Bacillus amyloliquefaciens fmb50 produces a high yield of surfactin, a lipopeptide-type biosurfactant that has been widely studied and has potential applications in many fields. A foam overflowing culture has been successfully used in the combined production-enrichment fermentation of surfactin. In this study, the agitation and aeration rates were found to have relationships with foam formation and surfactin enrichment. A maximum surfactin concentration of 4.7g/l of foam was obtained after 21h of culture with an agitation rate of 150rpm and an aeration rate of 1vvm in fed-batch culture. By controlling the foam overflow rate (fout) of a fed-batch culture, surfactin concentration in the foam was continuously maintained above 4g/l.
Bacillus amyloliquefaciens fmb50 produce gran cantidad de surfactina, un biosurfactante de tipo lipopeptídico que ha sido objeto de estudios pormenorizados y tiene aplicaciones en muchos campos. El cultivo en espuma desbordante se ha utilizado con éxito en la fermentación combinada de producción-enriquecimiento de surfactina. En este estudio, se halló que las tasas de aireación y agitación tienen relación con la formación de espuma y el enriquecimiento de la surfactina. Se obtuvo una concentración máxima de surfactina de 4,7g/l de espuma después de 21 h de cultivo con una tasa de agitación de 150 rpm y una tasa de aireación de 1 vvm en un cultivo alimentado (fed-batch). Al controlar la tasa de espuma desbordante (fout) de un cultivo fed-batch, la concentración de surfactina en la espuma se mantuvo continua por encima de 4 g/l.
Biosurfactants are amphiphilic molecules widely produced by a variety of microorganisms, which have been considered as an alternative to chemical surfactants. Lipopeptides are one of the major types of biosurfactants14. As the most effective biosurfactant that has been found so far, surfactin can lower the surface tension of water from 72 to 27mN/m5,12. Surfactin exhibits antibacterial and antiviral properties and biodegradability, and appears to be promising for applications in areas such as bioremediation and oil recovery1,2,15. However, after almost 50 years, surfactin is not yet a viable alternative to chemical surfactants because of the low yield in bioreactors13, its relatively high medium cost, and severe foaming in aerated and stirred bioreactors16. Therefore, the production of surfactin is still limited to laboratory scale5. To cope with these problems, renewable substrates and foam overflow fermentation were used in recent years4,9.
Some studies on fed-batch culture of surfactin have been undertaken in the past few years4,5,16. In foam overflowing fed-batch culture (FOFC), the flow rates of the feed and of the overflow foam were equal, and thus the broth volume in the bioreactor was kept constant, and continuous enrichment of surfactin in foam overflow was accomplished6. It has been shown that by controlling the agitation and aeration rates, maximum surfactin productivity could be achieved when the oxygen volumetric mass transfer coefficient (kLa) value was 0.0132/s16. However, the effect of agitation and aeration rates on surfactin enrichment in the foam has not been reported.
In our previous studies, we identified a strain of Bacillus amyloliquefaciens as producer of five surfactin homologues by using high performance liquid chromatography (HPLC) and electrospray ionization mass spectrometry methods13. A high yield of surfactin from strain B. amyloliquefaciens fmb50 was obtained by genome shuffling, and a method for surfactin determination was established17.
In this work, a semi-defined medium, which is of low cost and high yield compared with the commonly used Landy medium, was initially combined with foam overflow batch culture. The effect of agitation and aeration rates on the foam overflowing rate (fout) and surfactin enrichment in the batch culture process were studied. By using fed-batch culture, a continuous, high concentration enrichment of surfactin could be achieved. This novel fermentation technology has a potential for the industrial application of surfactin production.
Materials and methodsMicroorganism and culture mediaB. amyloliquefaciens fmb50 (CGMCC No. 6249), the surfactin producer used in this study, is registered by the China Committee for Culture Collection of Microorganisms.
The seed medium (BPY) consisted of: beef extract 5.0g/l, peptone 10.0g/l, yeast extract 5.0g/l, glucose 10.0g/l and NaCl 5.0g/l (pH 7.0).
The semi-defined medium (IBM) used for fermentation was optimized by the Taguchi method in our previous work, and consisted of: corn powder 35g/l, ammonium nitrate 15g/l, urea 6g/l, KCl 1.47g/l, NaH2PO4 20mmol/l, MnSO4 0.5mmol/l, MgSO4 0.1mmol/l, CuSO4 12.8μmol/l, FeSO4 1μmol/l and CaCl2 0.5μmol/l (pH 7.0).
Flask and bioreactor culture conditionsIn the primary inoculum, a loop of colonies from a fresh potato dextrose agar-slant was transferred into 50ml BPY medium in shaken flasks (250ml) and cultured at 37°C and 180rpm for 12h. For the secondary inoculation, 10ml of the primary culture was inoculated into 200ml BPY medium in shaken flasks11, and cultured in the same conditions as those in the primary inoculum.
Bioreactor cultivation was performed in a 19 l bioreactor containing 12l of medium (L1523, Bioengineering AG, Switzerland); the temperature was controlled at 32°C and the pH was maintained at 7.0 with the automatic addition of 4.0mol/l NaOH. Three control strategies were adopted in batch cultivation to investigate the effects of agitation and aeration rates on fout and surfactin enrichment in foam from overflowing cultures. The agitation and aeration rates were controlled as shown in Table 1. In control 1, the agitation and aeration rates were controlled at 200rpm and at 0.66vvm respectively. In control 2, the agitation and aeration rates were controlled at a relatively high level; the agitation rate was 300rpm and the aeration was increased from 1.2 to 2.66vvm as the foam overflowed and the medium volume in the bioreactor was reduced.
Analytical methodsThe colony forming units (CFU) were calculated using plate colony-counting methods. Dry cell weight (DCW) was obtained by collecting the fermentation broth with different incubation times in the IBM medium. After centrifugation at 1000×g for 15min, the collected pellets were dried for 8h at 90°C and the dry cells weighed8. Then, the dry cell weight (DCW) was determined using a pre-determined standard curve relating the number of colony forming units (CFU) to the dry weight: DCW=2.43×log CFU/ml−18.63, with an R2 value of 0.92. Results were represented as CFU per milliliter.
Culture samples were precipitated and then extracted with methanol as described by Cooper et al.3 in the extraction of surfactin. Surfactin concentration in crude samples was determined by reverse phase HPLC (U-3000, Dionex, United States) equipped with an Agilent C18 column (4.5mm×250mm, Agilent, United States) and a UV detector. About 20μl of the surfactin sample was injected into the column and then eluted with acetonitrile with 0.1% TFA at a flow rate of 0.84ml/min. Eluent absorbance was monitored at 210nm17. Quantization was performed based on a standard curve using a standard sample of surfactin (Sigma–Aldrich Co., United Sates). The standard curve equation is: y=17.771x−38.742 (R2=0.999). The equation for the calculation of the specific growth rate (μ/h) was: μ=dX/(X·dt); the equation for the calculation of the specific growth rate (qp/g/(g*h)) was: qp=dP/(X·dt), X: cell yield (g/l); P: product yield (U/ml); t: fermentation time (h).
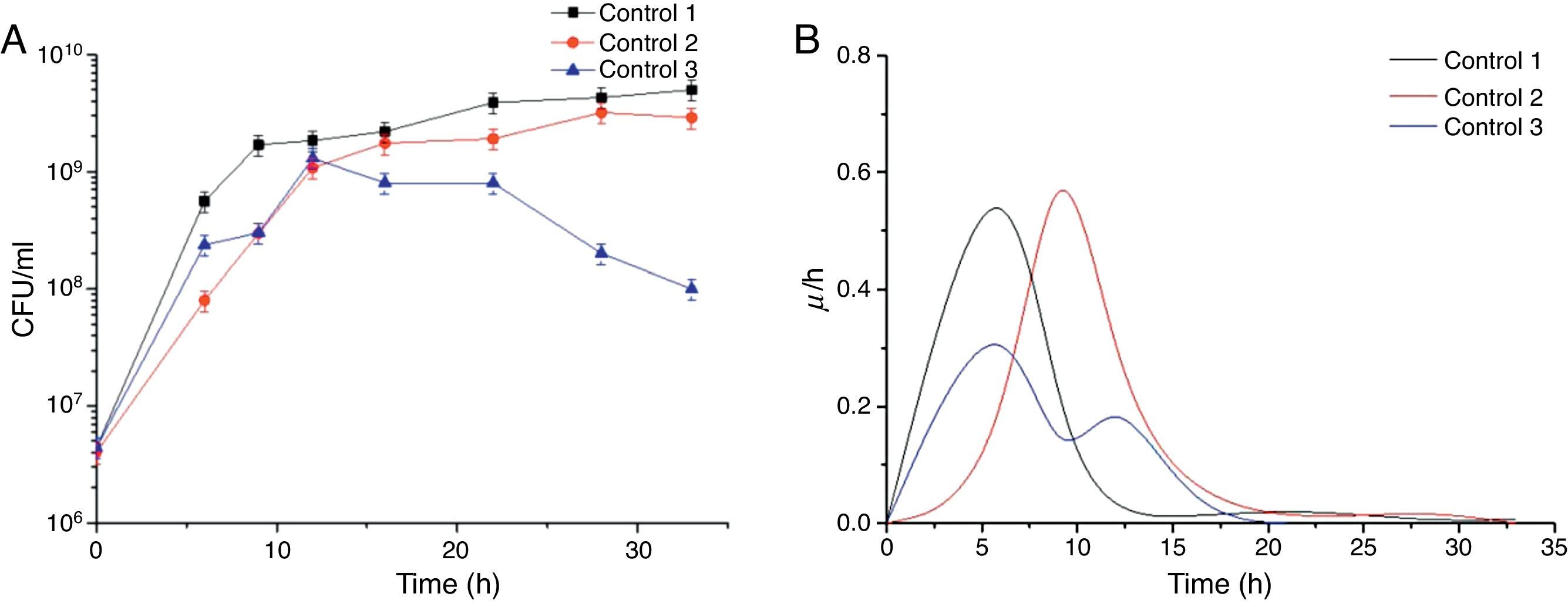
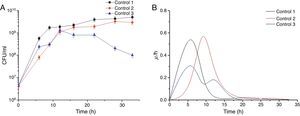
Results and discussionEffect of the control strategy on cell specific growth rateThe effect of agitation and aeration rates on cell growth in the three control strategies adopted in this study in batch culture is shown in Figure 1. In controls 1 and 2, B. amyloliquefaciens fmb50 in the bioreactor reached up to 109CFU/ml after 15±0.2h culture, and then the cell growth went into stationary phase (Fig. 1A). However, in control 3, cell growth declined from 12h of cultivation. That may be due to the agitation rate changing between 150 and 250rpm; normal cell growth seemed to be inhibited and the biomass decreased due to the simultaneous increase of the overflow. B. amyloliquefaciens fmb50 reached its maximum specific cell growth rate at 6h of cultivation in control 1, which was 4h earlier than in control 2 (Fig. 1B). In control 3, where cell growth was disturbed, a lower specific cell growth rate was observed. The maximum specific cell growth rate was lowered nearly by half with respect to controls 1 and 2. Although the increase in the agitation rate promoted quadratic cell growth, this one was not delayed.
Time courses of CFU (A) and cell specific growth rate (B) by Bacillus amyloliquefaciens fmb50 in batch culture with three control strategies. In control 1, the agitation rate was 200rpm, and the aeration rate was 0.66vvm; in control 2, the agitation rate was 300rpm, and the aeration rate increased from 1.2 to 2.66vvm; in control 3, the agitation rate changed between 150 and 250rpm to keep the overflowing flow rate at a relatively moderate level, and the aeration rate was kept at 1.8vvm.
Surfactin production is essentially associated with cell growth7,16, although high biomass does not necessarily mean high surfactin production. It seems that a lower specific growth rate is more conducive to the production of biosurfactants such as mycosubtilin4. The effect of agitation and aeration rates on cell growth and cell specific growth rate was studied in this paper and the best control strategy was chosen to enhance surfactin production.
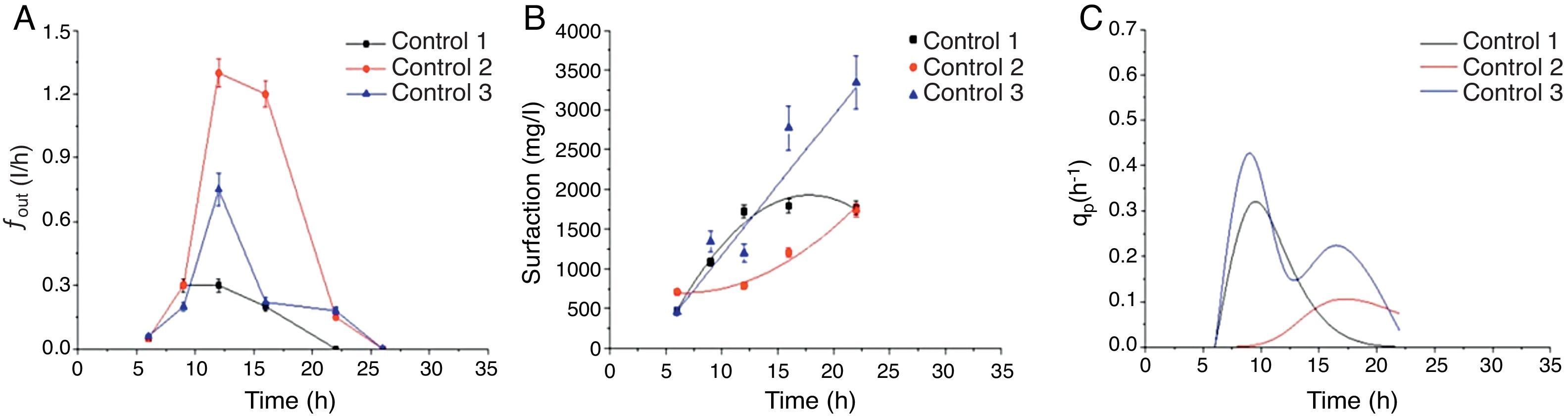
Effect of the control strategy on fout and the concentration and production of enriched surfactin in foamFoam began to overflow outside the bioreactor after 6h of cultivation (Fig. 2A). The maximum fout in control 1 was 0.3l/h while in control 2 it was 1.3l/h, which indicated that the foam overflow was significantly affected by the agitation and aeration rates used in different strategies. The surfactin concentration in the overflow foam also changed with time. As shown in Figure 2B, different trends were observed in the three control strategies. In control 3, surfactin concentration increased linearly and showed the highest specific production rate, reaching a maximum of 0.426l/h (Fig. 2C).
Time courses of foam overflowing flow rate (A), surfactin concentration in the foam (B) and specific production rate (C) in batch culture of Bacillus amyloliquefaciens fmb50 with three control strategies. In control 1, the agitation rate was 200rpm, and the aeration rate was 0.66vvm; in control 2, the agitation rate was 300rpm, and the aeration rate increased from 1.2 to 2.66vvm; in control 3, the agitation rate was changed between 150 and 250rpm to keep fout at a relatively moderate level, and the aeration rate was kept at 1.8vvm.
The linear increase of surfactin concentration and the relatively high specific production rate in control 3 (Fig. 2B and C) indicated that high surfactin enrichment in foam may be achieved by a combined control of the agitation and aeration rates at a reasonable level. As shown in Figure 2B, the maximum surfactin concentration in foam reached 3.342g/ml and the maximum surfactin production in foam reached 2.525g (approximately overflowing 7.6l foam) in control 3. However, maximum surfactin concentration and maximum surfactin production were 1.8g/ml and 8.46g (approximately overflowing 4.7l of foam) in control 1, respectively. These results in control 3, which show the linear increase of surfactin concentration and the relatively high specific production rate, are similar to those in the study by Davis et al.6, the enrichment of surfactin in the foam was enhanced to 0.44g/l with the time going by supplementing culture broth in the stationary phase6. Foam stopped overflowing at 22h in control 1 and at 26h in controls 2 and 3. This was partly due to the medium level in the bioreactor dropping as overflowing foam: however; the surfactin in the foam was thus separated from the broth almost completely6. Apparently, continuous surfactin production in the bioreactor ensured an increasing trend of surfactin concentration in the foam. With the agitation and aeration rate controlled under different strategies, the surfactin production presented a different kinetic trend. Control 3, under which fout was below 0.7l/h, indicated that surfactin enrichment may be improved through stir speed and aeration control.
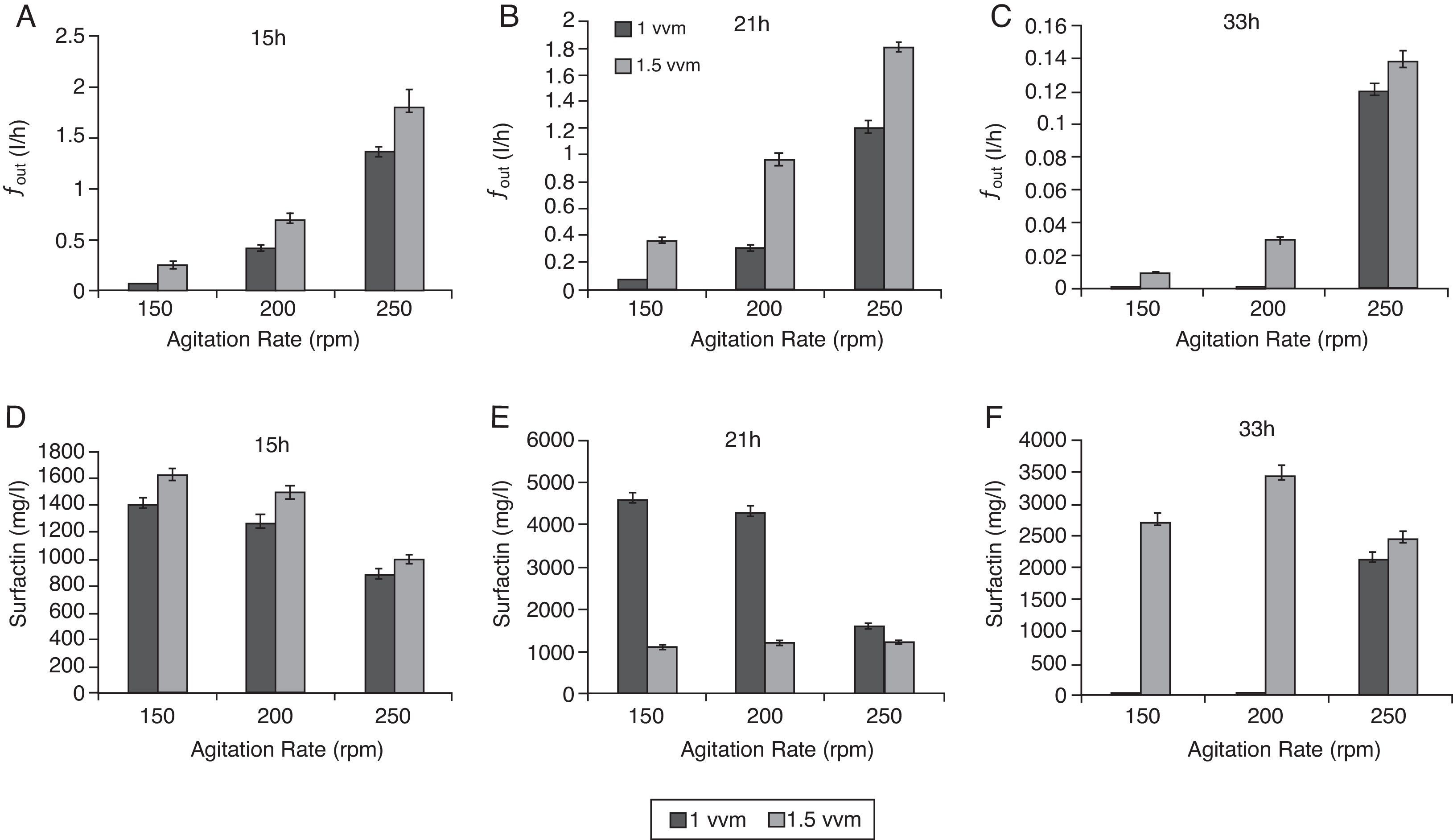
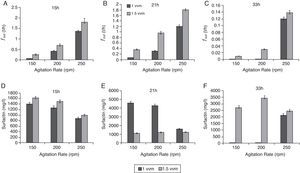
Influence of agitation and aeration rates on fout and concentration of enriched surfactinThe influence of agitation and aeration rates on fout and surfactin enrichment in the overflowing foam were investigated in batch culture experiments conducted using a series of combinations of aeration and agitation rates. Foam overflow began after 6h of cultivation (Fig. 2A). As indicated in Fig. 3(A–C), fout tended to increase with an increased aeration rate from 1 to 1.5vvm and agitation rate from 150 to 250rpm. At 33h, fout was extremely low, only a maximum 0.14l per hour (Fig. 3C); however, raising the aeration rate to 250rpm, the fout could increase to a maximum 1.76 per hour.
Agitation and aeration rates significantly affected the concentration of surfactin in the foam. In the early stage of fermentation (i.e. at 15h; Fig. 3A), an increase in agitation rate increased fout; however, the surfactin concentration was at a relatively low level and decreased from 1.6g/l to 1g/l with the agitation increase. At 21h, the maximum surfactin concentration of 4.7g/l occurred at 150rpm agitation and 1vvm aeration. With the increase of agitation to 250rpm, the surfactin concentration was reduced to 1.5g/l. Interestingly, the change in the agitation rate had no effect on the surfactin concentration at 1.5vvm aeration (Fig. 3E). At 33h, the surfactin concentration in the foam was maintained at >2.5g/l with an aeration rate of 1.5vvm and it did not drop with an increasing agitation rate. However, under the condition of aeration rate at 1vvm, the surfactin concentration in the foam was 2.5g/l only with the agitation rate of 250rpm. Apparently, the agitation and aeration rates significantly affected the concentration of surfactin enriched in the foam.
The agitation and aeration rates in the aerobic fermentation greatly affected the foam formation rate and the surfactin concentration in the foam. Similar to our case, a previous study observed that a higher stirring speed or aeration rate led to a higher fout6. However, surfactin enrichment in this study presented a different kinetic relationship with fout compared to that in the basic study by Davis et al.6, in which the foam continuously overflowed under a constant agitation rate. In the late stages of batch FOFC, such as at 33h (Fig. 3F), the surfactin concentration in the foam was lower than at an earlier stage (21h), because > 50% of the culture broth in the bioreactor was gone. Thus, the broth level in the bioreactor was too low to maintain the overflowing foam and further foam formation was also limited because almost all the surfactin had already been drained away in the foam. Both the surfactin concentration in the foam and fout were determined by the surfactin produced in the broth. Furthermore, the fout level affected the surfactin enrichment, and at the same time, fout was influenced by the agitation and aeration rates.
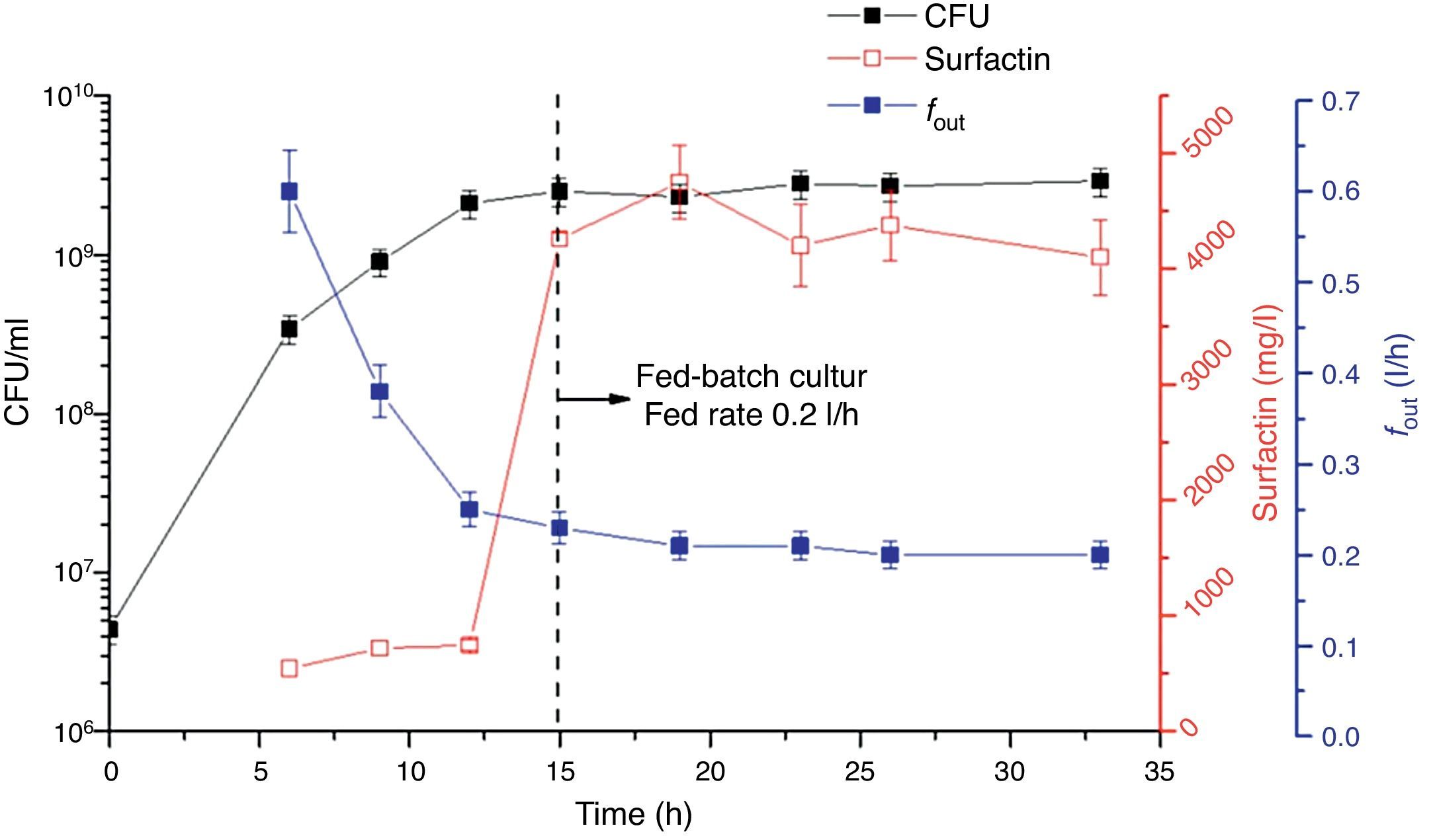
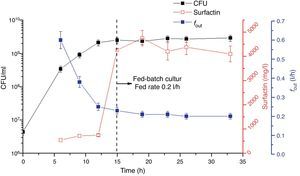
Fed-batch culture with controlled agitation and aeration ratesA fed-batch culture process was also adopted in this study. Figure 4 shows that foam began to overflow after 6h of cultivation. Afterwards, fout gradually dropped from 0.6l/h to 0.2l/h by real-time control of the agitation and aeration rates and was maintained at this rate. From 15h, the IBM medium was fed into the bioreactor at a 0.2l/h feed rate (matching fout). In this culture, the biomass was kept above 109CFU/ml. Foam continued to overflow until the cultivation terminated and surfactin was enriched in the foam with a concentration >4g/l; the maximum concentration reached 4.7g/l. At the end of the fermentation, the surfactin concentration in the culture broth in the bioreactor was very low (data not shown), which means that almost all the surfactin was transferred into the foam.
In other studies of foam overflowing cultures, although the product was enriched and separated in foam, the foam overflowed spontaneously, and thus the surfactin concentration in it fluctuated6,10,16. For this reason, surfactin in the foam was not enriched to a very high level; in previous studies, in which surfactin was produced by potato process effluent, it was 1.67g/l and 0.9g/l6,10. Thus, controlled foam overflow in this work achieved almost 3 and 4 times the surfactin concentration compared with the previous studies, respectively6,10. Furthermore, the medium feeding in our study overcame disruption of cell growth, which ensured that surfactin was continuously produced. Combined with control of fout at a low level, the surfactin concentration in the foam was maintained above 4g/l, which will significantly reduce production costs and improve industrial production capacity.
ConclusionsOur study focused on the control of agitation and aeration rates in foam overflowing fermentation to improve surfactin enrichment and continuous high-level production. In batch mode, the agitation and aeration rates were found to have a close relationship with cell growth and surfactin production. In further research, our study revealed that fout does not always negatively impact on the surfactin concentration in the foam. The broth level in the bioreactor and the surfactin residue in the broth also affect both fout and the surfactin enrichment of the foam.
Through feeding the medium into the bioreactor, a fed-batch fermentation process was successfully established in which foam overflowed at a controlled flow rate of 0.2l/h, and surfactin in the enriched foam was kept at a level above 4g/l.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was supported by grants from the National Natural Science Foundation of China (grant no. 31271828) and the National Science and Technology Support program (grant no. 2011BAD23B05).