Campylobacter fetusfetus (Cff) is a major infectious cause of abortion in sheep worldwide, and an opportunistic human pathogen. Information on Cff as an ovine abortifacient in South America is limited. We describe a case of abortion caused by a multidrug resistant strain of Cff in a sheep in Uruguay. In August 2017, 3/57 pregnant ewes (5.3%) aborted whithin one week. Histopathologic examination of the placenta of an aborted ewe revealed severe neutrophilic and fibrinonecrotizing placentitis with vasculitis and thrombosis of the chorionic arterioles. Cff was isolated on microaerobic culture in Skirrow agar, and further confirmed by 16S rDNA PCR amplification and sequencing, and endpoint and real time PCR assays. Antimicrobial sensitivity testing revealed resistance to tetracyclines, nalidixic acid, telithromycin and clindamycin. Other abortifacients were not detected. Further studies are necessary to determine the geographic distribution, ecology, epidemiology, economic impact, and antimicrobial resistance of Cff in sheep flocks in Uruguay.
Campylobacter fetus fetus (Cff) es una importante causa de abortos en ovinos y un patógeno oportunista en humanos. La información sobre Cff como abortifaciente en ovinos en Sudamérica es limitada. Describimos un caso de aborto causado por una cepa de Cff multirresistente a antibióticos en una oveja en Uruguay. En agosto de 2017, 3/57 ovejas preñadas (5,3%) abortaron en una semana. El examen histopatológico de la placenta de una de ellas reveló placentitis neutrofílica fibrinonecrosante severa, vasculitis y trombosis. Cff fue aislado en microaerobiosis en agar Skirrow, y confirmado mediante amplificación del ADNr 16S por PCR seguida de secuenciación, y por PCR punto final y qPCR. Las pruebas de sensibilidad antimicrobiana revelaron resistencia a tetraciclinas, ácido nalidíxico, telitromicina y clindamicina. No se detectaron otros abortifacientes. Son necesarios más estudios para determinar la distribución geográfica, ecología, epidemiología, el impacto económico y la resistencia antimicrobiana de Cff en majadas ovinas de Uruguay.
The genus Campylobacter comprises a diverse group of gram negative, microaerophilic, motile, and non-spore-forming epsilon-proteobacteria, highly adapted to animals and humans20. Currently, the genus includes 32 species and 13 subspecies; the type species, Campylobacter fetus, contains three subspecies: C. fetus subsp. fetus (Cff), C. fetus subsp. venerealis (Cfv) and C. fetus subsp. testudinum (Cft)14. Cff and Cfv cause reproductive disorders in ruminants, while Cft has been detected in reptiles and humans18.
Although both Cfv and Cff are reproductive pathogens of cattle and sheep, they have different habitats and transmission routes. The natural niche of Cfv, the causative agent of bovine genital campylobacteriosis, is the genital tract of cattle, where it causes infertility and abortion after venereal transmission18. The primary reservoir of Cff is the intestinal tract of sheep, cattle, and a wide range of other species, causing reproductive disease after reaching the pregnant uterus through bacteremia20. In sheep, Cff causes late gestation abortion, stillbirths, and birth of weak lambs16,20.
Cff is also an opportunistic human pathogen causing diarrhea, bacteremia, abortion, and perinatal mortality22. Even though the pathogenesis and sources of human exposure are largely unknown, foodborne and occupational (zoonotic) transmission has been suggested22. The use of antimicrobials in food animals and the livestock-to-human transmission of bacterial pathogens raises concerns about the possible transfer of determinants of antimicrobial resistance, even across bacterial genera. Antibiotic resistance, including multidrug resistance, has been widely documented in Campylobacter species isolated from ruminants4,11,23 and humans21. To the best of our knowledge, multidrug resistance has not been documented in Cff isolated from sheep.
Currently, Cff is recognized as one of the leading causes of ovine abortion worldwide20. In South America, Cff-associated abortion in sheep has only been reported once in Argentina7 and Brazil9, respectively. The reproductive impact of ovine campylobacteriosis is probably underreported in South America. In Uruguay, there is little information regarding the causes of ovine abortion, which is worrying considering not only the economic impact but also that many infectious abortifacients of sheep are zoonotic.
The aim of this paper is to describe a case of ovine abortion caused by Cff in Uruguay, expanding the understanding on the infectious causes of abortion in sheep in this country and broadening the current knowledge on the geographic distribution of ovine campylobacteriosis in South America. We also report on the antimicrobial susceptibility of the isolated Cff strain.
In August 2017, three Finnish ewes aborted within one week in a flock of 62 sheep in the department of Colonia, Uruguay. After a breeding period of 34 days (from March 24 to April 27), at least 57 of the 62 sheep became pregnant (minimum pregnancy rate=91.9%). The fetuses of the aborted ewes could not be recovered; however, a section of the retained placenta from one of the sheep that aborted at 103–137 days of gestation was pulled manually and aseptically from the vagina/vulva by the animal caretaker, placed in a sterile plastic bag and carried to the veterinary laboratory for diagnostic investigation.
Approximately 1h after extraction, a fresh sample of placenta was inoculated onto Skirrow agar [Columbia blood agar base (CM0331, Oxoid) with 7% defibrinated sheep blood, supplemented with Skirrow Campylobacter selective supplement (SR0069, Oxoid) and Campylobacter growth supplement (SR0232, Oxoid)] and incubated in a microaerobic atmosphere at 37°C using sealed jars and commercial sachets (CampyGen, CN0025, Oxoid), as described previously18. A sub-sample of the same specimen was incubated aerobically on blood and MacConkey agars at 37°C for 7 days. Large numbers of round, smooth bacterial colonies of approximately 1mm diameter were isolated under microaerobic conditions in Skirrow agar after 48h. Light microscopic examination revealed gram negative curved rods; darting motility was observed under dark-field microscopy. The colonies were subjected to biochemical tests including catalase, oxidase, and hydrogen sulfide production (triple-sugar-iron – TSI – medium), growth in the presence of 3.5% sodium chloride or 1% glycine, and growth in Skirrow medium at 25 and 42°C18. The bacteria tested positive for catalase and oxidase and negative for hydrogen sulfide production, grew at 25°C but not at 42°C, grew in the presence of 1% glycine but not in 3.5% sodium chloride. Thus, the isolate was phenotypically identified as C. fetus, and thermophilic Campylobacter species such as C. jejuni and C. coli were ruled out. No other bacteria were isolated on Skirrow agar incubated under microaerobic conditions. Only Escherichia coli was isolated in blood and MacConkey agars.
To further identify the agent at the molecular level, DNA extraction was performed from bacterial colonies isolated on Skirrow agar using a commercial kit (Quick-DNA Fecal/Soil Microbe Miniprep Kit, Zymo Research). Identification of Campylobacter spp. was performed by amplifying and sequencing a fragment of the 16S rRNA gene. Amplicons of approximately 1500bp were sequenced (Macrogen Inc., Seoul, South Korea), and compared with the available 16S rDNA sequences using the BLASTn tool (blast.ncbi.nlm.nih.gov/Blast.cgi), as previously described1. The amplified sequence was 99.7% identical to several C. fetus complete genomes (i.e. those identified with accession numbers CP000487.1 and HG004426.1). Furthermore, species and subspecies identification were performed using multiplex PCR10 and species was also identified by real time PCR13; these tests confirmed Cff, and other Campylobacter species and subspecies were ruled out.
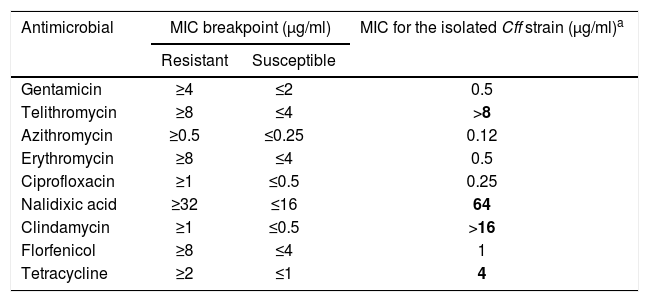
The broth microdilution method was performed on the Cff isolate to assess antimicrobial sensitivity to nine antimicrobials in seven antimicrobial classes, including aminoglycosides (gentamicin), ketolides (telithromycin), macrolides (azithromycin and erythromycin), quinolones (ciprofloxacin and nalidixic acid), lincosamides (clindamycin), phenicols (florfenicol), and tetracyclines (tetracycline). The minimum inhibitory concentrations (MICs) to these antimicrobials were determined using Sensititre™ Campylobacter CAMPY AST Plate (Thermo Fisher Scientific), following procedures suggested by the Clinical and Laboratory Standard Institute2. Briefly, plates were incubated at 37°C for 48h and the MIC was defined as the lowest concentration of each antimicrobial resulting in complete inhibition of visible growth on the medium. For quality control, we used strain ATCC 33560 of C. jejuni. Results were interpreted based on the breakpoint values proposed by the National Antimicrobial Resistance Monitoring System (NARMS) of the U.S. Centers for Disease Control and Prevention (CDC) for C. jejuni17, as no breakpoints have been established for C. fetus. The isolated Cff strain was resistant to tetracyclines, nalidixic acid, telithromycin and clindamycin, and sensitive to azithromycin, ciprofloxacin, erythromycin, gentamycin, and florfenicol (Table 1).
Minimal inhibitory concentration (MIC) breakpoints to determine antimicrobial susceptibility in the isolated Campylobacter fetus subsp. fetus strain.
| Antimicrobial | MIC breakpoint (μg/ml) | MIC for the isolated Cff strain (μg/ml)a | |
|---|---|---|---|
| Resistant | Susceptible | ||
| Gentamicin | ≥4 | ≤2 | 0.5 |
| Telithromycin | ≥8 | ≤4 | >8 |
| Azithromycin | ≥0.5 | ≤0.25 | 0.12 |
| Erythromycin | ≥8 | ≤4 | 0.5 |
| Ciprofloxacin | ≥1 | ≤0.5 | 0.25 |
| Nalidixic acid | ≥32 | ≤16 | 64 |
| Clindamycin | ≥1 | ≤0.5 | >16 |
| Florfenicol | ≥8 | ≤4 | 1 |
| Tetracycline | ≥2 | ≤1 | 4 |
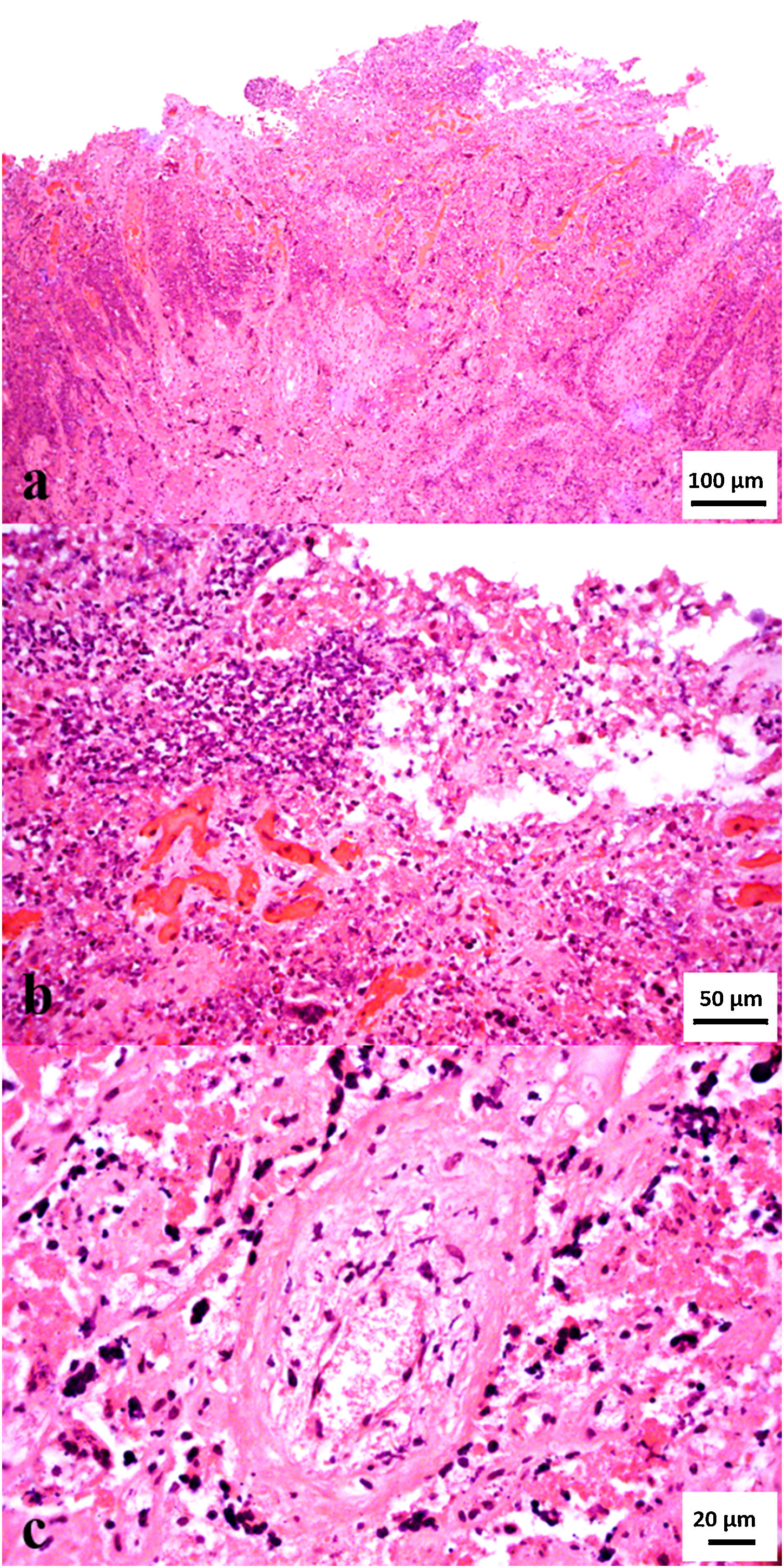
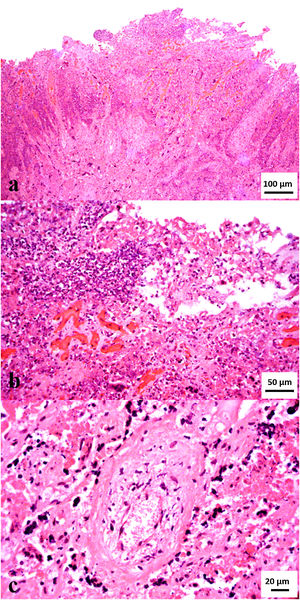
Upon arrival to the laboratory, sections of placenta were fixed in 10% neutral buffered formalin for 48h, dehydrated, embedded in paraffin, microtome-sectioned at 4–5μm, mounted on glass slides, and stained with hematoxylin and eosin for routine histologic examination, as well as Steiner's silver stain (SSS). Microscopically, there was severe and extensive fibrinonecrotizing and neutrophilic cotyledonary placentitis, with severe neutrophilic vasculitis and thrombosis in the chorionic arterioles (Fig. 1); numerous curved bacilli morphologically resembling Campylobacter spp. were observed intralesionally in the sections stained with SSS. Additionally, serial sections of formalin-fixed paraffin-embedded placenta were processed by immunohistochemistry (IHC) for the detection of antigens of other abortifacients of sheep, including Toxoplasma gondii5, Chlamydia spp.8 and Coxiella burnetii15 using adequate positive and negative controls. Positive controls consisted of ovine tissues infected with the agents investigated. Negative controls were sections of placenta incubated with normal instead of hyperimmune serum. The IHCs were all negative in the tested case and the negative controls, while positive in the positive controls.
(A) Histologic section of the cotyledonary region of the placenta showing severe extensive inflammatory infiltrate. (B) Higher magnification of Figure 1A. Note abundant degenerate neutrophils admixed with fibrin exudate and karyorrhectic (necrotic) cellular debris (severe extensive fibrinonecrotizing and neutrophilic cotyledonary placentitis. (C) Cross section of a chorionic arteriole (center) depicting concentric necrosis of the tunica media and mural neutrophilic infiltrate (arteriolitis), with abundant fibrin and necrotic debris in the surrounding stroma (hematoxylin and eosin stain, A: 200×, B: 400×, C: 630×).
To the best of our knowledge, this is the first description of ovine campylobacteriosis by Cff in Uruguay and the third in South America, where cases were previously reported in Brazil in 20109 and Argentina in 20147. In the case described here, the diagnosis was based on pathological and bacteriological findings, including molecular testing, which allowed unequivocal etiologic confirmation. Additionally, the complete genome of the Cff strain isolated from this sheep was sequenced and deposited in GenBank under the BioProject number PRJNA5541553. The isolation of E. coli was probably a consequence of environmental contamination and was not unexpected in a field sample of retained placenta.
The placental lesions we described are typical of those induced by Campylobacter spp., including vasculitis (arteriolitis) which is a frequent finding16. Arteriolitis of chorionic arterioles has also been described in cases of Chlamydia spp. and Coxiella burnetii placentitis, which were ruled out in our case. In this context, the intralesional identification of large numbers of curved bacilli morphologically resembling Campylobacter spp. with SSS in our case is a strong indicator of causality. Interestingly, previous works from South America did not report these lesions either because histological examination was not performed9 or perhaps due to advanced autolysis of the placentas examined in the laboratory7. The spectrum of lesions induced by Cff in aborted fetuses includes multifocal necrotizing and neutrophilic hepatitis, suppurative bronchopneumonia, and fibrinous peritonitis/pleuritis16. Unfortunately, the fetuses were not submitted to the laboratory in this outbreak. In extensive, pasture-based production systems, such as those prevailing in Uruguay, identifying recently aborted sheep, and retrieving fetuses and their placentas before scavenging is often difficult, unless there is placental retention as described in this case. Scavenging and fetoplacental autolysis decrease the chances of finding quality samples for laboratory investigation and reaching an etiologic diagnosis, which remains undetermined in a high proportion of abortion cases.
Ovine campylobacteriosis can manifest as abortion storms affecting up to 50% of the flock, or as sporadic abortions20. Since ewes that aborted due to Cff infection develop an effective immune response, outbreaks usually occur when flock immunity wanes or susceptible ewes are incorporated16,20. In recovered flocks, abortion outbreaks in successive years is uncommon. However, a cyclical pattern of abortions every 4–7 years is reported. In contrast, sporadic losses occur in endemic situations20. In this flock 3/57 pregnant ewes (5.3%) aborted in a week suggestive of an abortion outbreak. However, diagnostic samples were submitted from just one abortion. Thus, the etiology of the abortion in the other two ewes remained undetermined. The remainder 54 pregnant ewes delivered 117 lambs throughout the lambing season (prolificacy rate=2.17). No abortions were recorded in the same flock in 2018.
Abortion is often the only clinical manifestation of Cff infection in sheep20. Vaginal discharges, retained placentas and metritis have been sporadically reported and have occasionally led to the death of affected ewes16,20. The aborted ewe in this report had a retained placenta, as described also in the outbreak in Argentina7, but mortality of ewes was not reported.
The strain of Cff we isolated was resistant to four antimicrobials in four different antimicrobial classes, including a ketolide (telithromycin), a quinolone (nalidixic acid), a lincosamide (clindamycin), and a tetracycline (tetracycline), fulfilling the criterion to be regarded as a multidrug resistant strain. Resistance to tetracyclines has been reported in a Cff strain isolated from an aborted ewe in Turkey23; however, none of the seven Cff strains analyzed in that study evidenced multidrug resistance. The Uruguayan strain was not resistant to gentamicin, as was previously identified in a strain of Cff isolated from an aborted ovine fetus23. Different C. fetus strains isolated from feedlot cattle in Canada were resistant to tetracyclines, enrofloxacin, ciprofloxacin and erythromycin, although none was resistant to multiple antibiotics11. These strains were not identified to the subspecies level11. Tetracycline and ciprofloxacin resistance have been identified in Cff strains isolated from humans in Canada21. Multidrug resistance has been observed in a C. fetus strain isolated from an immunocompromised human patient in the USA, although the bacterium was not identified at the subspecies level. De Brito et al.4 isolated multidrug resistant C. fetus strains from calf feces and the genital tract of adult cattle in Brazil4. Twenty-one strains (fourteen fecal and seven genital) were evaluated, resistance was found in 85.7% of the strains to nalidixic acid, 71.4% to cefoperazone, 42.9% to tetracycline, 19.1% to ampicillin, 9.5% to chloramphenicol, and 4.8% to erythromycin4. Multidrug resistance was found in ten (eight fecal and two genital) of these strains, of which all were resistant to cefoperazone, eight to tetracycline, eight to nalidixic acid, four to ampicillin, and one each to erythromycin and chloramphenicol. These strains were not identified at the subspecies level.
Since tetracycline is a recommended therapeutic agent to treat Campylobacter spp. infections, further studies should be conducted to determine if resistance is more widespread in the region. Tetracycline resistance has been rarely reported in Cff and is considered less relevant than the resistance generated by C. jejuni to this antimicrobial. In the USA, the high selection pressure caused by the frequent use of tetracyclines has led to the emergence of a tetracycline-resistant C. jejuni clone19. Currently, this clone of C. jejuni has spread replacing Cff as one of the main causes of ovine abortion in the USA19. To the best of our knowledge, ours is the first description of antibiotic resistance in a Cff isolated from an aborted ewe in South America7,9.
Since cattle and sheep are the main reservoirs of Cff, the products from these animals are potential sources of infection to humans22. Furthermore, Cff strains with identical genotypes have been identified in cattle and humans, suggesting a possible link6. In Uruguay, infection by a bovine strain of Cff was described in a rural worker undergoing cancer treatment, and zoonotic transmission was hypothesized12. The role of sheep as potential sources of human campylobacteriosis, particularly in the context of antimicrobial resistance, should be further explored in South America.
Further studies are needed to clarify the geographic distribution, ecology, epidemiology, economic impact, and antimicrobial resistance of Cff in sheep flocks in Uruguay.
FundingThis work was financially supported by grants N-15156 PL 15 0 00 and PL 27 N-23398 of the Instituto Nacional de Investigación Agropecuaria (INIA, Uruguay).
Conflict of interestThe authors declare that they have no conflicts of interest
The authors thank Karen Sverlow and Juliann Beingesser from CAHFS, UC Davis for technical assistance with the immunohistochemical techniques.