect against schizophrenia.23 This would support the hypothesis that the genes in that region have a direct association with psychotic symptoms.
Conclusions22q11.2DS is a clinically heterogeneous and relatively frequent syndrome in our setting. Psychiatric manifestations, although frequent in these patients and severe in some cases, have long been underestimated by physicians. This underscores the need to increase awareness of the high prevalence and severity of these symptoms among healthcare professionals, who should watch closely for any of these manifestations and inform patients and their parents about any risk of psychiatric symptoms developing.
The association between 22q11.2DS and schizophrenia has been clearly established. However, presence of other signs of the syndrome is rarely evaluated in cases of newly-diagnosed schizophrenia. The same is true of ADHD, mood disorders, anxiety, and ASD. Correct diagnosis requires information about other symptoms associated with the syndrome. This involves a comprehensive physical examination, an appropriate medical history, and a high level of suspicion in patients with psychiatric disorders who also display other typical features of 22q11.2DS, such as cleft palate, cardiac alterations, or a history of hypocalcaemia.
Genetic diagnosis, which is available in Spain, not only helps determine the aetiology of the syndrome and anticipate other associated comorbidities, but also enables the provision of genetic counselling, given the autosomal dominant inheritance pattern of the syndrome.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bertrán M, Tagle FP, Irarrázaval M. Manifestaciones psiquiátricas del síndrome de deleción 22q11.2: una revisión de la literatura. Neurología. 2018;33:121–128.