Patients with interstitial lung disease (ILD) may exacerbate due to infection, heart failure, pulmonary embolism, pneumothorax or drugs. Oftentimes, however, no etiology is found, and such event is interpreted as an accelerated progression of the underlying ILD, as in idiopathic pulmonary fibrosis (IPF), nonspecific interstitial pneumonia or sarcoidosis. Now, encountering a patient who has recently taken a 5h flight and presents in acute respiratory failure makes you wonder if the hypoxic environment in the aircraft could have favored the episode.
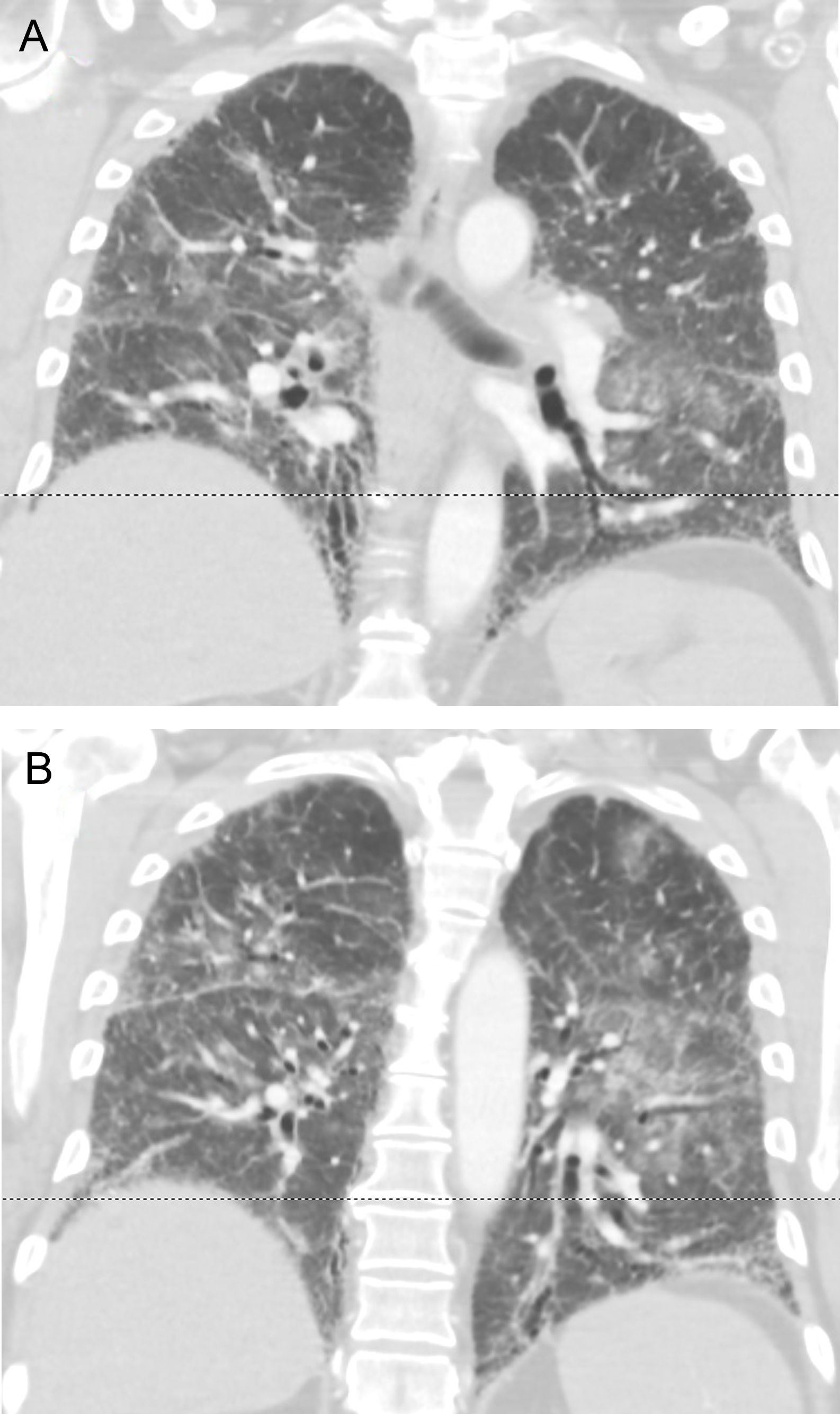
A 61 year-old male from The Netherlands with IPF diagnosed by a typical HRCT pattern and on treatment with nintedanib for the previous 6 months was taken to the emergency room two days after landing in Gran Canaria. He admitted to worsening dyspnea over the previous month and yet decided to embark with his family. On arrival to the ER he referred cough with scanty phlegm and loss of appetite. On the physical exam he was tachypneic, Tª: 37°C. Diffuse crackles were noted on auscultation. Oxygen therapy with FiO2 50% through a Venturi mask was required to secure adequate oxygenation. Sputum cultures, urinary antigens and serology for atypical pneumonia were negative. Acute pulmonary embolism and left heart failure were ruled out by angioCT, echocardiography and serum pro-BNP. Taken together with the thoracic imaging (Fig. 1A, B), the findings were interpreted as an acute exacerbation of IPF. He was treated with pulse steroids and antibiotics, made a partial recovery over 2 weeks and was repatriated by air ambulance to a hospital in his country when the oxygen requirements fell to FiO2 35%. Upon questioning, he referred to have felt increased dyspnea during the flight. Herein, we suggest that in-flight hypobaric hypoxia could have played a relevant role in the exacerbation.
In most healthy individuals, the decrease in PAO2 seen at the cabin pressure – equivalent to 8000 feet (2438m) of altitude – is of little physiological consequence, as the compensatory mechanisms lead to increased minute ventilation, increased heart rate and cardiac output. These mechanisms may be less effective in patients with pulmonary or cardiac disease, increasing the risk of significant hypoxemia.1,2 In ILD, a compensatory increase in minute ventilation may be blunted by decreased sensitivity to hypoxemia or by ventilation/perfusion defects.3,4 Moreover, the recurrent mechanical stretch of the lung is considered a pathogenic factor for IPF exacerbation.5
In acute mountain sickness (AMS), another condition related to hypobaric hypoxia, several studies indicate that an inflammatory reaction contributes to its pathogenesis. Other findings underscore the importance of endothelial dysfunction induced through the hypoxic inducible factor-1 (HIF-1) in both the systemic and the pulmonary circulation, with an imbalance between vasoconstrictors (e.g., endothelin 1) and vasodilators (e.g., NO), and a greater production of reactive oxygen species.6,7 Sleep apnea, arrhytmias, and systemic and pulmonary arterial hypertension are also linked to increased plasma levels of HIF-1.7 In fact, the hypoxic pulmonary vasoconstriction may be exaggerated in some individuals and subclinical interstitial pulmonary edema has been detected at moderately high altitudes.8 Likewise, individuals with altered respiratory transepithelial sodium transport are prone to high altitude pulmonary edema (HAPE), and certain polymorphisms in the genes of the renin angiotensin system are more frequent in subjects who develop HAPE.9–11 Lastly, a higher incidence of AMS is reported in those with a patent foramen ovale, a defect highly prevalent in the general population.12 So one can theorize that, in significantly hypoxemic patients at sea level, any of these mechanisms could play a role to increase dyspnea and/or cause symptoms of AMS in a medium or long haul flight, and accelerate the inflammatory and hemodynamic processes characteristic of an exacerbation.13 We think that our patient may have experienced an ongoing deterioration of his pulmonary function before flying, which could have worsened during air travel, possibly with transient HAPE, triggering the process that led to exacerbation of IPF.
Coker et al. reported that unscheduled health demands were more frequent in the first month after flying in a group of 431 COPD and ILD patients, especially in the ILD group.14 In an previous series, 17 patients with chronic restrictive ventilatory impairment and mean baseline PaO2 of 10.4kPa (77mmHg) were studied at rest and during 20W bicycle exercise at sea level and at 2438m simulated altitude in a hypobaric chamber. The mean PaO2 at rest in altitude fell to 6.5kPa (48mm Hg). This and other studies show that exertion under hypoxic conditions further worsens oxygen desaturation, both in COPD and ILD patients.1,3,4 Recently, Barrat et al. reported 106 patients with ILD (69 with IPF) with a mean baseline PaO2 of 9.3kPa (69.7mm Hg) and TLCO of 46% pred. submitted to hypoxic challenge testing (HCT), and found that 51% of them fulfilled the indication for in-flight supplemental oxygen. Interestingly, 27% of patients with room air SpO2≥96% fell into this category.4 Finally, Kelly et al. studied 14 ILD patients during air travel and found that sea level SpO2 was predictive of in-flight SpO2 – mean 95% and 85%, respectively. Seven patients were eligible for in-flight oxygen according to HCT results, out of whom 3 reported dyspnea while in the aircraft.15
Apart from the relative immobility of patients during flights – left aside pulmonary thromboembolism, there is not a definite explanation for the low prevalence of respiratory emergencies in aircrafts, and there is not a strong evidence that in-flight oxygen will avert hypoxic complications during or after the travel. However, conducting a preflight assessment should be done for those with paO2 70mmHg or SpO2 92–95% and for those with PaO2 70mmHg or SpO2 95% with high risk features such a FEV1 30% predicted, bullous lung, concomitant cardiac disease or significant symptoms during previous air travel. Likewise, declaring fitness to fly is not appropriate if a requirement of more than 4lpm of oxygen is expected.1,2 The prescription of oxygen may be burdersome and superfluous for some patients, but we believe that if HCT is not available, a low threshold to endorse it is a prudent decision for patients at risk.
Conflicts of interestsThe authors of the manuscript entitled “Air travel and acceleration of lung injury” declare not to have any conflicts of interest.