Human parechovirus (HPeV) is one of the members of the family Picornaviridae that has been associated with fever of unknown origin, gastroenteritis, clinical sepsis, meningitis, or encephalitis in very young infants. HPeV detection is not routinely performed in most clinical microbiology laboratories in Argentina and, therefore, its real prevalence is unknown. We here report three cases of HPeV CNS infection that presented to our hospital with different clinical features after the implementation of a multiplex PCR meningitis/encephalitis panel. Molecular diagnostic techniques could help improve patient care and understand the real prevalence of this infection in Argentina.
Los parechovirus humanos (HPeV) son virus de la familia Picornaviridae, que se han asociado a diferentes cuadros clínicos, como fiebre de origen desconocido, gastroenteritis, sepsis, meningitis o encefalitis en niños pequeños. Su detección no está disponible de rutina en la mayoría de los laboratorios de nuestro país, por lo que su prevalencia es desconocida. Reportamos 3 casos de infección del sistema nervioso central por HPeV con diferentes características clínicas, que se presentaron luego de la implementación de un panel molecular para el diagnóstico sindrómico de meningitis/encefalitis. Las técnicas de diagnóstico molecular podrían ayudar a mejorar el abordaje y el cuidado de estos pacientes, así como también a conocer la prevalencia de esta infección en Argentina.
Human parechovirus (HPeV) is a small non-enveloped RNA virus that belongs to the family Picornaviridae. It was first isolated in 1956 and the first two viruses were classified as echoviruses 22 and 23 of the genus Enterovirus (EV). In 1999 they were reclassified into the genus Parechovirus, as HPeV-1 and HPeV-2 respectively. Currently, 19 genotypes have been identified, being HPeV-3 particularly neurotropic and associated with more severe disease10.
Infection is prevalent among young children. It is often asymptomatic or associated with mild disease of the gastrointestinal and respiratory tracts. However, HPeV can also cause severe diseases such as sepsis, encephalitis, and meningitis4,10 with long term neurological sequelae in young infants, including cerebral palsy, central visual impairment, and gross motor impairment3,11,13. Therefore, it represents a diagnostic and therapeutic challenge for the treating physician.
HPeV detection is not routinely performed in most clinical microbiology laboratories and, therefore, its real prevalence is unknown. Recent studies have shown that HPeV-3 might be one of the main agents involved in neonatal neurological infections worldwide1,7,14.
We report three cases that presented with different clinical features in our hospital after the implementation of a multiplex PCR meningitis/encephalitis panel (ME) (Filmarray) between December 2018 and December 2019. The panel includes the detection of 7 viruses (HPeV, EV, herpes simplex virus 1 and 2, human herpesvirus 6, varicella-zoster virus), 6 bacteria (Escherichia coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae) and one yeast (Cryptococcus neoformans/gattii).
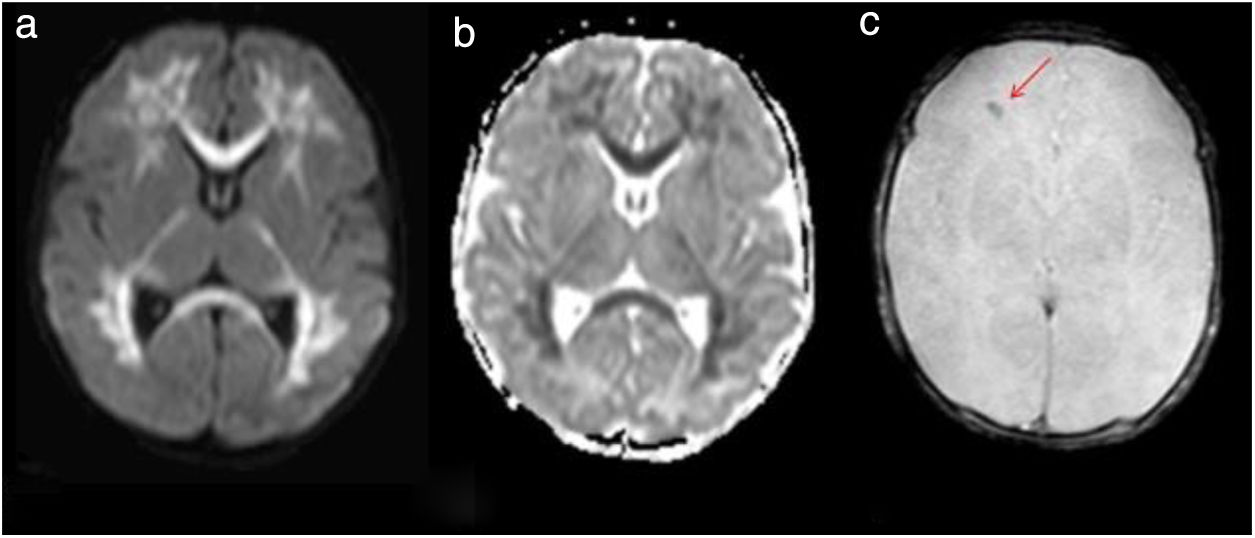
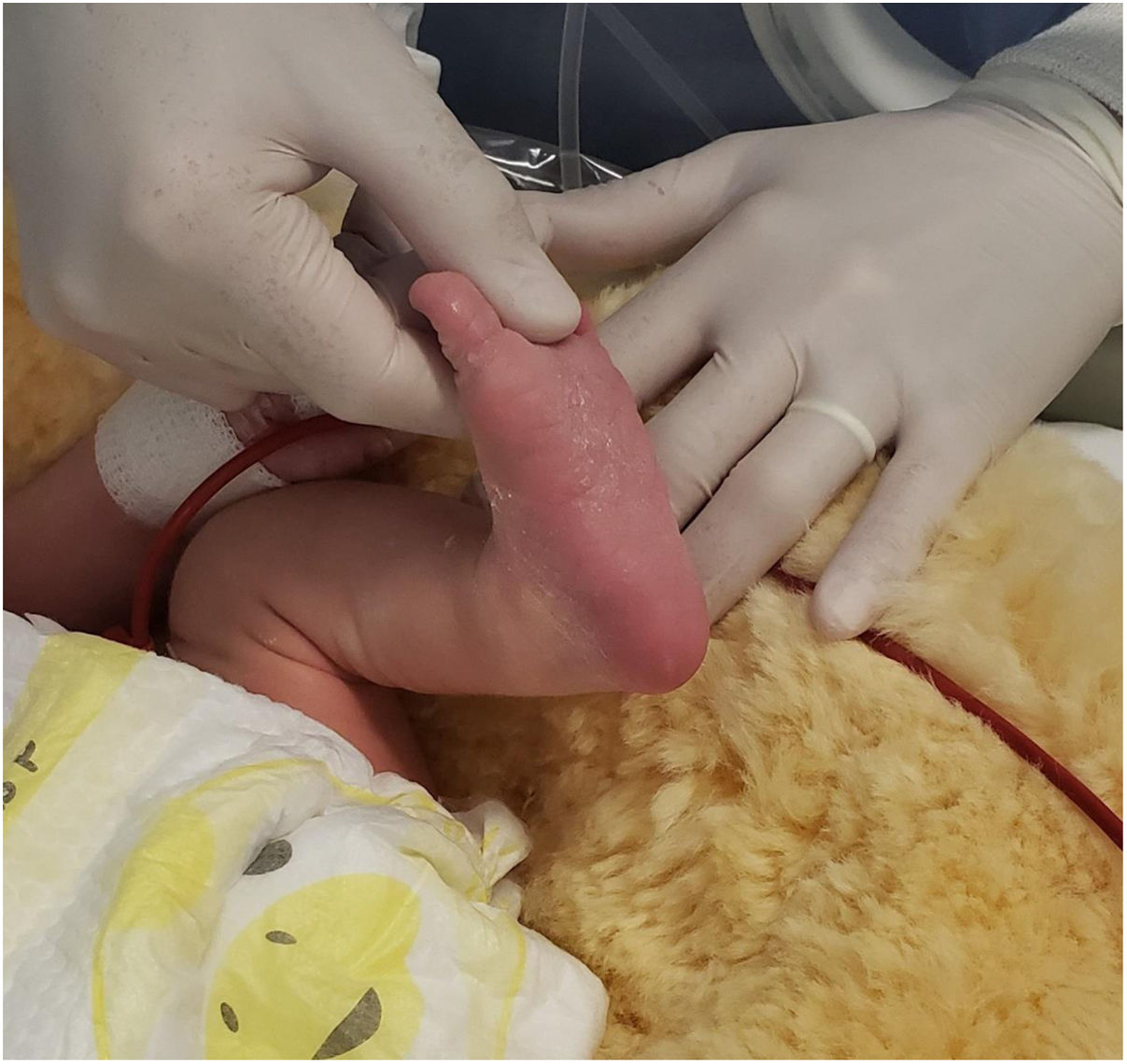
A 6-day-old boy was brought to the Department of Pediatrics for a neonatal jaundice follow-up examination. His mother reported decreased oral intake and a febrile episode of 37.9°C without gastrointestinal or respiratory symptoms. He was cesarean-born and his mother had a Streptococcus group B (SGB)-positive culture during pregnancy. His mother refused to hospitalize him and returned the following day reporting fever, irritability, and decreased feeding. He also presented with mild aqueous diarrhea that was self-limiting. He was admitted to the neonatal ICU. Laboratory tests included lumbar puncture, urine, stool, and a set of blood cultures. His white blood cell count (WBCc) was 8.7×103WBC/mm3 and his C-reactive protein (CRP) level was 1.6mg/l. He was administered intravenous ampicillin and gentamicin. The cerebrospinal fluid (CFS) was clear and colorless, had no pleocytosis, only mild proteinorrachy (87mg/dl). Bacterial cultures of blood, urine, and CFS were negative and his chest X-ray was normal. The ME panel was positive for HPeV and rotavirus antigen was detectable in the stool. On day 2 of admission, he developed left tonic–clonic movements and episodes of bradycardia and desaturation. Phenobarbital was administered (15mg/kg). Brain ultrasound showed subcortical white matter hypoechogenicity of both cerebral hemispheres, corpus callosum, and both temporal lobes. Brain magnetic resonance imaging (MRI) showed acute leukoencephalitis (Fig. 1). He also presented an erythematous palmar–plantar rash (Fig. 2). Levetiracepam was added to his treatment scheme as he manifested new desaturation episodes and hypertonia. He gradually improved and was discharged on day 15. Due to the severity of the case, HPeV was identified by real-time RT-PCR as HPeV-3. He is currently followed-up in Child Neurology, continues on an anticonvulsant treatment, and receives early stimulation.
Case 1 – axial magnetic resonance imaging. Diffusion sequence (a) and apparent diffusion coefficient (ADC) map (b) where extensive restrictive areas of diffuse and symmetric distribution are observed, compromising the supratentorial bihemispheric white matter, dorso-lateral nuclei of both thalamus and corpus callosum. T2 * Sequence (c) showing the bleeding area (red arrow).
A 16-day-old girl was admitted to the emergency department for a history of decreased oral intake, irritability, and fever of 38°C without gastrointestinal or respiratory symptoms. She was born full-term by vaginal delivery without complications and her mother had an SGB-positive culture during pregnancy. On physical examination, no rash was observed. Laboratory tests included lumbar puncture, urine, and a set of blood cultures. Her WBCc was 6.2×103WBC/mm3 and her CRP level was 2.0mg/l. She was administered intravenous gentamicin and cefotaxime. Her CFS was clear and colorless and without pleocytosis. Bacterial cultures of blood, urine, and CFS were negative. The ME panel was positive for HPeV. Her chest X-ray was normal and a nasopharyngeal swab was negative for respiratory viruses. She was admitted for 3 days, and after 24h with no fever and a favorable evolution, she was discharged.
A 4-month-old boy, born full-term by vaginal delivery, was admitted to the emergency department after 4h of persistent crying and two febrile episodes of 38°C in the last 24h. He had normal weight-for-age. On physical examination, he was irritable with abdominal tenderness and no rash was observed. His brother was having an upper respiratory tract infection. His WBCc was 6.2×103WBC/mm3 and his CRP level was 5.2mg/l. Abdominal ultrasound only showed meteorism. A lumbar puncture was performed, and CFS was clear and colorless, without pleocytosis. The ME panel was positive for HPeV. Bacterial cultures of blood, urine, and CFS were negative. A cerebral ultrasound was performed and was normal. He had intermittent sub-febrile episodes and was discharged on day 4 of admission, after 30h with no fever.
HPeV is one of the picornaviruses that has been associated with fever of unknown origin, gastroenteritis, clinical sepsis, meningitis, or encephalitis in very young infants. We report three different cases of HPeV CNS infection that presented in our hospital between December 2018 and December 2019.
HPeV infections have been reported worldwide, and previous studies have shown a prevalence of HPeV in CSF samples ranging from 0 to 17%10,14. Gutierrez et al. reported HPeV as the etiologic agent of meningitis and sepsis-like illness in infants with a detection rate of 18% in South America, which is higher than any other series worldwide6. However, the prevalence rates differ depending on the design of the study, sampling sites, and demographic characteristics. As HPeV infection is not a notifiable disease in Argentina and the test is not widely accessible, epidemiological information is not available. For the same age group in the studied period, the incidence of HPeV and EV infections was similar, as there were three viral encephalitis cases due to EV. We, therefore, conclude that further studies are needed to estimate the burden of HPeV infections.
The three cases were recorded during the spring, as it has been previously described6,8. However, most HPeV infections tend to occur in late summer and autumn months7. The age distribution of patients with HPeV infection is characterized for presenting in children under three months of age, and mainly in neonates8. In this cohort, the three patients were less than 4 months of age.
Transmission of HPeV occurs through the fecal–oral route and possibly infective respiratory droplets2,10. There is scant data regarding respiratory transmission, however, it is supposed to be the route in children with CNS symptoms10. The risk of transmission is probably highest during acute symptomatic illness. Confirmed cases should be under standard, contact, and respiratory precautions to prevent transmission to other young infants during admission2.
All cases presented with fever and irritability as common clinical features. However, the severity of case 1 was quite different, reflecting the wide range of clinical features of this viral infection. Although most mild HPeV infections have no specific symptoms, severe infections have some clinical features that may suggest the etiology. Clinical syndromes caused by HPeV vary by genotype, and HPeV-3 illness can be severe with up to 20–50% of admitted patients presenting with sepsis-like syndrome2. The combination of fever, rash, and severe irritability with characteristics of sepsis in neonates should lead clinicians to consider HPeV infection as a differential diagnosis7. Long term follow-up is strongly advised, as neonates are considered a vulnerable population at risk for neurodevelopmental delay and neurological sequelae3.
The absence of CSF pleocytosis and normal protein and glucose levels in CSF are commonly reported in most cases of HPeV CNS infections, as observed in these three cases5. HPeV should be considered for testing in CFS samples of neonates as it can lead to severe encephalitis associated with white matter injury in the neonatal period12. Syndromic molecular testing allowed us to establish the specific cause of encephalitis at around 3h since sample collection. A shorter time to result could lead to shorter hospital stays and a decrease in the use of antimicrobial therapy9.
Molecular diagnostic techniques could help improve patient care and understand the real prevalence of this infection in Argentina.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank María Cecilia Freire, Servicio de Neurovirosis, INEI-ANLIS Dr. Carlos G. Malbran, for genotyping HPeV-3 from case 1.