This article describes recent research which informs our understanding of changes in social cognition during adolescence. The focus will be on mentalising, the ability to attribute and manipulate mental states in the self and others. Mentalising is supported by the medial prefrontal cortex (MPFC) and both anterior and posterior regions of the temporal lobes. In the past decade, studies have demonstrated development during adolescence of white and grey matter brain structure, with most protracted changes observed in frontal and temporal lobes, including those regions supporting mentalising. This article presents evidence that certain aspects of social cognition continue to change during adolescence, highlighting results from recent research investigating the use of theory of mind information in a communicative context. The findings highlight how adolescence, and not only childhood, is a time of continued maturation of brain and behaviour, when education and the environment can have an impact on cognitive development.
Este artículo describe resultados de investigaciones recientes sobre cambios en la cognición social durante la adolescencia. Se centra en la mentalización, la capacidad de atribuir y manipular estados mentales en uno mismo y en los demás. La mentalización está asociada con la corteza prefrontal media (MPFC) y las regiones anterior y posterior de los lóbulos temporales. En el último decenio hay estudios que demuestran que a lo largo de la adolescencia se desarrolla la estructura cerebral tanto de la sustancia blanca como de la gris, observándose los cambios más notables en los lóbulos frontal y temporal, que incluyen regiones en las que se asienta la mentalización. Este artículo demuestraque determinados aspectos de la cognición social siguen desarrollándose durante la adolescencia, presentando los resultados de estudios recientes que investigan la utilización de la teoría de la mente en un contexto comunicativo. Los resultados subrayan cómo la adolescencia, y no sólo la niñez, es una etapa de maduración continua del cerebro y del comportamiento, cuando la educación y el entorno pueden influir en el desarrollo cognitivo.
Adolescence can be defined as the period of life that starts with the beginning of puberty and ends when a stable, independent role in society is attained (Steinberg, 2010). The transition from childhood to adulthood is a period of changes, both in terms of the environment and in terms of brain and cognitive development. In a proportion of individuals, adolescence is the time for the first onset of mental disorder, with an analysis by Kessler et al. (2005) revealing that 75% of adult mental disorder, including anxiety and mood disorders, schizophrenia, impulse-control and substance-use disorders, have their onset before 24 years of age. In addition, the leading causes of death in adolescence are accidents, violence, and suicide (Patton et al., 2009). These findings highlight the importance of increasing our understanding of both typical and atypical behavioural and brain development during adolescence.
Advances in the field of magnetic resonance imaging (MRI) in the last two decades have permitted for the first time to study the healthy living human brain during development. Research has been divided between structural MRI, which investigates anatomical changes in the brain (Giedd & Rapoport, 2010) and functional MRI, which investigates changes in neuronal activity via changes in blood flow. More recently, researchers have attempted to link these two measures and further explore the link between structural and functional changes during development, and how structural and functional brain maturation may account for behavioural changes observed in a wide range of situations and paradigms.
Two particular aspects of cognition are thought to show prolonged changes during adolescence. One aspect is cognitive control, which encompasses a range of cognitive processes (“executive functions”), including inhibition, working memory, planning, and attention (see Anderson, 2002; Luna, Padmanabhan, & O’Hearn, 2010 for reviews). The second aspect is social cognition, and will be the focus of this article. Social cognition encompasses all those cognitive processes that allow individuals to interact with one another (Adolphs, 1999; Frith & Frith, 2007). These range from the perception of facial expression, body posture, and eye gaze, to mentalising, the ability to attribute and manipulate mental states in the self and others (Frith & Frith, 2007). A wide network of brain regions have now consistently shown to be recruited during social cognition tasks (Frith & Frith, 2007; Van Overwalle, 2009). These regions form the “social brain”, which is the brain basis for the capacity to process social signals (Frith & Frith, 2007).
Over the past 15 years, a large number of independent studies have shown remarkable consistency in identifying the brain regions that are involved in theory of mind or mentalising. These studies have employed a wide range of stimuli including stories, sentences, words, cartoons, and animations, each designed to elicit the attribution of mental states (see Amodio & Frith, 2006, for review). In each case, the mentalising task resulted in the activation of a network of regions including the posterior superior temporal sulcus (pSTS) at the temporo-parietal junction (TPJ), the anterior temporal cortex (ATC), or temporal poles, and the dorsal medial prefrontal cortex (MPFC) (Frith & Frith, 2007; Gallagher & Frith, 2003; Saxe, 2006). The agreement between neuroimaging studies in this area is remarkable and the consistent localisation of activity within a network of regions including the pSTS/TPJ and MPFC, as well as the temporal poles, suggests that these regions are key to the process of mentalising. However the specificity of these regions for processing social information is still investigated. For example the ATC supports semantic knowledge, including, but not limited to, social concepts and social scripts, and the dorsal MPFC may be more broadly recruited in meta-cognitive processes of reflecting on thoughts and intentions, that may not be social in nature (Frith & Frith, 2007).
In this article, I will first summarise the findings on structural brain development during adolescence, focusing in particular on those changes that take place in the mentalising regions of the social brain. I will then present the findings of early development of the understanding of false-beliefs and later developmental changes in theory of mind use, and describe the findings from functional neuroimaging studies investigating changes in social brain activation during adolescence. Finally, I will briefly highlight potential implications of these findings for education policy and practice.
Structural Development of the Social BrainPost-mortem brain studies in the 1970s and 1980s had shown that synaptic density, which corresponds to the number of connections (“synapses”) between neurons observed per unit of neuronal surface, increases drastically in the first few months and years of life and then progressively decreases during childhood in the visual and auditory cortex, and during adolescence in the prefrontal cortex (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997; see Petanjek et al., 2011 for more recent data). Despite this evidence for early dendritic arborisation and later prolonged pruning of synaptic connections, it was until recently widely held that the brain was anatomically mature during adolescence, and that changes in social behaviour during this period of life were a result of hormones, social experience, and the changing social environment. These factors are likely to play a major role, but neuroanatomical development may also play a role. In the last couple of decades, results from large MRI studies investigating the development of the brain have provided further evidence that the structure of the brain continues to change during adolescence. Notably, the frontal and temporal lobes, which support social cognition, undergo the most protracted development in humans (Giedd et al., 1999; Gogtay et al., 2004; Shaw et al., 2008; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999). Report that the peak cortical thickness, which is an index of the developmental timecourse of grey matter changes, is first reached in the occipital lobes, at age 7-9 years old, then in the parietal lobes (age 8-11), in the frontal lobes (age 8-13), and last reached in the temporal lobes (age 11-15).
A recent study provides further evidence of the prolonged development of those brain regions supporting mentalising (Mills, Lalonde, Clasen, Giedd, & Blakemore, 2014). This study analysed 857 structural scans from 288 participants aged 7-30 years old and focused on those four regions of the social brain described above: ATC, MPFC, TPJ, and pSTS. Developmental changes in three structural measures were investigated: grey matter volume, cortical thickness, and surface area. The findings indicated that grey matter volume and cortical thickness in MPFC, TPJ, and pSTS decreased from childhood into the early twenties, while the ATC increased in grey matter volume until adolescence and in cortical thickness until early adulthood. Surface area in all four regions followed a cubic trajectory, with a peak in late childhood or early adolescence before a decrease into the early twenties. Changes in grey matter structure are thought to correspond partly to synaptic reorganisation and to enable the fine-tuning of grey matter tissue according to experience and the environment.
A second aspect of brain structure that has been investigated using MRI is white matter volume and white matter fibres. Early studies had shown that during development neuronal axons, which make up the white matter fibres connecting brain regions, become coated in myelin sheath, and that myelination occurs progressively throughout the brain, with latest myelination observed in the higher association areas (Bonin, 1950). Longitudinal MRI studies have shown that white matter volume increases during development until the third decade of life (Giedd et al., 1999; Lebel & Beaulieu, 2011). This increase is thought to reflect myelination but also other processes such as increasing axon diameter, and may be associated with an increasing speed of signalling between neurons, which would in turn increase processing speed during development. More recent studies have used novel MRI techniques such as diffusion tensor imaging (DTI) and have shown that, similar to grey matter, the developmental timecourse of white matter changes vary across brain regions (e.g., Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008).
In their recent study, Mills et al. (2014) note that the ATC is an area of the cortex which is linked to both the MPFC and limbic structures via the uncinate fasciculus, one of the last white matter tracts to reach maturity (Lebel et al., 2008), and suggest that perhaps the late myelination of the ATC projections allow a long window for learning social scripts. This highlights the importance for future studies of attempting to combine and integrate both white matter and grey matter data when studying structural changes in the brain during development.
To summarise, MRI research in the last two decades has shown that there are prolonged changes in both grey and white matter structures that occur during adolescence, and that these developmental changes are particularly protracted in the frontal and temporal lobes, which support, among other cognitive processes, mentalising, the ability to manipulate and reflect on our own or other people's mental states.
Development of Social CognitionDevelopmental psychology research on theory of mind has demonstrated that the ability to understand others’ mental states develops over the first four or five years of life (e.g., Happé, 1995). While certain aspects of theory of mind are present in infancy (Baillargeon, Scott, & He, 2010), it is not until around the age of four years that children begin to explicitly understand that someone else can hold a belief that differs from one's own, and which can be false (Barresi & Moore, 1996). An understanding of others’ mental states plays a critical role in social interaction because it enables us to work out what other people want and what they are about to do next, and to modify our own behaviour accordingly (Frith & Frith, 2007). In the context of learning and education, there is evidence that social interaction is special and plays a particular role in learning (e.g., Kuhl, Tsao, & Liu, 2003). One possible explanation for this role is that social interaction increases infants’ motivation through enhanced attention and arousal. Social interaction also directs the adult trainer to focus on the learner's individual needs and tailors the training content for the learner (see Kuhl, 2007, for review). In addition, by nine months, infants start to understand that pointing to, or looking in the direction of, an object indicates that this object is being referred to. This is one of the first building blocks of theory of mind (see Frith & Frith, 2007, for review), and this understanding is thought to be linked to the development of language, in particularly the acquisition of new vocabulary (see Akhtar & Gernsbacher, 2007, for discussion).
There is a rich literature on the development of social cognition in infancy and childhood, pointing to step-wise changes in social cognitive abilities during the first five years of life (Frith & Frith, 2007). However, there has been surprisingly little empirical research on social cognitive development beyond childhood. Only recently have studies focused on the development of the social brain beyond early childhood, and the results support evidence from social psychology that adolescence represents a period of significant social development. Adolescence is characterised by psychological changes in terms of identity, self-consciousness, and relationships with others (Sebastian, Burnett, & Blakemore, 2008; Steinberg, 2010). Compared with children, adolescents are more sociable, form more complex and hierarchical peer relationships and are more sensitive to acceptance and rejection by peers (Steinberg & Morris, 2001).
Most developmental studies of social cognition focus on early childhood, possibly because children perform adequately in even quite complex mentalising tasks at around age four (Happé, 1995). On the one hand, this could be attributed to a lack of suitable paradigms: in order to create a mentalising task that does not elicit ceiling performance in children aged five and older, the linguistic and executive demands of the task may need to be increased. However, such increases in task complexity render any age-associated improvement in performance difficult to attribute solely to improved mentalising ability. On the other hand, the good performance of 4-5 year-olds could be attributed to qualitative differences in 2nd person perspective abilities, with basic abilities emerging very early, and more complex abilities, potentially associated with executive functioning, gradually improving during childhood and adolescence. The protracted structural development of the brain regions involved in theory of mind in adolescence and early adulthood (Giedd & Rapoport, 2010; Shaw et al., 2008, see above) might indeed be expected to affect mental state understanding. In addition, evidence from social psychology studies shows substantial changes in social competence and social behaviour during adolescence (Steinberg, 2010), and this is hypothesised to rely on a more sophisticated manner of thinking about and relating to other people – including understanding their mental states.
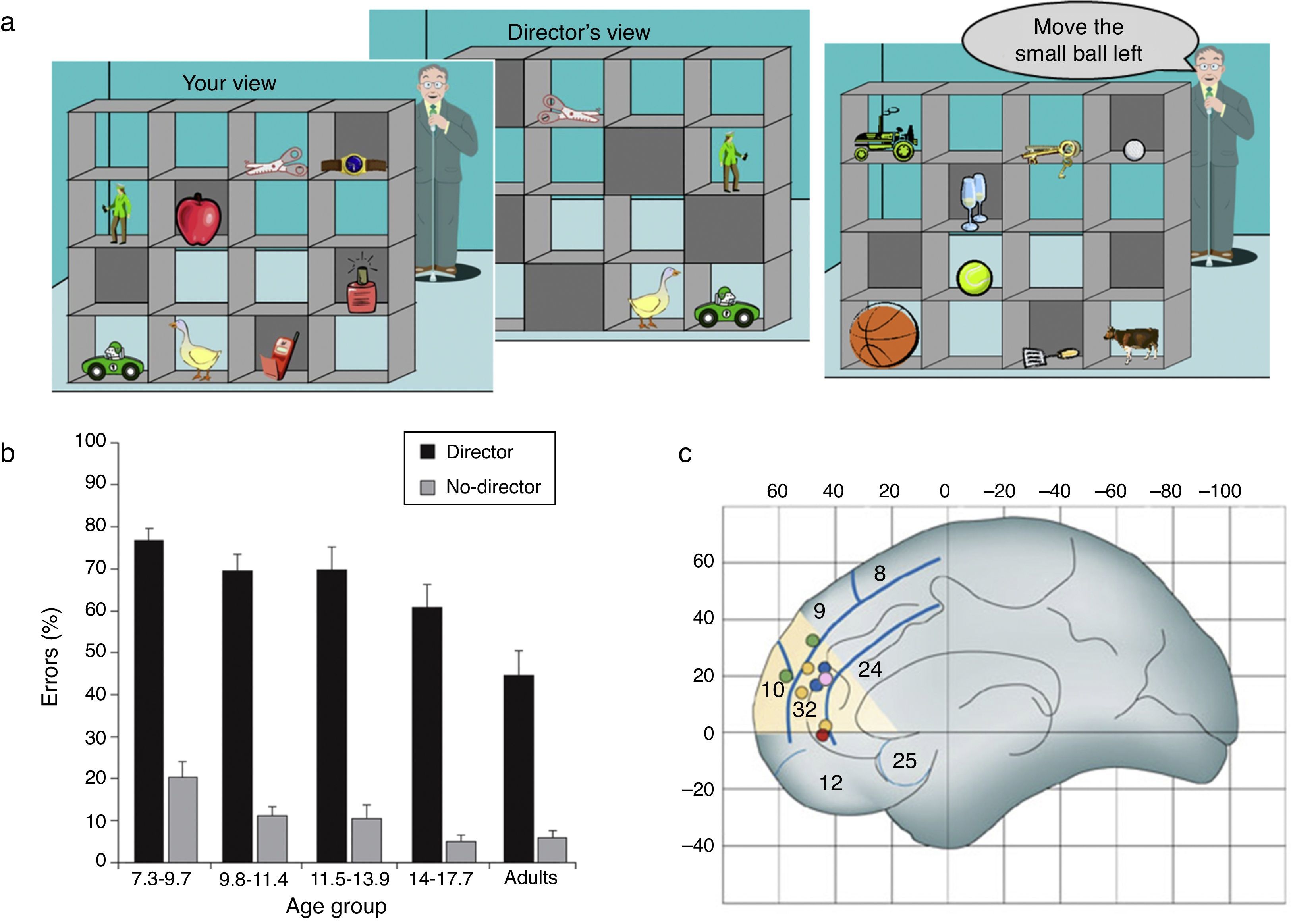
Recently, we adapted a task that requires the online use of theory of mind information when making decisions in a communication game, and which produces large numbers of errors even in adults (Keysar, Barr, Balin, & Brauner, 2000; Keysar, Lin, & Barr, 2003). Keysar et al. (2000, 2003) report that adults frequently fail to use their conceptual competence for theory of mind, or mentalising, in an online communication game in which they need to take account of the speaker's visual perspective. In our computerised version of the task, participants view a set of shelves containing objects, which they are instructed to move by a “director,” who can see some but not all of the objects (Apperly et al., 2010; Dumontheil, Apperly, & Blakemore, 2010). Correct interpretation of the instructions requires participants to use the director's perspective and only move objects that the director can see (the director condition) (see Figure 1a). Although clearly capable of understanding that the director has a different perspective, adult participants frequently fail to use this information when interpreting the director's instructions (Apperly et al., 2010). This can be considered as evidence that humans are prone to egocentric bias.
Social cognition development
(a) Online mentalising task. Participants have to take into account the perspective of another person (the Director) when following his instructions to move objects. The left panel shows the participant's view of a set of shelves containing objects. The middle panel shows the Director's view – some objects are occluded from his viewpoint. The right panel shows an example trial. The director cannot see the golf ball, so when he asks the participant to “move the small ball left” the participant should move the tennis ball. (b) Behavioural results. Adolescents aged 14-17 years old make more errors than adults when they are required to take into account the perspective of a speaker (the Director) asking them to move objects in a set of shelves. In contrast, no difference in accuracy is found between these two age groups between these age groups in a non-social control task (No-Director condition) (adapted from Dumontheil, Apperly, & Blakemore, 2010). (c) A section of the dorsal MPFC that is activated in studies of mentalising: Montreal Neurological Institute (MNI) ‘y’ coordinates range from 30 to 60, and ‘z’ coordinates range from 0 to 40. Dots illustrate regions where activity decreases between late childhood and adulthood during six mentalising tasks(see Blakemore, 2008, for references). The mentalising tasks ranged from understanding irony, which requires separating the literal from the intended meaning of a comment, thinking about one's own intentions, thinking about whether character traits describe oneself or another familiar other, watching animations in which characters appear to have intentions and emotions and thinking about social emotions such as guilt and embarrassment (Burnett & Blakemore, 2009b). (Adapted from Blakemore, 2008).
We used this paradigm to investigate developmental changes in theory of mind use (Dumontheil, Apperly et al., 2010). One critical aspect of the study was the use of a control, non-social condition which had similar inhibitory control and general task demands to the director condition. This enabled us to specifically identify developmental changes in accuracy related to the social perspective taking component of the task. We tested participants aged between 7 and 27 years and found that while performance in the director and control conditions followed the same trajectory (improved accuracy) from mid-childhood until mid-adolescence, the mid-adolescent group (aged 14-17 years old) made more errors than the adults in the director condition only (see Figure 1b). These results suggest that the ability to take another person's perspective to direct appropriate behaviour is still undergoing development at this relatively late stage. Interestingly, a recent study (Furumi, 2012) suggests that performance on the Director task may be associated with performance on other more standard social cognition tasks such as understanding the difference between irony and lying and understanding second-order false belief. Children aged 8-11 years old were tested on a variant of the Director task and divided into low vs. high scorers on the standard social cognition tasks. The results showed that the low score group made significantly more errors on the Director task than the high score group. Further work will be needed to better characterise the cognitive processes involved in the Director paradigm, but we have proposed that a critical element of this task is that participants are not explicitly asked about their own or somebody else's mental states, but instead that they need to use that information in the context of performing an action.
To summarise, developmental research has typically focused on the early maturation of the understanding of false-belief and shown that performance reaches ceiling in early childhood. Using a novel paradigm investigating the use of perspective taking information in a communicative context, we have shown that performance on this task has still not reached adult levels by mid-adolescence.
Neuroimaging Studies of Social Cognition DevelopmentA number of functional MRI (fMRI) studies have investigated the development during adolescence of the functional brain correlates of mentalising (see Blakemore, 2008 for review). For example, Burnett and colleagues (Burnett, Bird, Moll, Frith, & Blakemore, 2009) compared brain activity of adolescent (aged 11-17 years old) and adult (aged 21-37 years old) participants while they were reading and rating the strength of the emotion they would feel in situations described in short written scenarios. Scenarios involved either social emotions, which require representation of other people's mental states (guilt, embarrassment), or basic emotions (disgust, fear). The results of this fMRI study indicated that although regions of the social brain were more active in the social emotions condition compared to the basic emotions condition in both adult and adolescent groups, activity in the MPFC in this comparison decreased with age, while activity in the left ATC increased with age. Notably, these results were observed in the absence of difference in the ratings made by the two groups of participants (Burnett et al., 2009).
Despite using a variety of mentalising tasks, a large number of studies have now consistently shown that MPFC activity during mentalising tasks decreases between adolescence and adulthood. In each of these studies, MPFC activity was greater in the adolescent group than in the adult group during the mentalising task compared to the control task (see Figure 1c and Blakemore, 2008). The mentalising tasks ranged from understanding irony, which requires separating the literal from the intended meaning of a comment (Wang, Lee, Sigman, & Dapretto, 2006), thinking about one's own intentions (Blakemore, den Ouden, Choudhury, & Frith, 2007), thinking about whether character traits describe oneself or another familiar other (Pfeifer, Lieberman, & Dapretto, 2007; Pfeifer et al., 2009), watching animations in which characters appear to have intentions and emotions (Moriguchi, Ohnishi, Mori, Matsuda, & Komaki, 2007), performing an affective theory of mind task based on vignettes (Sebastian et al., 2012), and thinking about social emotions such as guilt and embarrassment (Burnett et al., 2009), as described above. In addition, there is evidence for differential functional connectivity between MPFC and other parts of the mentalising network across age (Burnett & Blakemore, 2009a).
One issue that is raised when studying adolescence is that age may not always be the best predictor of brain development. Puberty is the biological process resulting in physical maturity and reproductive competence. Puberty is hormonally driven and is partly dissociable from age: in humans, there is a 4-5 year normal variation in the timing of onset of puberty (Tanner & Whitehouse, 1976). Further, there is evidence that some of the brain structural changes that occur during adolescence may be related to increasing pubertal hormones; however, only few functional neuroimaging studies of the adolescent brain have included puberty measures (see Goddings, Burnett Heyes, Bird, & Blakemore, 2012, for review). Goddings et al. (2012) used the social emotion paradigm described above (Burnett et al., 2009) to investigate the relationship between puberty and social emotion processing. Forty-two females aged 11.1 to 13.7 years took part in the fMRI study and were assigned to early or late puberty groups on the basis of their pubertal stage and menarcheal status. In addition, concentrations of testosterone, oestradiol, and dehydroepiandrosterone (DHEA) were used as hormonal pubertal indicators. The results showed that while the decrease in MPFC activation with age in the contrast social vs. basic emotions was replicated, even in this small age range, activation in the ATC was positively correlated with hormone levels rather than age (Goddings et al., 2012). Chronological age and pubertal hormones were found to have different influences on the mentalising network in adolescence.
It is not yet understood why MPFC activity decreases between adolescence and adulthood during mentalising tasks, but two non-mutually exclusive explanations have been put forward (see Blakemore, 2008, for details). One possibility is that the cognitive strategy for mentalising changes between adolescence and adulthood. For example, adults may rely more on previous experiences and the retrieval of social scripts in the ATC to interpret social situations than adolescents, who instead might base their judgement on novel computations performed in the MPFC. This possibility may be related to the skill learning hypothesis (Johnson, 2011), whereby one region first supports a certain function, but another brain region may take over later in development. In addition, this hypothesis suggests the PFC may be particularly involved during the learning of new abilities. A second possibility is that the functional change with age is due to neuroanatomical changes that occur during this period. Decreases in activity are frequently interpreted as being due to developmental reductions in grey matter volume, presumably related to synaptic pruning. However, there is currently no direct way to test the relationship between number of synapses, synaptic activity, and neural activity as measured by fMRI in humans (see Blakemore, 2008, for discussion), although structural MRI measures could already provide some information. Understanding the link between structural and functional changes is critical in understanding the mechanisms of neurocognitive development, yet very few studies have directly compared structural and functional data within the same individuals (Lu et al., 2009; Olesen, Nagy, Westerberg, & Klingberg, 2003).
An alternative account of the decrease in MPFC recruitment during adolescence relates to the nature of the experimental paradigms used in the studies described above. These tasks typically required participants to make explicit judgements regarding the mental states of a character in a scenario presented in different formats (Blakemore et al., 2007; Burnett et al., 2009; Moriguchi et al., 2007; Sebastian et al., 2012; Wang et al., 2006), or to judge how much a phrase, such as “I am popular”, described themselves (Pfeifer et al., 2007; Pfeifer et al., 2009), or else to judge a person's emotion from photos of their eyes (Gunther Moor et al., 2011). In sum, in these studies participants were asked to reflect on their own or someone else's thoughts or emotions in an explicit and somewhat detached manner. In contrast, in everyday situations we often have to use theory of mind or mentalising to make top down decisions and select actions that are appropriate for the inferred mental states of the people we interact with. This requires a combination of executive functions and online mentalising.
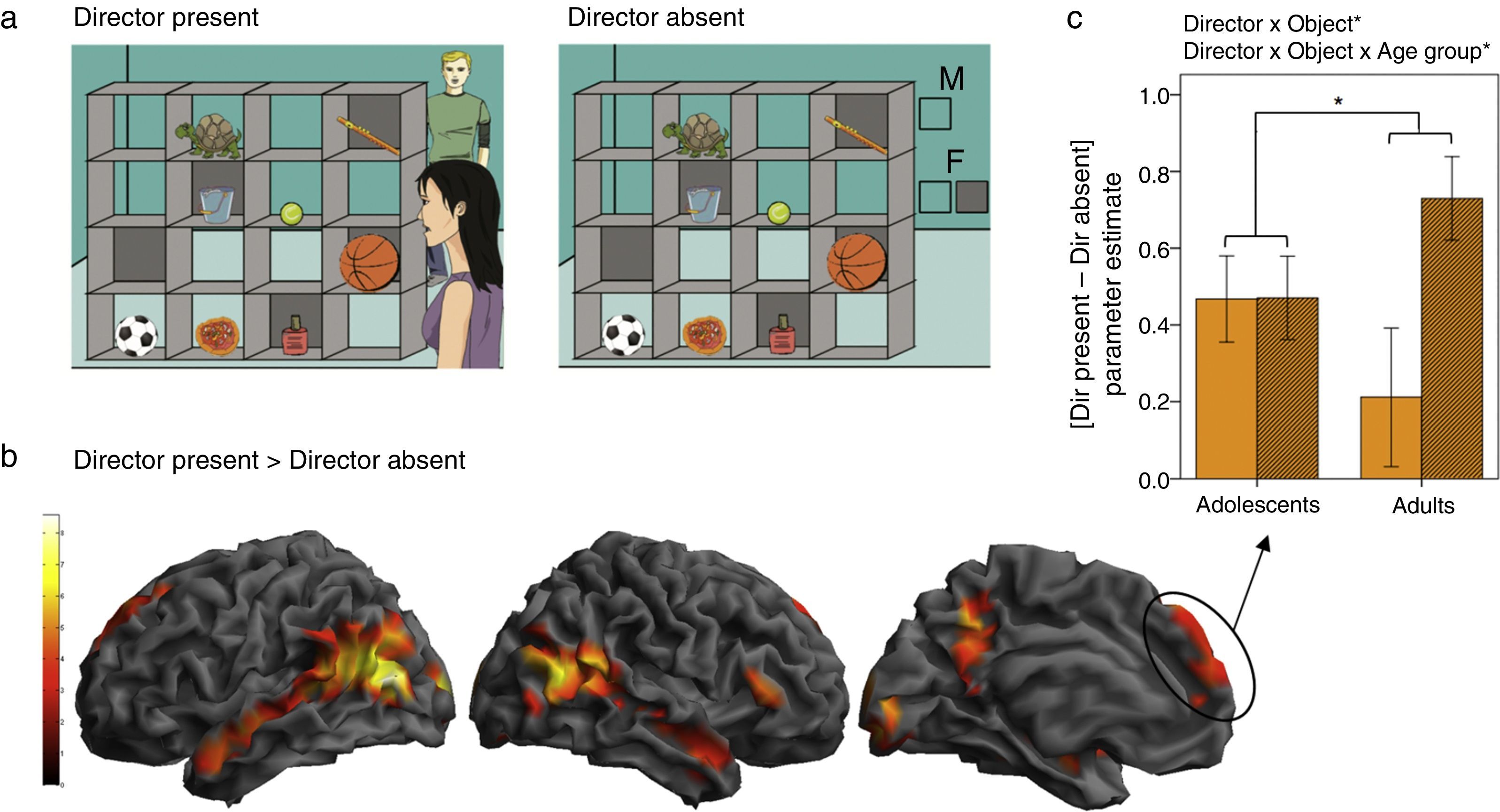
To investigate the developmental neural correlates of theory of mind use in a more implicit communicative context, we tested adolescent (11-16 years old) and adult (21-30 years old) participants in a variant of the Director task adapted for fMRI (Dumontheil, Hillebrandt, Apperly, & Blakemore, 2012; Dumontheil, Küster, Apperly, & Blakemore, 2010). A second director was introduced, and participants were required, on a trial-by-trial basis, to consider whether the director was standing on the same side of the shelves as them, or on the opposite side (Figure 2a). As in the behavioural study (Dumontheil, Apperly et al., 2010), a control condition was rule-based and matched the inhibitory control and general task demands of the Director condition. The critical findings were first that regions of the social brain were more active in the Director than the control condition, in particular in the dorsal MPFC, and along the superior temporal sulcus bilaterally (Figure 2b). Regions of interest analyses further showed that the dorsal MPFC cluster was differentially recruited in the adult and adolescent groups. Adults showed greater activation in this region specifically when the participants had to take into account the perspective of the director to pick the correct object out of three possibilities, compared to when only one object could be chosen (Dumontheil, Küster et al., 2010). Adolescents instead showed dorsal MPFC activation in both conditions (Figure 2c; Dumontheil et al., 2012). These results suggest that adolescents showed less specificity of MPFC activation for mentalising than adults, and that adolescents may show “over-mentalising” activation in the MPFC during social cognition tasks.
Neuroimaging variant of the Director task
(a) Examples of a 3-object trial in the Director Present and the Director Absent (control) conditions. In both conditions in this example, participants hear the instruction: “Move the large ball up” in either a male or a female voice. In both examples, if the voice is female, the object to be moved would be the basketball, since in the Director Present condition (left) the female Director is standing in front of the shelves and can see all the objects, while in the Director Absent condition (right) the two boxes below the “F” (for “female”) indicate that all objects can be moved by the participant. If the voice is male, the object to be moved would be the football, since in the Director Present condition the male Director is standing behind the shelves and therefore cannot see the larger basketball in the occluded slot, while in the Director Absent condition the single clear box below the “M” (for “male”) indicates that only objects in open shelves can be moved. (b) Main effect of the Director factor across age groups. Regions showing increased BOLD signal in the Director Present compared to Director Absent blocks are rendered on the SPM8 surface mesh template. From left to right: lateral view of the left and right hemisphere, medial view of the left hemisphere. Activations were observed bilaterally along the superior temporal sulci and in the superior dorsal MPFC. Contrast thresholded at p < .001 uncorrected at the voxel-level, p < .05 FWE corrected at the cluster level. (c) ROI analysis testing for interactions between Director and Object, and between Director, Object, and Age group. Mean parameter estimates in the superior dorsal MPFC cluster of the Director Present > Director Absent contrast showed a significant interaction between Director and Object factors, with greatest activation in the DP 3-object condition, and a significant 3-way interaction between Director, Object, and Age group, with a significant increase in [DP - DA] in 3-object vs. 1-object blocks in the adults only. (Adapted from Dumontheil et al., 2012).
Interestingly, this pattern of increasing specialisation for mentalising has been observed in the TPJ in younger participants reading stories that involved mental states, people, or physical objects (Saxe, Whitfield-Gabrieli, Scholz, & Pelphrey, 2009). In this fMRI study, the right TPJ was found to be similarly activated in the People and Mental vs. Physical conditions in the younger participants (6-9 years old), but more activated in the Mental than People conditions in the older children (9-10 years old).
Thus to summarise, neuroimaging research has revealed that regions of the social brain show changes in activity during adolescence. In explicit mentalising paradigms, MPFC activation is reliably observed to decrease with age, whereas temporal cortex regions, in particular the ATC, show increased activity with age or with hormonal markers of puberty. In more implicit paradigms, the data suggest that regions of the adult mentalising network may show increased specificity for mentalising with age, rather than activation in response to social stimuli more generally.
Future Possibilities for Education Policy and PracticeThere are many questions that remain to be investigated in this new and rapidly expanding field exploring the development of social cognition during adolescence. This research is likely to have important implications for society in relation to education and the legal treatment of teenagers, as well as a variety of mental illnesses that often have their onset in adolescence.
Knowledge of how the brain develops and learns has the potential to have a profound impact on education in the future. As described above, social interaction with a real live person is critical for at least some types of early learning (Kuhl et al., 2003), suggesting that, while not necessarily harmful, DVDs and CDs aimed at teaching babies and young children may not be associated with optimal learning. More importantly, the time spent watching DVDs is time that could otherwise be spent in social interaction with a real person, and denying the developing brain of this might have negative consequences. We need to ask whether online social networking, which is particularly popular with teenagers, is the same as real live interaction, or whether it might be denying the developing teenage brain important real life interactions. There is as yet no research on this important question.
Understanding the brain basis of social functioning and social development may improve the fostering of social competence inside and outside the classroom, in support of previous social psychological research in this domain (e.g., Durlak, Weissberg, Dymnicki, Taylor, & Schellinger, 2011). Social functioning plays a role in shaping learning and academic performance (and vice versa), and understanding the neural basis of social behaviour may contribute to understanding the origins and process of schooling success and failure. The finding that changes in brain structure continue into adolescence (and beyond) has challenged accepted views, and has given rise to a recent spate of investigations into the way cognition (including social cognition) might change as a consequence. Research suggests that adolescence is a key time for the development of regions of the brain involved in social cognition and self-awareness, as well as in problem solving and abstract thinking. This is likely to be due to the interplay between a number of factors, including changes in the social environment and in puberty hormones, as well as structural and functional brain development and improvements in social cognition and reasoning abilities.
If early childhood is seen as a major opportunity – or a “sensitive period” – for teaching, so too should the teenage years. During both periods, significant brain reorganisation is taking place. The idea that teenagers should still go to school and be educated is relatively new. And yet the brain is still developing during this period, is adaptable, and can be moulded and shaped. Perhaps the aims of education for adolescents might usefully include a focus on abilities that are controlled by the parts of the brain that undergo most change during adolescence, including those described in this review: social cognition and the understanding and awareness of the potentially different perspective of others. Finally, it might be fruitful to include in the curriculum some teaching on the changes occurring in the brain during adolescence. Adolescents might be interested in, and could benefit from, learning about the changes that are going on in their own brains.
Resumen ampliadoLa adolescencia es el periodo en el que se muestran por primera vez la mayoría de los trastornos mentales del adulto. Un 75% de los trastornos mentales del adulto, incluyendo trastornos de la ansiedad y el ánimo, la esquizofrenia, el control de los impulsos y el uso de sustancias, tienen su inicio antes de los 24 años. Además, las principales causas de muerte en adolescentes son los accidentes, la violencia y el suicidio. Todo esto no puede sino resaltar la importancia de mejorar nuestra comprensión del desarrollo, tanto típico como atípico, del comportamiento y del cerebro, durante la adolescencia. Uno de los aspectos más destacables del desarrollo en este período es el control cognitivo, que abarca una serie de procesos cognitivos (también llamadas funciones ejecutivas), incluyendo la inhibición, la memoria de trabajo, la planificación y la atención. El segundo aspecto es la cognición social, que abarca todos aquellos procesos cognitivos que permiten a los individuos interactuar con los demás. Aquí se incluye desde la percepción de la expresión emocional, la postura corporal y la dirección de la mirada, hasta la mentalización, la capacidad para identificar y manipular los estados mentales de uno mismo y de los otros.
Durante muchos años se pensó que los cambios en el comportamiento social de los adolescentes eran fruto de las hormonas, la experiencia social y cambios en el entorno social. Es probable que estos factores contribuyan de manera importante, pero también es probable que el desarrollo neuroanatómico juegue un papel relevante. Los abundantes resultados obtenidos mediante imagen por resonancia magnética cerebral (MRI en sus siglas en inglés) acumulados en las dos últimas décadas evidencian que la estructura del cerebro continúa cambiando durante la adolescencia. El máximo grosor de la corteza, que es un índice del desarrollo de la modificación de la materia gris, se alcanza primeramente en los lóbulos occipitales a los 7-9 años de edad, seguidos de los lóbulos parietales (8-11 años), los frontales (8-13 años) y, por último, los temporales (11-15 años). Aparte de este patrón general, desde la niñez hasta los primeros años de la segunda década de vida, el grosor de la corteza y el volumen de la materia gris –un índice de la densidad sináptica– disminuyen en la corteza prefrontal media(MPFC), la unión témporo-parietal (TPJ) y el surco temporal superior posterior (pSTS), mientras que la corteza temporal anterior (ATC) aumenta su volumen de materia gris hasta la adolescencia y su grosor cortical hasta el comienzo de la edad adulta. La superficie de estas regiones cerebrales sufre cambios que siguen una trayectoria cúbica: su mayor valor se alcanza al final de la niñez o comienzo de la adolescencia, antes de disminuir hacia el comienzo de la segunda década. En resumen, a lo largo de la adolescencia se producen cambios estructurales significativos tanto en la materia gris como en la sustancia blanca del cerebro; los cambios se prolongan particularmente en los lóbulos frontales y temporales, que participan, entre otros procesos cognitivos, en la mentalización, la capacidad para manipular y reflexionar sobre nuestros propios estados mentales o los de otras personas (una forma compleja de los procesos de teoría de la mente o ToM, según sus siglas en inglés). La adolescencia se caracteriza por cambios psicológicos en cuanto a identidad, autoconciencia y relaciones con los otros. En comparación con los niños, los adolescentes son más sociables, forman relaciones más complejas y jerárquicas con las personas de su edad y son más sensibles a la aceptación y al rechazo por parte de sus pares. En la adolescencia y la adultez temprana, el retraso del desarrollo estructural de las regiones cerebrales implicadas en la teoría de la mente afecta la comprensión de los estados mentales.
Nuestro grupo ha adaptado recientemente una tarea que requiere el uso de la información sobre la teoría de la mente para tomar decisiones en un juego de comunicación y que produce altos índices de errores incluso en adultos. Para interpretar correctamente las instrucciones se requiere que los participantes utilicen la perspectiva de otra persona, ‘el director’, quien en la condición experimental da órdenes acerca del cómo mover objetos en una estantería; se tiene que tener en cuenta qué ve y qué no ve el director (figura 1a). Aunque los adultos son perfectamente capaces de saber que el director tiene otro punto de vista, fallan frecuentemente en esta prueba. Los resultados en esta tarea mejoran desde la niñez media hasta la adolescencia media (14-17 años), que a su vez lo hacen peor que los adultos. Tradicionalmente se había creído que la maduración de los procesos de teoría de la mente alcanzaban su zénit al comienzo de la niñez; utilizando un nuevo paradigma experimental en el que hay que utilizar la perspectiva de otras personas en un contexto comunicativo se comprueba, sin embargo, que la ejecución en este tipo de tareas en la adolescencia media no alcanza todavía la de los adultos.
Cuando realizamos este experimento utilizando MRI funcional pudimos comprobar que esta tarea activa especialmente regiones del cerebro social, particularmente la corteza medial prefrontal (MPFC). Los adultos activaban específicamente esta región sólo cuando era más compleja (tres objetos vs. un objeto). Sin embargo, los adolescentes activaban intensamente esa región independientemente de la complejidad. Este es un indicio de que los adolescentes muestran menor especificidad que los adultos en la activación de la MPFC durante la mentalización y de que los adolescentes pueden mostrar una “sobrementalización”. En definitiva, la investigación en neurociencia está poniendo de manifiesto que hay regiones del cerebro social que sufren cambios en su actividad durante la adolescencia.
Este tipo de investigaciones pueden tener importantes implicaciones para la sociedad en relación con la educación y la consideración legal de los adolescentes. Entender las bases cerebrales del comportamiento social y su desarrollo durante esta edad puede ayudar a mejorar la competencia social de los alumnos tanto dentro como fuera del aula. El funcionamiento social juega un papel importante en el aprendizaje y en el rendimiento académico (y viceversa). Conocer las bases neurales del comportamiento social puede contribuir a entender las raíces y los mecanismos de los procesos de éxito y fracaso escolar. El hecho de que los cambios en la estructura cerebral continúen a lo largo de la adolescencia (y más allá) desafía las ideas hasta ahora aceptadas y está dando lugar a un número creciente de investigaciones sobre la forma en que la cognición (incluida la cognición social) podría cambiar. La adolescencia es una etapa clave para el desarrollo de regiones del cerebro implicadas en la cognición social y la autoconciencia, así como en la resolución de problemas y el pensamiento abstracto. Si el comienzo de la niñez suele considerarse un período de importantes oportunidades –o “período sensible”– para la enseñanza, lo mismo debería decirse de la adolescencia ya que durante ambos períodos tiene lugar una reorganización cerebral importante. Quizá entre los objetivos de la educación de los adolescentes debería incluirse el desarrollo de habilidades que están controladas por las partes del cerebro que sufren los mayores cambios durante ese período, incluyendo aquellas que se exponen en esta revisión: la cognición social y la comprensión y la conciencia de las perspectivas potencialmente diferentes de los otros.
Conflict of InterestThe author of this article declares no conflict of interest.




![Neuroimaging variant of the Director task (a) Examples of a 3-object trial in the Director Present and the Director Absent (control) conditions. In both conditions in this example, participants hear the instruction: “Move the large ball up” in either a male or a female voice. In both examples, if the voice is female, the object to be moved would be the basketball, since in the Director Present condition (left) the female Director is standing in front of the shelves and can see all the objects, while in the Director Absent condition (right) the two boxes below the “F” (for “female”) indicate that all objects can be moved by the participant. If the voice is male, the object to be moved would be the football, since in the Director Present condition the male Director is standing behind the shelves and therefore cannot see the larger basketball in the occluded slot, while in the Director Absent condition the single clear box below the “M” (for “male”) indicates that only objects in open shelves can be moved. (b) Main effect of the Director factor across age groups. Regions showing increased BOLD signal in the Director Present compared to Director Absent blocks are rendered on the SPM8 surface mesh template. From left to right: lateral view of the left and right hemisphere, medial view of the left hemisphere. Activations were observed bilaterally along the superior temporal sulci and in the superior dorsal MPFC. Contrast thresholded at p < .001 uncorrected at the voxel-level, p < .05 FWE corrected at the cluster level. (c) ROI analysis testing for interactions between Director and Object, and between Director, Object, and Age group. Mean parameter estimates in the superior dorsal MPFC cluster of the Director Present > Director Absent contrast showed a significant interaction between Director and Object factors, with greatest activation in the DP 3-object condition, and a significant 3-way interaction between Director, Object, and Age group, with a significant increase in [DP - DA] in 3-object vs. 1-object blocks in the adults only. (Adapted from Dumontheil et al., 2012). Neuroimaging variant of the Director task (a) Examples of a 3-object trial in the Director Present and the Director Absent (control) conditions. In both conditions in this example, participants hear the instruction: “Move the large ball up” in either a male or a female voice. In both examples, if the voice is female, the object to be moved would be the basketball, since in the Director Present condition (left) the female Director is standing in front of the shelves and can see all the objects, while in the Director Absent condition (right) the two boxes below the “F” (for “female”) indicate that all objects can be moved by the participant. If the voice is male, the object to be moved would be the football, since in the Director Present condition the male Director is standing behind the shelves and therefore cannot see the larger basketball in the occluded slot, while in the Director Absent condition the single clear box below the “M” (for “male”) indicates that only objects in open shelves can be moved. (b) Main effect of the Director factor across age groups. Regions showing increased BOLD signal in the Director Present compared to Director Absent blocks are rendered on the SPM8 surface mesh template. From left to right: lateral view of the left and right hemisphere, medial view of the left hemisphere. Activations were observed bilaterally along the superior temporal sulci and in the superior dorsal MPFC. Contrast thresholded at p < .001 uncorrected at the voxel-level, p < .05 FWE corrected at the cluster level. (c) ROI analysis testing for interactions between Director and Object, and between Director, Object, and Age group. Mean parameter estimates in the superior dorsal MPFC cluster of the Director Present > Director Absent contrast showed a significant interaction between Director and Object factors, with greatest activation in the DP 3-object condition, and a significant 3-way interaction between Director, Object, and Age group, with a significant increase in [DP - DA] in 3-object vs. 1-object blocks in the adults only. (Adapted from Dumontheil et al., 2012).](https://static.elsevier.es/multimedia/1135755X/0000002100000002/v1_201511180050/S1135755X15000172/v1_201511180050/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
