Alzheimer disease (AD) causes progressive cognitive decline leading to loss of independence for activities of daily living; rivastigmine is one of the drugs used for symptomatic management.
ObjectiveTo assess the therapeutic use of different pharmaceutical forms of rivastigmine in patients with AD in normal clinical practice.
Patients and methodsCross-sectional, observational, multi-centre study conducted on patients with mild to moderate AD treated with rivastigmine in Spanish outpatient clinics specialising in Geriatrics, Psychiatry, and Neurology. Data regarding use of oral (OR) and transdermal (TDR) rivastigmine, compliance (degree of adherence), and caregiver satisfaction with treatment were evaluated.
ResultsIn total, 2252 patients with a mean age of 77.2 years were included; 60.2% were women. AD was moderate to moderately severe in 58.4%. Rivastigmine treatment was started orally in 54.4% of the patients and transdermally in 45.6%; 35.6% of those who started treatment by the OR route switched to TDR. A single dose adjustment was sufficient for 77.5% of patients on TDR treatment vs. 11.8% of patients receiving OR treatment. More patients on TDR treatment (80.8% vs. 57.1% on OR treatment) reached the maximum therapeutic dose of rivastigmine and did so in a shorter period of time (51.6 vs. 205.8 days). Compliance rates (60.5% vs. 47.2%) and caregivers’ satisfaction with treatment (89.4% vs. 81.9%) were also higher for TDR.
ConclusionsIn normal clinical practice, using the TDR route of administration improves dose titration and drug compliance, allowing more patients to reach the maximum recommended dose of rivastigmine in a shorter time period.
La enfermedad de Alzheimer (EA) produce un deterioro cognitivo progresivo que conlleva la pérdida de independencia para las actividades de la vida diaria, siendo la rivastigmina uno de los fármacos utilizados para su tratamiento sintomático.
ObjetivoEvaluar el manejo terapéutico con distintas formas galénicas de rivastigmina, en sujetos con EA en la práctica clínica habitual.
Pacientes y métodosEstudio transversal, multicéntrico realizado en consultas españolas de Geriatría, Psiquiatría y Neurología, en sujetos con EA de leve a moderadamente grave que recibían rivastigmina. Se recogieron datos sobre el modo de uso de rivastigmina oral (RO) y transdérmica (RTD), el cumplimiento terapéutico (grado de adherencia) y la satisfacción del cuidador.
ResultadosSe evaluaron 2.252 sujetos con edad media de 77,2 años; 60,2% mujeres. El 58,4% presentaban EA moderada-moderadamente grave. El 54,4% habían iniciado el tratamiento con RO y el 45,6% con RTD; el 35,6% de aquellos con RO cambiaron a la vía transdérmica. El 77,5% con RTD requirió un solo ajuste de dosis vs. 11,8% con RO. El 80,8% de los sujetos con RTD alcanzaron la dosis máxima de rivastigmina (vs. 57,1% RO) en menos tiempo (51,6 vs. 205,8 días). El cumplimiento fue mayor con RTD (60,5 vs. 47,2%) así como el porcentaje de cuidadores satisfechos con el tratamiento (89,4 vs.81,9%).
ConclusionesEn la práctica clínica habitual, la RTD facilita la dosificación y mejora el cumplimiento, permitiendo a un mayor porcentaje de sujetos alcanzar la dosis máxima recomendada de rivastigmina en un plazo de tiempo menor.
Alzheimer disease (AD) is currently the most frequent cause of dementia. It is characterised by cognitive disorders that progressively lead to impairment of other mental functions, inability to perform activities of daily living, and psychological and behavioural symptoms.1,2
Specific pharmacological management of AD is only symptomatic, and treatments include the cholinesterase inhibitors rivastigmine, galantamine, and donepezil (prescribed in cases of mild to moderately severe AD), and memantine (indicated for moderate to severe AD). Rivastigmine improves cognitive and functional state in AD patients and decreases their psychological and behavioural symptoms.3–6
Rivastigmine was initially marketed for oral delivery (capsules and solution) and more recently for transdermal delivery (patches). The mean drug exposure time is similar for both routes of administration. Drug plasma levels during 24hours for oral rivastigmine (OR) show spikes every 12hours, while transdermal rivastigmine (TDR) shows more sustained delivery.7,8 This may offer certain advantages in normal clinical practice.
TDR has been shown to have good efficacy, safety, and tolerance,9,10 with no differences in efficacy between oral and transdermal routes of administration. However, gastrointestinal disorders have been more frequently observed with OR treatment, with nausea and vomiting being 3 times more frequent than with TDR.11 Researchers conclude that tolerability is greater for TDR than for OR.
Drug compliance is fundamental to achieving effective treatment, especially when managing chronic diseases. One of the best strategies for optimising treatment compliance is to simplify drug regimens by using more comfortable administration routes or decreasing the number of doses per day, especially in dementia patients and the elderly.12,13 Transdermal administration may favour treatment compliance in diseases such as AD in which carers have to monitor treatment because of the patient's cognitive deficits and neuropsychiatric disorders.14
Although TDR offers theoretical advantages, few data show that these advantages have an effect on normal clinical practice. The current study presents information on the therapeutic management of patients treated with rivastigmine (oral or transdermal) by analysing dose adjustment methods, treatment compliance, and carers’ satisfaction with treatment.
Patients and methodsThis cross-sectional multi-centre observational study was carried out in neurology, psychiatry, and geriatric medicine outpatient clinics across Spain. Each researcher had the task of including 10 consecutive outpatients, of either sex and aged 18 or older, with a diagnosis of mild to moderately severe AD according to DSM-IV-TR15 (Mini Mental State Examination by Folstein≥10).16 Patients had to have undergone treatment with OR or TDR at stable doses within at least 3 months prior to their inclusion in the study, and informed consent was required. Each researcher was asked to include 5 patients treated with OR and 5 others treated with TDR.
The sample size was calculated according to the TRAIN17 study results which estimated a sample of 1587 subjects per drug delivery method, resulting in a projected total of 3174 patients.
Each researcher collected data in a single visit by consulting the patient's medical history and interviewing his/her carer. Researchers recorded sociodemographic data, previous illnesses, and history of treatment with rivastigmine (from starting treatment to study visit: route of administration, dose, adjustments, and changes in the route of administration during dose escalation).
Treatment compliance was evaluated using the Spanish version of the Morisky adherence scale and the primary carers’ satisfaction with treatment was analysed using an ad hoc questionnaire similar to the one used in the KAPPA19 study. The 4 items on the questionnaire addressed the following concepts: (1) ease of delivering treatment, (2) ease of monitoring treatment, (3) how often treatment interfered with the carer's daily life, and (4) overall satisfaction with treatment. Questions 1, 2, and 4 had 4 multiple-choice answers: two favourable responses (‘very easy/very satisfied’ and ‘easy/satisfied’), and two unfavourable responses (‘difficult/unsatisfied’ and ‘very difficult/very unsatisfied’). Questions 3 had 5 multiple-choice answers: ‘always’, ‘most of the time’, ‘sometimes’, ‘rarely’, and ‘never’. The first 3 responses were unfavourable. Responses to items on the carer satisfaction questionnaire were classified as either favourable or unfavourable.
Categorical variables were described using absolute and relative frequencies, while continuous variables were described using the mean, standard deviation, median, and interval (minimum and maximum), including the total number of valid values for each parameter. Subgroups were compared using the Mann–Whitney U test for quantitative variables and the chi-square test for qualitative variables. Statistical analyses were performed using SAS statistical software version 9.1.3 for Windows. Bilateral tests were applied with a significance level of .05.
The protocol was approved by the Clinical Research Ethics Committee at Hospital Universitario Ramón y Cajal, Madrid, Spain.
ResultsDescription of the sampleBetween March and September 2009, 268 researchers included 2708 subjects; of these, 2252 provided data determined to be valid for the statistical analysis. The remaining 456 patients were excluded due to not meeting one or more of the selection criteria, especially the requirement of having been taking stable doses of rivastigmine in the preceding 3 months.
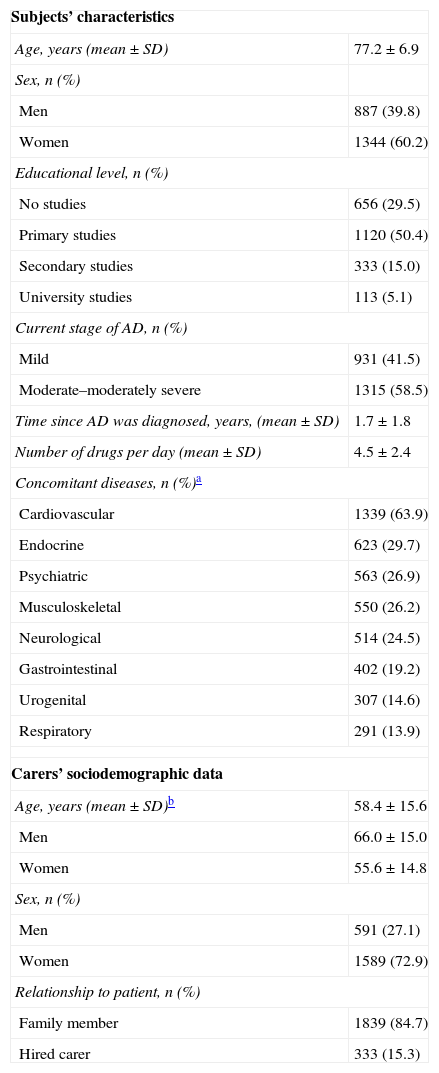
Table 1 shows patients’ sociodemographic and clinical characteristics; more than half were women, and they were significantly older than the male patients (77.6±6.8 years vs. 76.7±6.9 years; Mann–Whitney U test; P=.0004). Of the patient total, 55.8% were married or lived in domestic partnership and 37.7% were widowed. Almost all subjects lived in multi-person households (95.1%), while 4.9% lived alone. Most of the subjects who did not live alone resided with their families (78.1%); another 11.7% were institutionalised and 5.8% lived with hired carers. A total of 58.5% presented moderate to moderately severe AD and mean time since diagnosis was 1.7±1.8 years.
Subjects’ sociodemographic and clinical characteristics; carers’ sociodemographic data.
| Subjects’ characteristics | |
| Age, years (mean±SD) | 77.2±6.9 |
| Sex, n (%) | |
| Men | 887 (39.8) |
| Women | 1344 (60.2) |
| Educational level, n (%) | |
| No studies | 656 (29.5) |
| Primary studies | 1120 (50.4) |
| Secondary studies | 333 (15.0) |
| University studies | 113 (5.1) |
| Current stage of AD, n (%) | |
| Mild | 931 (41.5) |
| Moderate–moderately severe | 1315 (58.5) |
| Time since AD was diagnosed, years, (mean±SD) | 1.7±1.8 |
| Number of drugs per day (mean±SD) | 4.5±2.4 |
| Concomitant diseases, n (%)a | |
| Cardiovascular | 1339 (63.9) |
| Endocrine | 623 (29.7) |
| Psychiatric | 563 (26.9) |
| Musculoskeletal | 550 (26.2) |
| Neurological | 514 (24.5) |
| Gastrointestinal | 402 (19.2) |
| Urogenital | 307 (14.6) |
| Respiratory | 291 (13.9) |
| Carers’ sociodemographic data | |
| Age, years (mean±SD)b | 58.4±15.6 |
| Men | 66.0±15.0 |
| Women | 55.6±14.8 |
| Sex, n (%) | |
| Men | 591 (27.1) |
| Women | 1589 (72.9) |
| Relationship to patient, n (%) | |
| Family member | 1839 (84.7) |
| Hired carer | 333 (15.3) |
SD, standard deviation; AD, Alzheimer disease.
Mean age for carers was 58.4±15.6 years and most were women (72.9%). Female carers were significantly younger than their male counterparts (55.6±14.8 years vs. 66.0±15.0 years; Mann–Whitney U test; P<.0001). Most carers had completed primary studies (44.0%) or secondary studies (32.9%) and most were related to the patient (84.7%).
Treatment with rivastigmineOf the patient total, 54.4% of the subjects (n=1222) first began treatment with OR, whereas 45.6% (n=1026) were first treated with TDR. The most frequent initial dose of OR (for 88.9% of the patients) was 1.5mg/12hours. TDR was most commonly dosed at 4.6mg/12hours (for 97.0%).
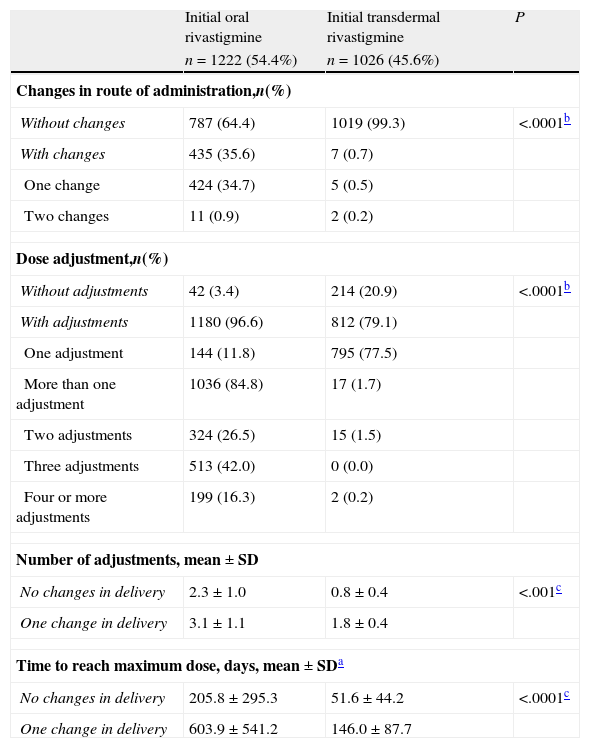
A total of 34.7% of the patients (n=424) who began treatment with OR changed to TDR and 0.9% (n=11) resumed OR treatment after having changed to the transdermal route. Only 0.7% of the patients (n=7) who began treatment with TDR changed the route of administration; 0.5% (n=5) changed to OR and 0.2% (n=2) resumed treatment with TDR after a temporary change to OR (Table 2). As a result, 36.8% of the patients were treated with OR (n=827) and 63.2% were treated with TDR (n=1421) at the time of the study visit. The mean time between starting rivastigmine treatment and the study visit was 2.0±1.7 years for subjects with OR and 0.4±0.3 for subjects with TDR; mean time was significantly higher for subjects treated orally (Mann–Whitney U test; P<.0001).
Changes in rivastigmine delivery and dose adjustments.
| Initial oral rivastigmine | Initial transdermal rivastigmine | P | |
| n=1222 (54.4%) | n=1026 (45.6%) | ||
| Changes in route of administration,n(%) | |||
| Without changes | 787 (64.4) | 1019 (99.3) | <.0001b |
| With changes | 435 (35.6) | 7 (0.7) | |
| One change | 424 (34.7) | 5 (0.5) | |
| Two changes | 11 (0.9) | 2 (0.2) | |
| Dose adjustment,n(%) | |||
| Without adjustments | 42 (3.4) | 214 (20.9) | <.0001b |
| With adjustments | 1180 (96.6) | 812 (79.1) | |
| One adjustment | 144 (11.8) | 795 (77.5) | |
| More than one adjustment | 1036 (84.8) | 17 (1.7) | |
| Two adjustments | 324 (26.5) | 15 (1.5) | |
| Three adjustments | 513 (42.0) | 0 (0.0) | |
| Four or more adjustments | 199 (16.3) | 2 (0.2) | |
| Number of adjustments, mean±SD | |||
| No changes in delivery | 2.3±1.0 | 0.8±0.4 | <.001c |
| One change in delivery | 3.1±1.1 | 1.8±0.4 | |
| Time to reach maximum dose, days, mean±SDa | |||
| No changes in delivery | 205.8±295.3 | 51.6±44.2 | <.0001c |
| One change in delivery | 603.9±541.2 | 146.0±87.7 | |
SD, standard deviation.
Percentages calculated based on total number of assessable patients beginning treatment, broken down by route of administration.
Doses were adjusted in 96.6% of the patients initially treated with OR (n=1180) and 79.1% of those treated with TDR (n=812); the difference between percentages was statistically significant (Mann–Whitney U test; P<.0001; Table 2). A total of 712 subjects (58%) treated with OR required 3 or more dose adjustments before reaching the target dose. Only 17 of the subjects treated with TDR (1.7%) needed two or more adjustments in order to reach the target dose. The number of adjustments was significantly higher for subjects treated orally (Mann–Whitney U test; P<.0001; Table 2).
At the time of the study visit, 72.4% of the patients (n=1628) were being treated with the maximum recommended dose according to the route of administration. Among them, the percentage of subjects treated with the maximum dose was significantly higher for patients treated with TDR (9.5mg/24hours) than for patients treated with OR (6mg/12hours): 80.8% vs. 57.1%, respectively (Mann–Whitney U test; P<.0001).
The mean time to reach the maximum dose in subjects who did not change delivery methods was significantly higher for OR (205.8±295.3 days) than for TDR (51.6±44.2 days) (Mann–Whitney U test; P<.0001). Where the drug delivery method was changed, the times to reach the maximum dose were longer both in patients initially treated with OR (603.9±541.2 days) and in patients initially treated with TDR (146.0±87.7 days).
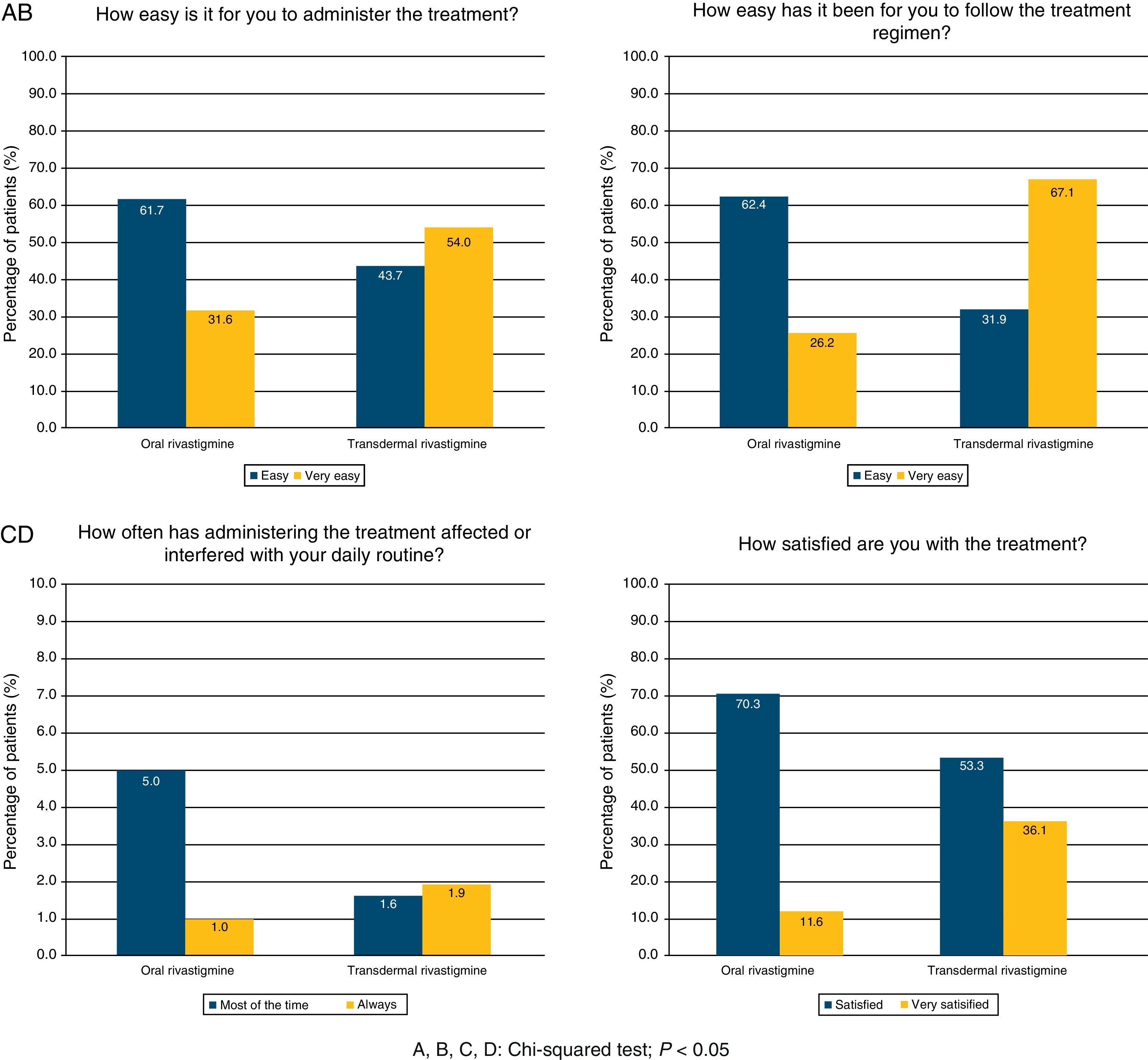
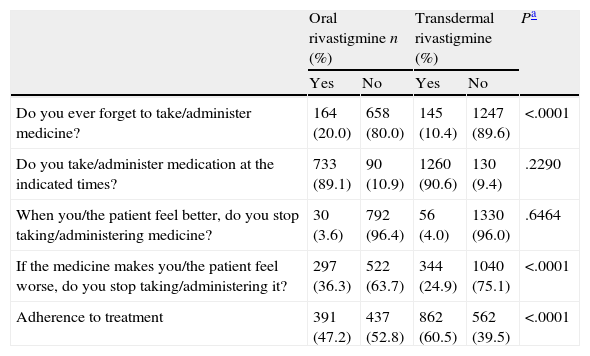
Treatment complianceOf the patient total, 22.1% of the subjects treated with OR and 23.8% of those treated with TDR were able to administer treatment themselves (chi-square test; P=.3468). Using the Morisky adherence questionnaire, we determined that 20.0% of the subjects treated with OR and 10.4% of those treated with TDR forgot to take their medication (chi-square test; P<.0001). Patients who stopped taking medication due to experiencing adverse effects accounted for 36.3% of all patients treated with OR and 24.9% of those treated with TDR (chi-square test; P<.0001). We found no differences between the two groups with regard to patients taking the medication at the indicated times or discontinuing the medication if they felt better. Overall treatment compliance was significantly higher in subjects treated with TDR (60.5%) than in subjects treated with OR (47.2%) (chi-square test; P<.0001; Table 3)
Morisky adherence questionnaire.
| Oral rivastigmine n (%) | Transdermal rivastigmine (%) | Pa | |||
| Yes | No | Yes | No | ||
| Do you ever forget to take/administer medicine? | 164 (20.0) | 658 (80.0) | 145 (10.4) | 1247 (89.6) | <.0001 |
| Do you take/administer medication at the indicated times? | 733 (89.1) | 90 (10.9) | 1260 (90.6) | 130 (9.4) | .2290 |
| When you/the patient feel better, do you stop taking/administering medicine? | 30 (3.6) | 792 (96.4) | 56 (4.0) | 1330 (96.0) | .6464 |
| If the medicine makes you/the patient feel worse, do you stop taking/administering it? | 297 (36.3) | 522 (63.7) | 344 (24.9) | 1040 (75.1) | <.0001 |
| Adherence to treatment | 391 (47.2) | 437 (52.8) | 862 (60.5) | 562 (39.5) | <.0001 |
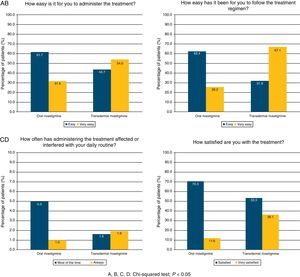
The questionnaire on the degree of satisfaction expressed by the patient's regular carer showed that 54.0% of the carers of subjects treated with TDR had no difficulties using the treatment and 36.1% were satisfied. For patients treated with OR, these percentages were 31.6% and 11.6%, respectively. Administering treatment was simpler with the TDR route (67.1% vs. 26.2%), whereas treatment with OR interfered more frequently with carers’ daily life (37.0% vs. 11.6%). TDR was assigned a significantly higher level of satisfaction than OR according to all items on the questionnaire (chi-square test; P<.0001; Fig. 1).
DiscussionThis study analyses treatment management with OR and TDR in subjects with mild to moderately severe AD in normal clinical practice.
Sociodemographic and clinical data revealed that the study population had characteristics similar to those of AD patients in other studies: old age, higher prevalence in women, low educational level, presence of concomitant illnesses (especially cardiovascular diseases), and polypharmacy.1,19 More than three-quarters of the subjects lived with their families (78.1%), with one family member entrusted with administering treatment. These results coincide with those from other studies, although percentages do vary from one country to another. More patients live with their families in semi-developed rather than developed countries, rural rather than urban areas, northern rather than southern Europe, and in countries offering fewer residences and less assistance for institutional living rather than the contrary.20–22 Most carers in this study were women, whether paid professionals or family members. These tendencies are consistent with results gathered by Alzheimer's Disease International in different countries.22
Ours is one of the first studies to address treatment management of rivastigmine with participation by all 3 of the medical specialties that are authorised to prescribe AD drugs in Spain in normal clinical practice. The purpose of the study is to better understand prescribing behaviours and how they affect patients and their carers. Overall, results seem to show that TDR is more beneficial than OR since it is associated with better scores on most of the treatment management parameters (compliance, optimal dose, time to reach the target dose).
According to its dose–response relationship, rivastigmine becomes more effective as doses increase, especially upon reaching the maximum recommended dose.9,11,23 However, an acceptable balance of both efficacy and tolerability must be achieved.24 In fact, preliminary data from a very recent study indicate that it may be possible to use higher doses than are currently recommended.25 In our study, the percentage of subjects treated with the maximum recommended dose was higher for patients with TDR than for those with OR. This dose was also reached in a significantly shorter time period (approximately 5 months). This finding is relevant because early drug treatment for AD is becoming increasingly important. In the IDEAL trial, the percentage of subjects who reached the maximum recommended dose was also higher for the transdermal route of administration.7,9 Results from the current study are similar, even though they were extracted from a normal clinical practice setting.
One of this study's important features is its detailed analysis of the administration route and dose management from the time treatment was first prescribed to each patient. Researchers recommend adjusting OR in a 3-step process and adjusting TDR in a single step. It is therefore surprising that nearly half of the subjects treated with OR (41.7%) underwent 1 or no adjustments, probably because dose scaling is more difficult for OR than for TDR. Our study was not designed to record the reasons for adjusting doses, but in any case, adjustments may reflect problems with treatment tolerance or compliance, lack of awareness of the optimal dose, and other care-related problems. Only 20.9% of the patients treated with TDR had no dose adjustments. This may be due to the high frequency of changes from OR to TDR, which at times makes it possible for doctors to choose the optimal dose according to a patients’ response and tolerance to the oral dose. The need for fewer adjustments may simplify the follow-up process and reduce the number of visits patients need during the early stages of treatment.
The SCALEX study showed that OR treatment with slow dose adjustment was accompanied by frequent interruptions due to low efficacy, which is the result of using subtherapeutic doses for extended periods of time instead of the currently recommended dose adjustment process.26 One of the advantages of TDR is that therapeutic doses can be reached in shorter times than with OR, which is clinically beneficial for the patient.
This study shows that both treatment compliance and carers’ satisfaction with the treatment were significantly higher for patients treated with TDR than for those on OR. Borah et al.27 observed that 42% of a group of patients with AD did not comply with oral treatment and suggested using transdermal delivery to improve treatment compliance. Rivastigmine's poor digestive tolerance, and the difficulty of adjusting OR doses according to clinical trials, are factors that may partially explain why carers are more satisfied with transdermal treatment.
Given that there is no specific method for assessing treatment compliance in AD, this study used the Morisky scale, which is brief, valid, and frequently used with different types of chronically ill patients.18 Data from the scale show treatment non-compliance among 52.8% of the patients treated with OR; this percentage was significantly lower in patients treated with TDR (39.5%). These differences were mainly due to the fact that subjects treated with OR forgot their doses more frequently and tended to stop taking the medication if it caused adverse effects. This indicates that one of TDR's advantages is that it is administered once daily, which seems to promote better treatment compliance. A 13.3% improvement in treatment compliance can be considered relevant, especially in an area in which compliance is so problematic.28 Our results coincide with those recently published in a study of Spanish patients with AD.29
Our study also shows that carers prefer the transdermal route of administration. The IDEAL clinical trial showed that 72% of carers preferred TDR at the end of the study. The most frequently cited reasons were ease of use and dosing simplicity. IDEAL was completed using the Alzheimer's Disease Caregiver Preference Questionnaire (ADCPQ).30,31 In contrast, we opted not to use that questionnaire since some of its items assess the efficacy of the two dosage forms, which is not the purpose of the study. Instead, we chose a questionnaire similar to that used in the KAPPA study.32 It features the same framework as the ADCPQ and addresses carers’ satisfaction with treatment. Results from the two studies were comparable.
Our study's weaknesses included, firstly, not having reached the expected sample size of 3174 subjects. However, enough patients were included in the study to analyse the study objectives with a 2.6% margin of error and the same statistical power described by the protocol. The sample size was sufficiently large and the study provides a good model of the AD population given that its characteristics were similar to those described in other international studies.1,19 On the other hand, a selection bias could be present since some patients may have been chosen for inclusion even though the study protocol specified recruiting subjects in consecutive order. This limitation is present in many studies carried out in normal clinical practice, in contrast with clinical trials. In addition, doctors may have initially transmitted a more positive impression of the transdermal dosage form than of the oral form, which could have influenced the results for carer satisfaction.
Based on the results of this study, we can conclude that using TDR rather than OR in normal clinical practice allows patients to reach the maximum recommended dose more quickly. In addition, TDR is associated with improved treatment compliance and better carer satisfaction.
Conflict of interestsThis study received financial support from Novartis Farmacéutica, S.A.
Alfonso J. Cruz Jentoft has participated as a moderator or speaker in educational activities organised by Grunenthal, Janssen-Cilag, Lundbeck, and Novartis, which market products for the treatment of Alzheimer disease. Basilio Hernández is employed full time by Novartis Farmacéutica, S.A., Barcelona (Spain).
We would like to thank all the researchers who contributed to the ENTERPRISE study (see Appendix 1). The company ADKNOMA provided effective logistical support and data analysis. Mirle Ferrer was the medical writer of this article and Mercè Viladrich provided the statistical analysis.
J. Abella Corral, H. Arquitecto Marcide, Ferrol; I. Abellan Miralles, H. San Vicente, Alicante; E. Aguera Morales, H. Reina Sofía, Córdoba; J.J. Aguirre Sánchez, H.U. Infanta Cristina, Badajoz; A. Alayon Fumero, C. Privada, Santa Cruz Tenerife; J. Alberca de Castro, C. Salud Mental Linares, Linares; J. Almajano Martínez, J. Benito León, H. 12 Octubre, Madrid; F. Alonso de Teso, C. Especialidades, Valladolid; J. Álvarez Gurtierrez, R. Andrés Celda, H. El Bierzo, Fuentes Nuevas; M.S. Amor Andrés, H. Virgen del Valle, Toledo; D. Apolinar García Estévez, H. Monforte, Lugo; S. Arabceta Arilla, CAP Cerdanyola-Ripollet, Ripollet; C. Arenas Cabrera, A. Marques de Paradas, Sevilla; J.A. Arenas Sánchez, Clínica Ponenet, Lleida; G. Ariza Zafra, H. Virgen del Perpetuo Socorro, Albacete; M.A. Arizcuren Domeño, R.G. Landazabal, Burlada; A. Arjona Padillo, A. Especialidades Bola Azul, Almería; M. Amaldos Paya, H. Santa María del Rosell, Cartagena; V. Arroyo Martínez, C. Privada, Bilbao; A. Avila Rivera, H.G. L’Hospitalet, L.Hospitalet; L. Benítez Rangel, H. Juan Grade, Jerez de la Frontera; J.A. Bergache Yarza; H.C. Bidasoa, Hondarribia; M.T. Bernal Bernal, F. Sanitaria Igualada, Igualada; V. Bertol Alegre, H.U. Miguel Servet, Zaragoza; E. Bescansa Heredero, H. Reina Sofía, Códoba; J.A. Boltes Alandi, H.G. Granollers, Granollers; M. Bonet Valls, H. Arnau Vilanova, Valencia; S. Boyero Durán, H. Cruces, Barakaldo; H.J. Bueno Perdomo, H.N. Señora de la Candelaria, La Laguna; M. Bujanda Alegría, H. Navarra, Pamplona; A. Burriel Roselló, H. Clínico Universitario, Zaragoza; N. Caballol Pons, CAP La Rambla, Sant Feliu de Llobregat; P. Cacabelo Pérez, H.C. Universitario de Salamanca, Salamanca; A. Callen Soto, H. Sant Boi, Sant Boi de Llobregat; J.M. Camacho Cuartero, C. Salud Mental Torrent, Torrente; A.B. Caminero Rodríguez, H. Ntra. Señora de Sonsoles, Ávila; J. Campdelacreu Fumadó, H. Bellvitge, L’Hospitalet de Llobregat; A. Cano Orgaz, H. Mataró, Barcelona; S. Cantanero Duque, H. Móstoles, Móstoles; F. Cañadillas Hidalgo, H. Reina Sofía, Córdoba; A. Cardozo Dodera, H. Provincial Santa María, Lleida; F.J. Carrillo Padilla, H.U. de Canarias, Santa Cruz de Tenerife; T. Casadevall Codina, H. Sant Jaume de Cellla, Barcelona; V. Casado Ruiz, H. Mataró, Barcelona; C. Castejón Gabriel, ABS Chafarinas, Barcelona; L. Castilla Guerra, H. Ntra Señora de la Merced, Osuna; J. Cerda Fayos, H. Provincial de Castellón, Castellón de la Plana; R.P. Chamarro Lazaro, H.C.U. Valencia; A. Da Silva González, R. Geriátrica Fuentes Blancas, Burgos; R.M. de Eugenio Huelamo, R. Geriátrica Palamós, Palamós; I. de la Serna de Pedro, M. de Toledo Heras, H. Ramón y Cajal, Madrid; R. de la Vega Cotarelo, H. Punta de Europa, Algeciras; B. del Amo Martínez, C. Padre Menni, Navarra; V.F. del Olmo García, C. Privada, Valladolid; M.A. del Real Francia, H. G. de Ciudad Real, Ciudad Real; E. Delgado Parada, H. Comarcal Jarrio, Coaña; C. Díaz Marín, H. de la Marina Baixa, Vila Joiosa; S. Díaz Nicolás, O. Fabre Pi, H.G. de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria; A. Díaz Ortuño, H. Reina Sofía, Córdoba; M. Diez Lopez, C. Privada, Burgos; L. Docasar Bertolo, U.Salud Mental Monforte, Monforte de Lemos; J.A. Dominguez Moran, H. de la Ribera, Alzira; E. Duaso Magaña, H. Sant Llatzer, Terrassa; J.C. Duran Alonso, H. Juan Grande, Jerez de la Frontera; C. Echeandía Ajamil, H. Central de la Defensa Gómez Ulla, Madrid; P. Eguia del Río, H.G. Lanzarote, Arrecife; J. Escudero Torrella, A. Especialidades Juan Llorens, Valencia; F.J. Espada Oliván, H. Sant Jaume de Calella, Calella; J. Esparza Rodríguez, H. Provincial Divino Valles, Burgos; A. Espino Montoro, H. Nuestra Señora de la Merced, Osuna; M.C. Espinosa Val, H. Sant Jaume, Barcelona; M.J. Esteban Dombriz, H.G.U. Guadalajara, Guadalajara; J. Estrany Bauza, Policlínica Miramar, Palma de Mallorca; E. Fages Caravaca, H. Santa María del Rosell, Cartagena; Y. Fernández Bullido, A. Moratalaz, Madrid; S. Fernández González, H. Creu Roja, Barcelona; D. Fernández Uria, H. Cabueñes, Gijón; J.M. Fernández-Cuesta Cuesta, A. Centro Diagnóstico Santander, Santander; E. Ferreira González de Viñaspre, H. Txagorritxu, Vitoria-Gateiz; A. Ferreros Vilar, Centro Salud Xativa, Xàtiva; A. Formica Martínez, A. Nuestra Señora del Coro, Donostia-San Sebastián; F. Formiga Pérez, H. Duran i Reynals, L’Hospitalet de Llobregat; J. Foronda Bengoa, H. Ciudad de Jaén, Jaén; M. Fragoso Martínez, Consorci Sanitari de Terrassa, Terrassa; J.I. Franch Valverde, H. Clínico, Valladolid; E. Franquet Gómez, H. Residencia Sant Camil, Sant Pere de Ribes; C.A. Gahete Jiménez, H. Virgen del Puerto, Plasencia; M.A. García Alhambra, H.G.U. Gregorio Marañón, Madrid; M.C. García de Cassasola García, H. Rambla, Santa Cruz de Tenerife; A. García de Vinuesa Matute, A. Especialidades Villanueva de la Serena, Villanueva de la Serena; G. García Gómez, R. Ancianos San Juan de Dios, El Álamo; C. Gacía Guijo, H.G. Jerez de la Frontera, Jerez de la Frontera; M.T. García López, A. Especialidades Bola Azul, Almería; H. García Miranda, H. Zumarraga, Zumarraga; J.M. García Moreno, H. Virgen de Macarena, Sevilla; M.I. García Tomás, H.G. Almansa, Albacete; F.J. Garzón, Maldonado, H. Virgen de la Victoria, Málaga; J. Gascón Bayari, CAP Ramona Via i Pros, El Prat de Llobregat; M.I. Gastón Zubimendi H. Virgen del Camimo, Pamplona; F. Gazquez Martínez, C. Salud El Ejido, El Ejido; J.M. Giménez García, H. Virgen de Altagracia, Manzanares; J.J. Giron Ubeda, H.G. Jerez de la Frontera, Jerez de la Frontera; M. Gómez Beldarrain, H. Galdakao, Galdakao; J.L. González Gutiérrez, H.C. San Carlos, Madrid; F. González Martínez, H. Cuenca, Cuenca; M. González Platas, H. Comarcal Blanes, Blanes; V. González Torres, H. Ciudad de Jaén, Jaén; F. Gracia Fleta, H.G. de Alicante, Alicante; M. Gracia Naya, A. San José, Zaragoza; C. Guerrero castaño, H. Sant Jaume de Calella, Calella; D. Guillen Mesado, C. Especialidades Peña Prieta-Hermanos Sangro, Madrid; J. Gutiérrez García, H.U. San Cecilio, Granada; L. Gutiérrez Rojas, A. Baza, Baza; J. Hernández Vara, H. Vall d’Hebron, Barcelona; F. Herrero Cerezo, H. Mataró, Mataró; R.E. Ibañez Alonso, H.G. Ciudad Real, Ciudad Real; R. Ibañez Corrales, C.S. Alcalá la Real, Alcalá la Real; J.A. Iniesta Valera, C.M. Jurado Cobo, H.G. Reina Sofía, Murcia; A. Jaen Peraire, CAP Numancia, Barcelona; J. Jara González, P. Pablo Luengo, Navalmoral de la Mata; S. Jauma Classen, CAP La Gabarra, Cornellà de Llobregat; J.M. Jiménez Paez, H. Cruz Roja, Córdoba; L. Lacruz Ballester H. San Francisco de Borja, Gandia; F. Lacruz Bescos, H. Navarra, Pamplona; L. Lillo Triguero, H.G. Univ. Gregorio Marañón, Madrid; J.M. Lomba Borrajo, C. Saude A Ponte, Ourense; J. Lominchar Espada, C.S.H.G. Univ. Valencia, Valencia; J.M. Lopez Arlandis, H. Virgen de los Lirios, Alcoy; A.I. López Fraile, H. Virgen de Altagracia, Manzanares; A.M. López Real, H. Juan Canalejo, A Coruña; M. López Roa, H. Psquiátric Febles Campos, Santa Cruz de Tenerife; I. Lopez Zuazo, H.G. Univ. Guadalajara, Guadalajara; J.M. Losada Domingo, H. Cruces, Barakaldo; P. Lozano Sanmartín, C. Sagrado Corazón, Sevilla; A. Luengo Dos Santos, A. Henares, Madrid; A. Luna Rodríguez, H. Palamós, Palamós; O. Llamazares de la Fuente, C. San Francisco, León; M. Llanero Luque, C.P.S.R. Calvo, Madrid; M.A. Llaneza González, H. Arquitecto Marcide, Ferrol; A. Maestre Martínez, H. Ciudad de Jaén, Jaén; S. Manzano Palomo, H.C. San Carlos, Madrid; M.M. Marcos Toledano, H. Mérida, Mérida; J. Marey López, H. Juan Canalejo, A Coruña; C. Margarito Rangel, H. Virgen del Perpetuo Socorro, Albacete; C. Marques, Clonus, Palma de Mallorca; M. Márquez Martínez, H. Virgen de la Victoria, Málaga; C. Marsal Alonso, H. Virgen de la Salud, Toledo; S. Martin Balbuena, H. Vega del Río Segura, Cieza; J.C. Martín Berra, C. Santa María de la Asunción, Tolosa; M.E. Martin Correa, H. Virgen del Valle, Toledo; J.J. Martín Fernández, J. Meca Lallana, H. U. Virgen de la Arrixaca, El Palmar; J. Martín Polo, H.G. Rio Carrión, Palencia; J.R. Martínez Calvo, H. Calde, Calde; R. Martínez Fernández, H. Figueres, Figueres; D. Martínez Lozano, H. G. Castellón, Castelló de la Plana; J. Matarredona Catalá, U.S.M. Alcoy, Alcoy; J.A. Mauri Llerda, H.C.Univ. Lozano Blesa, ZaragozaV. Medrano Martínez, H. Elda, Alicante; P.A. Megia López, H. Prov. San Telmo, Palencia; J.C. Miñana Climent, H. Monte Naranco, Oviedo; F.J. H. Son Dureta, Palma de Mallorca; T. Molina Nieto, H. Reina Sofía, Córdoba; A. Molins Albanell, H. Josep Trueta, Girona; J.A. Monge Argiles, H.G. Univ. Alicante, Alicante; E. Montes Latorre, H. Univ. Virgen del Rocío, Sevilla; M.D. Morales Martínez H. Virgen de la Macarena, Sevilla; A. Moya Rodrigo, C. Salud Xàtiva, Xàtiva; C. Muñoz Fernández, Amb. Especialidades Bola Azul, Almería; C. Obiol Madrid, UFISS C. de la Mercè, Barcelona; J.J. Ochoa Amor, J.J. Ochoa Sepúlveda, H. Reina Sofía, Córdoba; T. Ojea Ortega, H. G. Málaga, Málaga; J.A. Olivan Usieto, H. Comarcal Alcañiz, Alcañiz; C. Oliveras Ley, H. del Mar, Barcelona; C. Oreja Guevara, H. La Paz, Madrid; JC Ortigosa Digon, C.S. Mental Naranco, Oviedo; M. Ortigosa Luque, H. Princesa de España, Jaén; A. Oterino Duran, H. Marqués de Valdecilla, Santander; P. Otermin Vallejo, H.G. Granollers, Granollers; F. Padilla Parrado, H. Clínico, Málaga; S. Palau Duarte, H. San Vicente del Raspeig, San Vicente del Raspeig; A. Pampliega Pérez, H.G.U. de Alicante, Alicante; M.C. Pardo Bustamante, H. de Sant Andreu, Manresa; G. Pardo Castillo, Amb. Baza, Baza; LF Pascual Millan, H.C.U. Lozano Blesa; B. Pascual Sedano H Santa Creu i Sant Pau, Barcelona; J.C. Pastor de la Fuente, Residencia Ancianos San Juan de Dios, El Álamo; A. Pato Pato, H Povisa, S.A., Vigo; J.M. Paz González, H. Xeral de Lugo, Lugo; R. Pego Reigosa, H. Xeral de Lugo, Lugo; C. Peiro Vilaplana, H. de la Ribera; J. Peña Martinez, H. San Agustin, Avilés; D. Perez Martinez, H. Infanta Cristina de Parla, Parla; M.C. Perez Vieitez, H.G. de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria; C. Perla Muedra, H. Arnau de Vilanova, Valencia; V.C. Peset Mancebo, C.S. Aldaia - Antic Regne, Aldaia; S. Piles Galdon Fundació Privada H. de Mollet, Mollet del Vallès; L. Piqueras Rodriguez, H. de Elda, Alicante; M. Platero Roman, H.G. de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria; J.F. Plaza Nieto, H. del Sureste, Arganda del Rey; A. Ponz de Tienda, H.C.U Valencia, Valencia; R. Portillo Rivero, H.Reina Sofia, Córdoba; N. Prieto Mestre, C.S. Alamedilla, Salamanca; P. Quiroga Yañez, C. Saude A Ponte, Ourense; R. Reñe Ramirez, H.U. de Bellvitge, L’Hospitalet de Llobregat; A. Revuelta Argomaniz, H.U. Getafe; C. Ríos Gómez, H. de Barbastro, Huesca; M.C. Riveira Rodriguez, H.C.U de Salamanca, Salamanca; R.M. Rodriguez Fernandez, C. Hospitalario Ourense, Ourense; E. Rodriguez Moreno; H. Nuestra Señora de Valme, Sevilla; J. Rodriguez Vico, H.G. de Segovia, Segovia; A. Rojo Pantoja, Amb. Mollabao, Pontevedra; A. Rojo Sebastian, H.U. Príncipe de Asturias, Alcalá de Henares; J.J. Roldan Larreta, C. Psicogeriatrica Josefina Arregui; G. Romero Caballero, Residencia Pensionistas Inserso, Albacete; I. Rouco Axpe, H. de Cruces, Barakaldo, G. Rubio Esteban, H.G. Jerez de la Frontera, Jerez de la Frontera; M. Ruibal Salgado, H. de Zumarraga, Zumarraga; N. Ruiz Lavilla, H. Nuestra Señora de la Candelaria, La Laguna; M.J. Saenz San Juan, H. Virgen de los Lirios, Alcoy; J.M. Sanchez Alvarez, H. de Cabueñes, Gijón; C. Sánchez Castellano, H. Ramón y Cajal, Madrid; O Sanchez del Valle, Instituto de Ciencias de la Salud, Talavera de la Reina; P. Sanchez Ladeira, H. Psiquiatrico Conxo, Santiago de Compostela; F. Sanchez Lopez, C. Sanchez Ortiz, H. Reina Sofia, Córdoba; S.I. Sanchez Valiente, C.M. Especialidades Grande Covian, Zaragoza; C. Santafé Mara, H.C.U. Valencia, Valencia; L. Santolaria Martinez, Mutua Adeslas Zaragoza, Zaragoza; M.P. Sanz Cartagena, H. de Mataró, Barcelona, C.L. Sanz de la Garza, H. de Jove, Gijón; M. Seijo Martinez, H. Comarcal Do Sanes, Pontevedra; C. Sistiaga Berasategui, H. Comarcal de Bidasoa, Hondarribia; J.M. Soler Insa, H.G. Sant Joan de Deu, Manresa; G. Soriano Hernández, H. de Navarra, Pamplona; J. Súarez Muñoz, H.G. de Gran Canaria Dr. Negrín, Palmas de Gran Canaria; A.I. Tercero Uribe, Corporació Sanitaria Parc Taulí; F. Terriza Garcia, H. Santa Maria del Puerto, Cádiz; B. Tijero Merino, H de Cruces, Barakaldo; J .Tort Llobet, CAP Quevedo, Barcelona; D. Tortosa Conesa, H.U. Virgen Arrixaca, El Palmar; R. Trias Sanchez, H. Santa Clotilde, Santander; E. Ugalde López, H.G .Santa Cruz de la Palma, Santa Cruz de Tenerife; F. Uriz Otano, H. San Juan de Dios, Navarra; V. Valverde Moyar, C.S. Mental Miraflores, Alcobendas; O. Vega Lopez, Consultas Medicas Córdoba, Córdoba; J.M. Vega Perez, H. Ciudad de Jaén, Jaén; F. Veiga Fernandez, H. de Calde, Calde; R. Vela Yebra, Torrevieja Salud, Alicante; F. Velasco Juanes, H. de Cruces, Barakaldo; F.J. Viguera Romero, H. Virgen de la Macarena, Sevilla; E.M. Villar Villar, C. Especialidades Móstoles, Móstoles; A. Villarejo Galende, H.U. 12 de Octubre, Madrid; I. Villegas Rodriguez, H. Ciudad de Jaén, Jaén; M. Viñuela Beneitez, Amb. El Carmen, Palma de Mallorca; B. Zarza Sanz, H. Ramón y Cajal, Madrid; M.A. Zea Sevilla, H.U La Paz, Madrid.
Partial results from this study were presented at the 2010 Annual Meeting of the Spanish Society of Neurology.
Please cite this article as: Cruz Jentoft A, Hernández B. Manejo terapéutico con rivastigmina en pacientes con enfermedad de Alzheimer de leve a moderadamente grave en condiciones de práctica clínica habitual. Estudio ENTERPRISE. Neurología. 2014;29:1–10.