The study of outer membrane vesicles (OMVs) became relevant because of their probable important role in the transfer of virulence factors to host cells. Campylobacter fetus is mainly a mammal pathogen whose virulence characterization is still limited. The aim of this study was to evaluate and to characterize the secretion of OMVs in this bacterium. By transmission electron microscopy, we confirmed the production of OMVs in all the strains assayed. Purified OMVs showed a spherical shape and variable size, although comparable to those of other gram-negative bacteria. We also confirmed the presence of the S-layer on the surface of the OMVs of all the strains assayed with the exception of those derived from the NTCC reference strain. In addition, we demonstrated their immunoreactivity by the dot-blot assay. Hence, C. fetus OMVs could contribute to the modulation of the host response and constitute a candidate to be evaluated as an adjuvant of current vaccines used in the veterinary field. This work represents a platform to drive future studies towards the role of these subcellular structures in C. fetus-host interaction.
El estudio de las vesículas de membrana externa (VME) tomó un rol protagónico, ya que se las ha relacionado con la transferencia de factores de virulencia a la célula hospedadora. Campylobacter fetus es, principalmente, un patógeno de mamíferos cuya virulencia solo ha sido caracterizada de forma limitada. El objetivo de este trabajo fue evaluar y caracterizar la secreción de VME en esta bacteria. Mediante microscopía electrónica de transmisión confirmamos la producción espontánea de VME en todas las cepas estudiadas. Las VME purificadas mostraron una morfología esférica y un tamaño variable, pero compatible con el reporte de otras bacterias gram negativas. Asimismo, hemos demostrado que estas vesículas conservan la capa S en todas las cepas, menos en la cepa de referencia NCTC y hemos confirmado su inmunorreactividad por dot-blot inmunoblot. Estas VME de C. fetus podrían contribuir a la modulación de la respuesta del hospedador y constituir un buen candidato como adyuvante de las actuales vacunas empleadas en el campo veterinario. Este trabajo representa una plataforma para impulsar estudios futuros en torno al rol de estas estructuras subcelulares en la interfase C. fetus-hospedador.
The biogenesis and release of outer membrane vesicles (OMVs) is a common feature of several gram-negative bacteria. Through the release of OMVs and the subsequent fusion of membranes, different bacterial components could be delivered and incorporated into the host cells.
Since their first description in the literature, OMVs have been associated to relevant functions related to host-pathogen (and bacteria–bacteria) interaction, such as delivery of virulence factors and immune modulators, disruption of host tissue, gene and RNA transfer among others14,25.
Campylobacter are microaerophilic, motile, non-fermentative gram-negative organisms. Most species of Campylobacter can cause disease in both humans and animals. Campylobacter fetus comprises two subspecies associated to mammal hosts (Campylobacter fetus fetus and Campylobacter fetus venerealis). In animals, they have been associated to bovine infertility and bovine/ovine abortion21. C. fetus fetus has been isolated from blood, abscess fluid, bone marrow, cerebrospinal fluid, joint prostheses or implanted catheters and biopsies or stool from humans with different clinical conditions such as fever, colitis, venous thrombosis, erysipelas, meningitis, pleuritic chest pain, lethargy, endocarditis, photophobia and gastrointestinal problems8,31.
Campylobacter spp. have an outermost surface component, which is called the surface layer (S-layer), which consists of a crystalline array of proteinaceous subunits (SLP or surface layer proteins)26 encoded by a diverse number of homologous genes in each strain29. In C. fetus, SLPs attach to either type A or B lipopolysaccharides28. This bidimensional structure, which is external to the cell wall, fulfils a variety of biological functions and roles9. The S-layer has been characterized as a virulence factor that mediates the evasion of the immune response in C. fetus3,4.
Different virulence strategies have been widely described in the emblematic member of the genus: C. jejuni. One of the most innovative strategies is the delivery of the biologically active toxin CDT through OMVs16. While C. jejuni virulence has been widely studied, the description of C. fetus-host interphase has been delayed. In addition, OMVs have immunological properties that are suitable for vaccine strategies27. Interestingly, a Neisseria meningitidis OMV-based vaccine, the first licensed vaccine based on these nanoparticles, has demonstrated its usefulness in human health12,23.
In Argentina, reproductive diseases impair livestock production and C. fetus is among the main bacterial pathogens isolated from abortions in one of the most productive regions of the country20. C. fetus is the etiological agent of the venereal disease called bovine genital campylobacteriosis. Vaccination is not compulsive; however, it is often applied when positive animals are detected in the herd. The limited available data around bacterin-based commercial vaccines suggest that they must be improved5,7. The use of novel adjuvants could improve the accuracy of current vaccines.
The aim of this study was to evaluate the biogenesis of OMVs in C. fetus, to characterize them morphometrically and to assay their immunoreactivity.
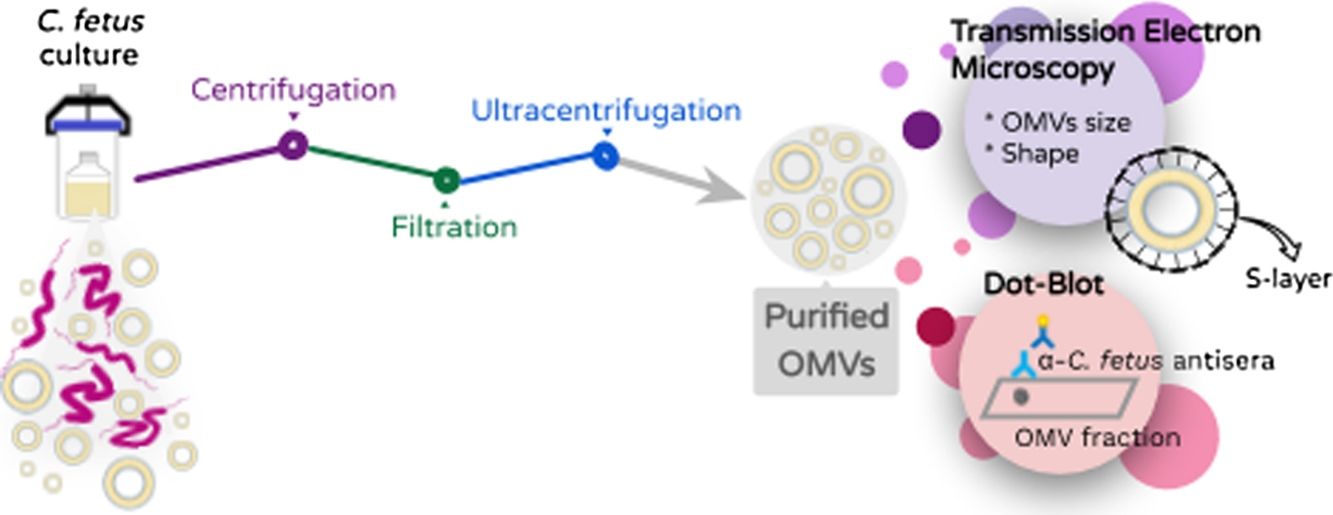
Material and methodsBacterial strains. Campylobacter fetus strains from the culture collection of the Bacteriology laboratory EEA-INTA Balcarce were used in this study. Three well-characterized C. fetus field strains obtained from tissue samples from bovine aborted fetuses were analyzed in the study: Campylobacterfetus venerealis biovar intermedius 06-341 (Buenos Aires), Campylobacterfetus fetus 13-344 (Buenos Aires), C. fetus fetus 08-421 (Santa Fe). The type strain C. fetus venerealis NCTC 10354 isolated from bovine vaginal mucus was added in the analyses. We have previously typed the strains following standard biochemical procedures21, whereas other studies have characterized them at molecular and genomic levels as well10,30.
Growth conditions and isolation of OMVs. OMVs were obtained by differential centrifugation from bacterial culture supernatants essentially as previously described by Elmi and co-workers for C. jejuni with some modifications6. Skirrow agar plates (Oxoid) were used for the recovery of the C.fetus strains. Strains were grown at 37°C for 72h (5% O2, 10% CO2 and 85% N2) and then the colonies were inoculated into 10ml Brucella broth (Oxoid) (starter culture). The cultures were grown on an orbital shaker (100rpm) under microaerophilic conditions at 37°C for 72h. Then, the starter culture was transferred to a flask containing 500ml Brucella broth. Because C. fetus is a slow growing bacterium, long-term cultures were grown to obtain the appropriate optical density (OD 0.3–0.4 reached after 72h–96h of culture). The bacterial culture was centrifuged at 10000g for 30min and the resulting supernatants were filtered through a 0.22μm membrane (Millipore) to remove cells and debris. Then, ultracentrifugation was carried out at 150000×g for 3h at 4°C using a 45Ti rotor (Beckman instruments) and the pellet containing OMVs was resuspended with a minimal volume of sterile phosphate-buffered saline pH 7.4 (PBS). Two further washing steps were performed with PBS before carrying out the immunoassays.
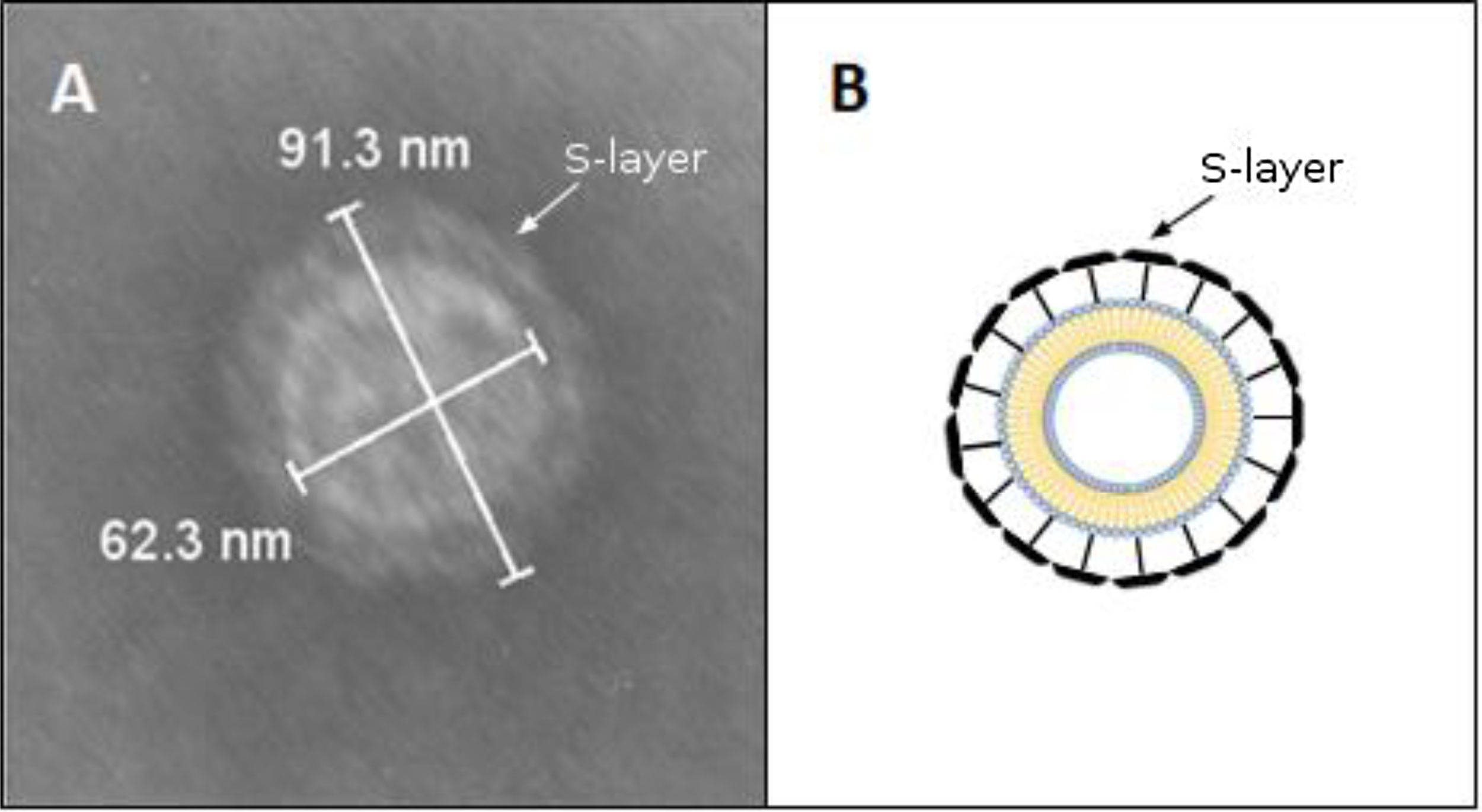
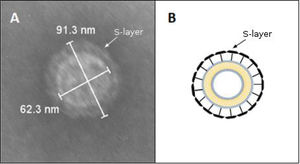
Transmission electron microscopy (TEM) and morphometry. The OMV fraction was fixed with one volume of 4% paraformaldehyde, mounted on grids and negatively stained with 1% phosphotungstic acid (PTA) adjusted to pH 7.5 with 1N NaOH. OMV production from bacteria grown on solid media was evaluated by picking up and suspending colonies in 50:50 4% paraformaldehyde PBS solution. In addition, a 200-μl fraction of the Brucella broth cell culture was centrifuged at 10000×g for 5min and resuspended in the same volume 4% paraformaldehyde to test the release of OMVs in planktonic conditions. These samples were processed for TEM as explained above. The samples were observed using a Jeol 1200 EX-II transmission electron microscope at 80kV (Jeol Ltd.) at the Service of the Laboratorio Integral de Microscopía, CICVyA, INTA, Argentina. The OMV size and S-layer thickness were expressed as mean±standard deviation. Thickness was calculated with the following formula Thickness=(total diameter−internal diameter)/2. All measurements were conducted by trained staff.
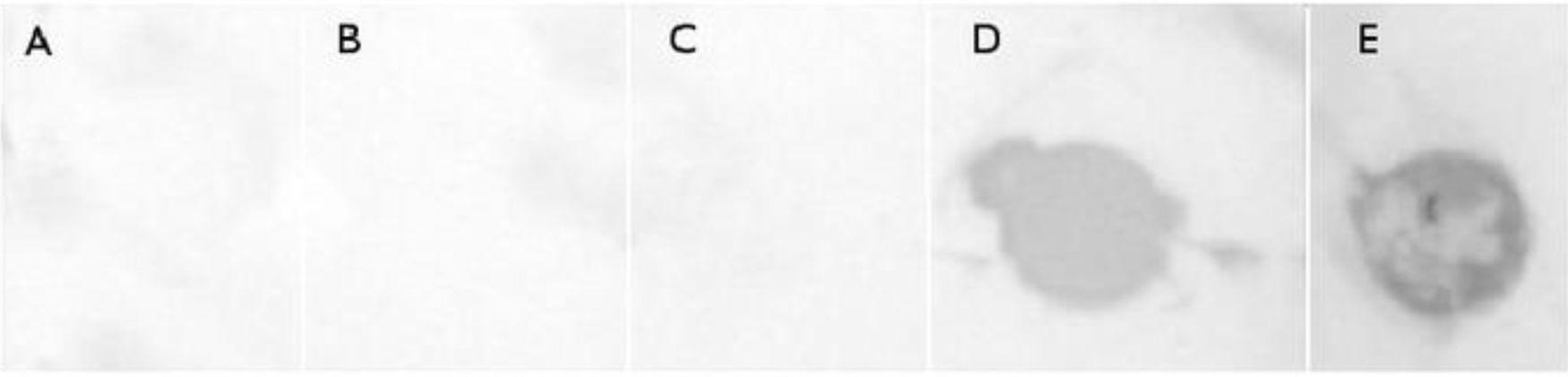
Antigenicity of OMVs (Immunodot). An OMV sample from C. fetus 08-421 (80μl) was blotted onto a nitrocellulose membrane using the microfiltration apparatus Bio-Dot (Bio-Rad, Life Science). The same volume of Brucella broth and PBS was used as negative controls and cell proteins of C. fetus were used as positive control. The blot was then blocked with 5% skimmed milk and incubated with a 1:100 dilution of serum anti whole cell proteins of C. fetus. Azul laboratories-Argentina gently donated the C. fetus serum, which was obtained by immunization of rabbits with two C. fetus strains (C. fetus fetus and C. fetus venerealis). After three washes, the blot was incubated with a 1:3000 dilution of alkaline phosphatase-conjugated anti-rabbit IgG (Sigma–Aldrich). After three additional washes, the blot was developed by adding an NBT/BCIP solution (Promega) as substrate.
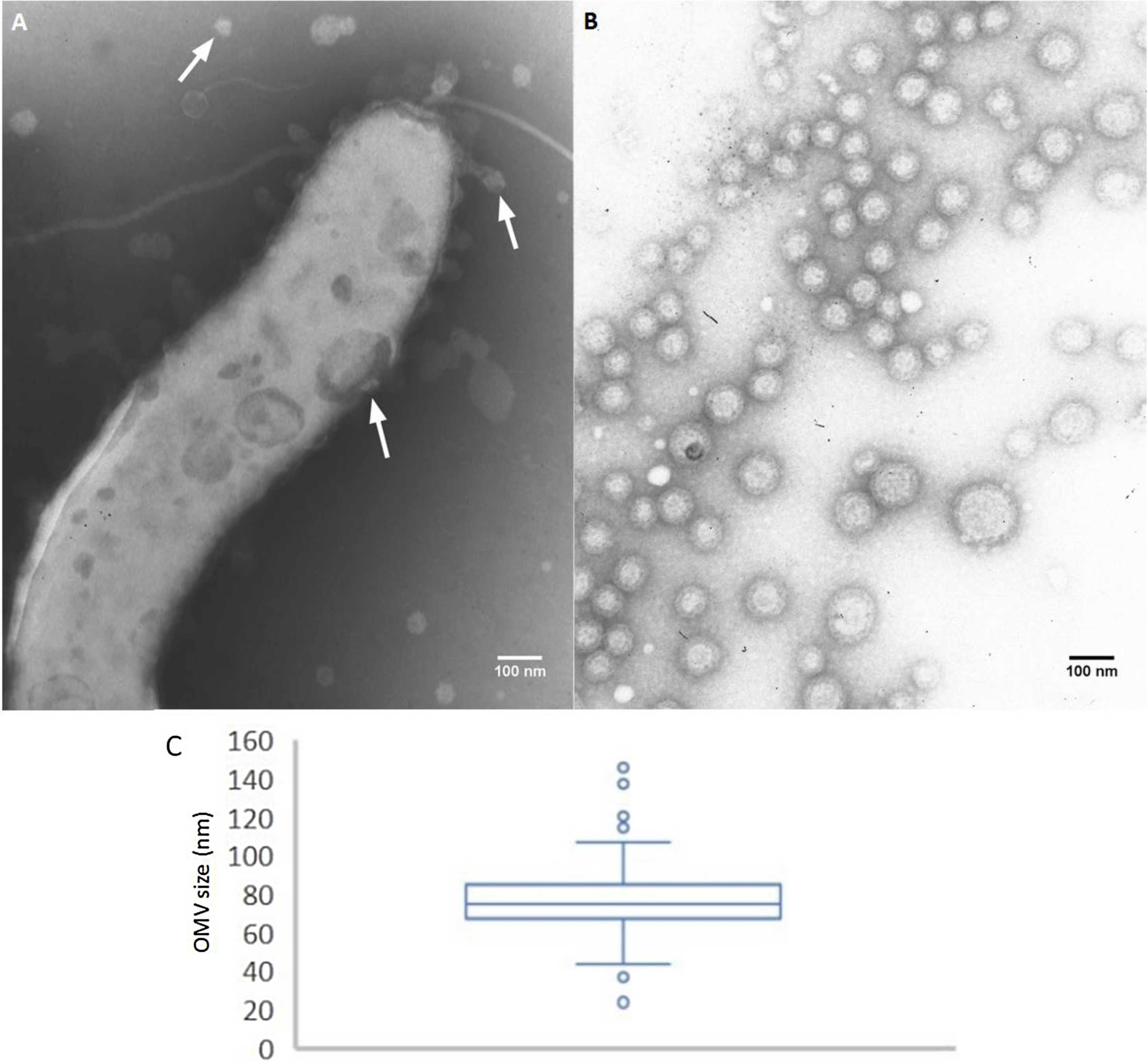
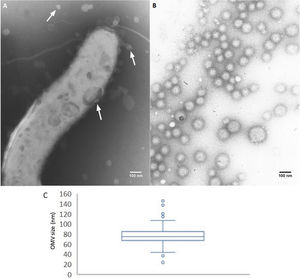
ResultsTo analyze the secretion of OMVs, we first studied the presence of OMVs in bacterium samples grown in solid or broth culture media (non-isolated OMVs). Through TEM, we detected OMVs both surrounding the bacterium cells and detached from cells. The presence of membrane vesicles budding off from the surface of the bacteria was also observed. These non-purified OMVs showed morphological variation (Fig. 1A). OMVs were also present in cultures grown in solid media (representative image in Fig. 2E).
Release and purification of C. fetus OMVs. (A) Non-purified OMVs (TEM micrograph, negative staining): bacterial fraction from C. fetus 08-421 culture grown in Brucella broth. A flagellate bacterium cell is surrounded by OMVs. The arrows indicate OMVs of different size and shape. (B) and (C) morphometry of purified OMVs. TEM micrographs of negative stained C. fetus 08-421 showing spherical OMVs covering a wide range of sizes (range 26–141nm), mean diameter: 76.58±11.53nm (n=244 from three micrographs).
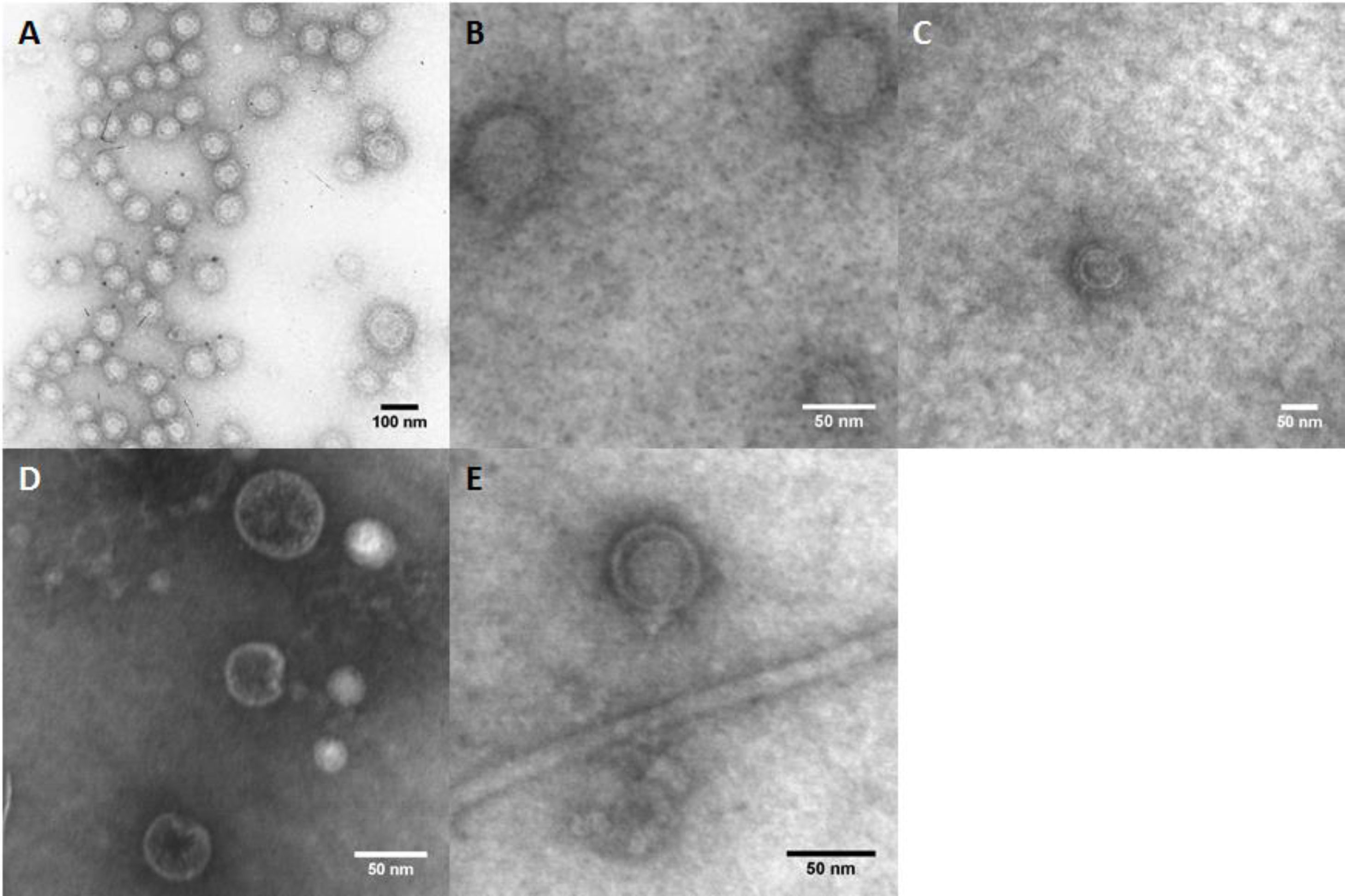
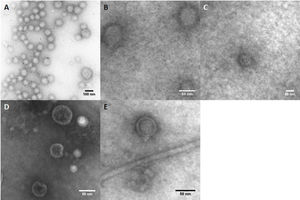
By contrast, the purified OMVs were homogeneous in shape but with variable size, ranging between 24 and 145nm with a mean diameter of 76.58±11.53nm (Fig. 1B). Regardless of the subspecies, all the studied strains produced OMVs (Fig. 2A–D). The analysis of the isolated OMVs revealed the presence of a typical motif of S-layer in all the strains, except for Campylobacter fetus venerealis NCTC 10354, which lacked this crystalline structure (Fig. 2D). The absence of the S-layer was also evident in non-purified OMVs from this strain grown on solid media (Fig. 2E). S-layer thickness was 15.99±8.91nm (n=20) (Fig. 3). This finding supports the fact that the S-layer is maintained during the biogenesis of OMVs.
Finally, a dot-blot immunoblot analysis to assess the antigenicity of released OMVs revealed a strong interaction with C. fetus-hyperimmune rabbit sera in the OMV fraction derived from C. fetus 08-421 (Fig. 4).
DiscussionEarlier electronic microscopy-based studies have provided evidence of the presence of ‘globular bodies’ in Campylobacter fetus, formerly Vibrio fetus22. Those structures were ignored at that time as OMVs and were characterized as membrane-associated structures that co-purified with flagella19. Through immunological techniques, Winter and co-workers32 have described the presence of the S-layer protein (former protein A) associated to non-spontaneously produced vesicles obtained by shearing of bacteria. Apart from these studies, no additional evidence of secretion of OMVs nor their isolation have been reported in C. fetus to date.
In this work, all the evaluated C. fetus field isolates produced OMVs in vitro and we were able to purify these vesicles successfully through standardized protocols. The shape and sizes obtained were similar to those previously described in other gram-negative bacteria18. However, it should be considered that OMV sizes could even be more variable (as shown in Fig. 1A); however, the filtration step of the purification process may have led to the elimination of larger OMVs.
A characteristic S-layer was evident on the surface of the OMVs in almost all the assayed strains (3/4). High-resolution electron microscopy and scanning probe microscopy have revealed that most S-layers are 5–25nm thick24, which coincides with our observations by TEM.
The S-layer is one of the first bacterial components to interact with the host. Examples of its different roles are cell adhesion, protection from feeding by protozoa or phagocytes, virulence factor, antigenic properties, anchoring sites for hydrolytic exoenzymes, receptors for phages, porin function and others2,9. This crystalline structure also provides the bacterium with a protective core against environmental conditions. Hence, some of these functions may also be valid for OMVs, e.g., increasing the lifetime of these transporters to reach distant targets.
In addition, the S-layer was absent or undetectable only in OMVs from C. fetus venerealis NCTC 10354. This result was observed both in culture and after purification from cell-free culture media. Interestingly, Blaser and Pei4 described spontaneous S-layer mutants obtained after multiple passages of a wild-type C. fetus strain on artificial media. That could be the case of the C. fetus venerealis NCTC 10354 strain. This strain probably expresses SLPs less efficiently, may not express them at all or may even express SLPs but the process of S-layer assembly could be impaired. Further research must be conducted in relation to the secretion of layered or non-layered OMVs. Thus, considerable attention should be paid when using this strain in future studies, especially when the presence of the S-layer is desirable. The presence of S-layered vesicles is relevant and could have important consequences such as the adhesion to cells or extracellular matrix and modulation of the host response.
To test the presence of immunoreactive components, we assayed the recognition of polyclonal sera raised with C. fetus. In our study, C. fetus 08-421 was the most active OMV-producing strain. Despite this fact, we had to employ a dot-blot assay to concentrate on a spot the low quantity of proteins present in the sample. Surface layer proteins have been previously described as important antigens in C. fetus3. Hence, the immunoreactivity of OMVs that we observed could be explained, in part, by the presence of the S-layer. Additionally, since we cannot discard that polyclonal antibodies could reach the inside of OMVs, other antigenic molecules could also contribute to this observed immunoreactivity. Moreover, surface layer proteins of different pathogens have been described as a pathogen-associated molecular pattern (PAMP). These PAMPs could be recognized by toll-like receptors (TLR) or ICAM-3-grabbing nonintegrin (DC-SIGN) present on macrophages and, in the case of DC-SIGN, on dendritic cells, thus resulting in cell activation and cytokine secretion11,13. Therefore, OMVs were described as structures capable of engaging innate and adaptive immunity and with intrinsic adjuvant capacity27. In this way, C. fetus OMVs could also fulfil important criteria to modulate both adaptive and innate immune responses and constitute a potential candidate to be evaluated as an adjuvant of current vaccines used in the veterinary field.
It is worth noting that obtaining OMVs is challenging since their productivity is low. Different culturing times, static growing vs. shacking, volume of culture, initial inoculum as well as different culture media were among the variables assayed to establish the final protocol. The purpose was to obtain native OMVs, thereby the low yields obtained with most of the strains were aligned with the expected results.
Stimulated secretion of OMVs could improve the obtained yields. Different stimuli such as nutrient depletion, exposure to antibiotics, hydrogen peroxide, heat shock or even detergent extraction, among others, have been proved to be useful in different bacteria17,33 and must be evaluated in C. fetus. However, it must be taken into account that the composition of the OMVs could be different33. For instance, the S-layer could be affected, which could have a detrimental effect on adhesion and immunogenicity.
In line with Thompson et al.28, we think that the engineering of OMVs producing-bacteria to generate OMVs is a promising strategy. Through this, a reduced linkage of the outer membrane to the peptidoglycan can increase OMVs release15 or could transform them in effective carriers of useful molecules1 (antigens, immunological mediators such as cytokines, among others) and even with a controlled host response (e.g., low LPS content)33. Consequently, its combination with vectored or traditional vaccines could be the best option to boost an overall immunological response.
Future proteomic studies could certainly contribute to determining the protein composition and roles of these nanoparticles in C. fetus. This study represents a starting point for future research in C. fetus focused on elucidating the host-pathogen interphase, the modulation of the host response and vaccine development.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank Bioq. M. Verónica Gal and Dr. Marcela Cucher (IMPAM, UBA-CONICET) for their valuable support in the early stage of this work. This study was supported by ANPCyT-Argentina Project PICT2015-1541, CONICET Project PIP2015–2017 11220150100316CO and INTA Project I102. Pablo Farace is fellow of CONICET.