This study evaluated the in vitro effect of three concentrations of atrazine, chlorpyrifos and endosulfan on the growth parameters of four non-toxigenic Aspergillus section Flavi strains. The ability of the strains to remove these pesticides in a synthetic medium was also determined. Growth parameters were measured on soil extract solid medium supplied with 5, 10 and 20mg/l of each pesticide, and conditioned to −0.70, −2.78, −7.06 and −10.0 water potential (MPa). Removal assays were performed in Czapek Doc medium (CZD) supplied with 20mg/l of each pesticide under optimal environmental conditions (−2.78 of MPa and 25°C). The residual levels of each pesticide were detected by the reversed-phase HPLC/fluorescence detection system. The lag phases of the strains significantly decreased in the presence of the pesticides with respect to the control media. This result indicates a fast adaptation to the conditions assayed. Similarly, the mycelial growth rates in the different treatments increased depending on pesticide concentrations. Aspergillus oryzae AM 1 and AM 2 strains showed high percentages of atrazine degradation (above 90%), followed by endosulfan (56 and 76%) and chlorpyrifos (50 and 73%) after 30 days of incubation. A significant (p<0.001) correlation (r=0.974) between removal percentages and growth rate was found. This study shows that non-toxigenic Aspergillus section Flavi strains from agricultural soils are able to effectively grow in the presence of high concentrations of atrazine, chlorpyrifos and endosulfan under a wide range of MPa conditions. Moreover, these strains have the ability to remove high levels of these pesticides in vitro in a short time.
En este estudio se evaluó los efectos in vitro de 3 concentraciones de atrazina, clorpirifós y endosulfán sobre los parámetros de crecimiento de 4 cepas no toxigénicas de Aspergillus sección Flavi. También se evaluó la capacidad de las cepas de remover los pesticidas. Los parámetros de crecimiento se ensayaron en medio agar extracto de suelo suplementado con 5, 10 y 20mg/l de cada pesticida y acondicionado a −0.70, −2.78, −7.06 y −10.0 de potencial de agua (MPa). Los ensayos de remoción se realizaron en medio Czapek Dox con 20mg/l de cada pesticida bajo condiciones óptimas de crecimiento (−2.78 de MPa y 25°C). Los niveles residuales de atrazina, clorpirifós y endosulfán se detectaron en un sistema HPLC con detección por fluorescencia. La fase de latencia de las cepas disminuyó significantemente en presencia de los pesticidas, indicando una rápida adaptación a dichas condiciones. La velocidad de crecimiento se incrementó considerablemente dependiendo de la concentración de pesticida. Las cepas Aspergillus oryzae AM1 y AM2 mostraron porcentajes elevados de degradación de atrazina (aproximadamente el 90%), seguidos por endosulfán (56 y 76%) y clorpirifós (50 y 73%). Se observó una correlación (r=0.974) significante (p<0.001) entre el porcentaje de pesticida removido y la velocidad de crecimiento. Este estudio muestra que cepas no-toxigénicas de Aspergillus sección Flavi aisladas de suelos agrícolas desarrollan eficientemente en presencia de altas concentraciones de atrazina, clorpirifós y endosulfán en un amplio rango de MPa. Además, presentan capacidad de remover in vitro altos niveles de pesticidas en corto tiempo.
During the last quarter of the 20th century, there was deep concern regarding the environmental problems arising from modern agricultural management, e.g. major changes in plant and animal communities as well as the deterioration of soil, water and air quality15. These problems are particularly related to the use of pesticides to control weeds and pests in crops. Pesticide residues have been found in soil matrices as a result of contamination followed by common application or sludge-derived soil fertilization17,33,36. The unsuitable use of pesticides has led to an increase in their bioaccumulation through the food chain, from where they can eventually represent a risk or threat to both animal and human life16. Some organochlorinated (OC) pesticides have been banned in most developed countries because of their highly persistent properties and potential threat to human health. Nowadays, organophosphate (OP) pesticides replaced OC pesticides in many countries since they can be easily degraded in the environment25.
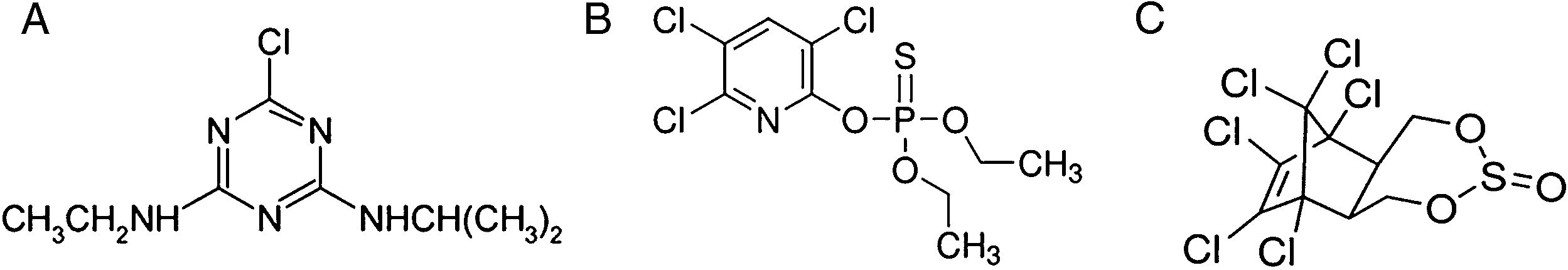
Atrazine [2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine] (Fig. 1A) is a selective herbicide extensively used in crop production to control many broad-leaf and some grassy weeds. Atrazine has long-term reproductive and endocrine-disrupting effects and has been reported to be a potential human carcinogen26.
The organophosphate insecticide chlorpyrifos (O, O-diethyl O-3,5,6-trichloro-2 pyridylphosphorothioate) (Fig. 1B) is used to control pests such as insects, nematodes, and mites on several agricultural crops as well as on lawns and ornamental plants. The use of this compound was banned for household use by the USEPA in 2000. Although its use has been restricted in several countries, chlorpyrifos is easily marketed in Argentina and in several developing and non-developing countries35. However, its presence has been related to toxicity in children, pets, wildlife, and the environment10.
Endosulfan(6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3 benzodioxathiepine-3-oxide) (Fig. 1C) is an OC insecticide and acaricide still used in agriculture in some countries. The European Union since 2006 and Argentina since 2013 have banned the use of endosulfan30. Several studies have documented the contamination with endosulfan of the atmosphere, soil, sediments, surface and rainwaters and foodstuffs. This chlorinated pesticide persists in the environment for very long periods, having a 60–800 day half-life21.
According to the Chamber of Agricultural Health and Fertilizers from Argentina9, in the last twenty-two years the use of pesticides has increased by 858%, while the cultivated areas of cereals and oilseeds and crop yields have only increased 50 and 30%, respectively.
The remediation of pesticide-contaminated sites to mitigate the hazardous effects of these toxic chemicals is required. Biotic degradation is one of the most viable options for the remediation of pesticides in soil and water. Several researchers have focused on microbial degradation, which has been reported as a primary mechanism of pesticide dissipation from soil and water environment. Microorganisms can obtain C, N, P or energy from pesticide molecules. Therefore, the enhanced degradation of organic compounds by indigenous microbes is one of the best soil remediation strategies19.
Fungal and insect contamination is one of the main problems in peanut production (Arachis hypogaea L.). Peanuts produce seed-bearing pods below the soil surface; therefore their pods are in direct contact with soil populations of Aspergillus section Flavi increasing the chances of seed colonization by these species before harvest14. From this section, A. flavus is the dominant species isolated from peanut seeds and agricultural soils in Argentina2,8.
Similarly, Carranza et al.6,8 evaluated the effect of glyphosate on the development of Aspergillus section Flavi strains isolated from agricultural soils. Several investigations informed the degradation capacity of Aspergillus niger of pyrethroid, fenitrothion, carbaryl dimethoate and endosulfan20,37. In a previous study, we evaluated the growth rate of Aspergillus section Nigri strains in the presence of glyphosate, atrazine and chlorpyrifos7. However, there is little information on the influence of other xenobiotic compounds on the development of Aspergillus section Flavi strains under different environmental conditions. Water potential (MPa) is one of the abiotic factors that most influences fungal growth. Optimal condition for fungal growth is between −0.7MPa and −2.8MPa. Lower values of MPa represent osmotic stress as well it is below the wilting point of the plants12. Therefore, the purpose of the present study was to evaluate the in vitro effect of atrazine, chlorpyrifos and endosulfan on the growth parameters of non-toxigenic Aspergillus section Flavi strains isolated from peanuts soils. In addition, the pesticide removal ability of these strains was determined.
Materials and methodsFungal strainsFour non-toxigenic Aspergillus section Flavi strains were evaluated: Aspergillus oryzae (AM 1, AM 2, GM 3) and Aspergillus flavus (GM 4)8. All of them were isolated from peanut soils in the southern region of Cordoba province, Argentina. These soils have been exposed to pesticides during the last decade. These strains were confirmed as non-producers of aflatoxin (AF) and cyclopiazonic acid (CPA)8. The strains belong to our culture collection at the Department of Microbiology and Immunology, in the National University of Río Cuarto, Córdoba, Argentina, and they are stored in 15% glycerol (Sigma–Aldrich, St. Louis, MO, USA) at −80°C.
The nucleotide sequences of the strains for the calmodulin and β-tubulin genes were deposited into GenBank under accession numbers KX298157–KX306816, KX298158–KX306817, KX298159–KX306818 and KX306820–KX306819 for strains AM 1, AM 2, GM 3 and GM 4, respectively.
PesticidesAtrazine, chlorpyrifos and endosulfan used in this study were obtained from the commercial formulation Icona FW®, (Icona S.A., Buenos Aires, Argentina), Hor-tal® (Buenos Aires, Argentina) and endosulfan® (Formulagro, Santa Fe, Argentina), respectively. Stock solutions from each pesticide (1g/l) were prepared in water and then the working solutions were done by performing the respective dilutions. These solutions were sterilized through a 0.2μm filter (Microclar, Argentina) and stored at 4°C. For HPLC analyses, standard solutions of atrazine, chlorpyrifos and endosulfan (Sigma–Aldrich, Argentina) were prepared in methanol.
Growth assaysA soil extract solid medium (SESM) was prepared using 200g of untreated and humid field soil in 400ml of water. This soil sample (Hapludol with a very fine sandy frank texture) was chosen from a field destined to agricultural production (without tillage in the last decade) in the south of the province of Cordoba, Argentina. The soil/water mixture was sterilized by autoclaving at 121°C for 30min, centrifuged at 2400×g for 20min and then filtered through filter paper (0.45μm, Microclar, Argentina), using a vacuum pump. Then, 20g agar was added to the soil/water mixture. The MPa of the medium was modified to −0.70, −2.78, −7.06 and −10.0 using the ionic solute KCl12. Media were sterilized by autoclave again using the same program. After cooling the media to 50°C, an aliquot of each pesticide solution was added to obtain final concentrations of 5, 10 and 20mg/l. Then, media were poured into 90-mm sterile Petri dishes. Control plates of each MPa value without pesticides were also prepared. The water potential of representative samples of each treatment was checked with an AquaLab Series 3 (Decagon Devices, Inc., WA, USA). In addition, MPa was measured at the end of the experiment to detect any significant deviation in this parameter.
The plates were needle-inoculated centrally with fungal spores (2μl) suspended in soft agar (106spores/ml), from 7-day-old cultures on malt extract agar (MEA). Inoculated Petri dishes of the same MPa were sealed in polyethylene bags and incubated at 25°C for 28 days. Each treatment was carried out in quadruplicate, and all the experiments were repeated twice.
For growth determination, two measures of colony radii, at right angles to each other, were taken from each replicate daily. The average radius of each colony was plotted against time, and a linear regression was applied to obtain the growth rate as the slope of the line to the X-axis. The lag phase (h) before growth was also determined1. The growth rate and lag phase analyses were performed on four non-toxigenic Aspergillus section Flavi strains, at three different concentrations of each pesticide (atrazine, chlorpyrifos and endosulfan) and four different MPa conditions, and the respective controls.
Pesticides removal by A. oryzae in synthetic mediumThe ability of A. oryzae (AM 1 and AM 2 strains) to remove atrazine, chlorpyrifos and endosulfan was tested in submerged cultures under optimal conditions of water potential and temperature for growth. These strains were selected since they showed the best growth parameters in media amended with pesticides. For the removal assays, Czapek Dox medium (CZD) (sucrose 30g, NaNO3 3g, K2HPO4 1g, MgSO4·7H2O 0.25g, KCl 0.5g, FeSO4·7H2O 0.01g, distilled water 1l) adjusted to −2.78MPa with the ionic solute KCl12 was used. Aliquots (50ml) of CZD were inoculated with one agar plug (3mm) taken from 7-day-old cultures in MEA. Incubation was done in an orbital shaker under agitation (60rpm) at 25°C for 3 days. After that period, 20mg/ml of each pesticide was added. Inoculated CZD without pesticides and non-inoculated CZD with pesticides were included as control treatments. One milliliter of each culture, 2h after the addition of the pesticides and after 30 days of incubation was collected. Then stored at 4°C until atrazine, chlorpyrifos and endosulfan residues analysis. All treatments were performed by triplicate and the experiment was repeated three times.
Extraction and detection of atrazineAtrazine residues in the CZD medium were extracted with three volumes of dichloromethane (5+3+3ml). The combined organic layer was passed through sodium sulfate, evaporated under vacuum and then re-dissolved in methanol. The extracts were filtered through a 0.45μm nylon syringe filter (Magnafarm S.R.L., Argentina) and analyzed using a reversed-phase HPLC/fluorescence detection system32. The detection limit of the analytical method was 1ng/ml of sample.
Extraction and detection of chlorpyrifosFor chlorpyrifos determination, 1ml of the medium was extracted with 2ml of a mixture of acetonitrile:methanol (80:20, v/v). The mixture was filtered using a 0.45μm nylon syringe filter (Magnafarm S.R.L., Argentina) and then it was analyzed by HPLC24. The detection limit of the analytical method was 1ng/ml.
Extraction and detection of endosulfanWith regard to endosulfan, 1ml of the liquid medium was extracted with the same volume of methanol. The extracts were filtered through a 0.45μm nylon syringe filter (Magnafarm S.R.L., Argentina) and then analyzed by HPLC24. The detection limit of the analytical method was 2.5ng/ml.
Recovery assay and HPLC systemRecoveries were evaluated by spiking the CZD medium before extraction with three concentrations (5, 10 and 20mg/l) of each pesticide. Spiking was carried out in triplicate and a blank sample was included. Then, the extraction and detection methods for each pesticide were applied as mentioned above.
Statistical analysisThe results were analyzed by analysis of variance (ANOVA). All data were transformed to log10 (x+1) to obtain the homogeneity of variance. Means were compared by the Fisher's protected LSD test to determine the influence of the abiotic factors assayed (MPa, pesticides and pesticide concentration) on growth rate, lag phase before growth and removal percentages by the strains tested. The Pearson correlation coefficient was used to evaluate the strength of the relationship between the removal percentages by A. oryzae (AM 1 and AM 2) in CZD medium and the growth rate of the same strains on soil extract solid medium. The analyses were conducted using PROC GLM in SAS (SAS Institute, Cary, NC)27.
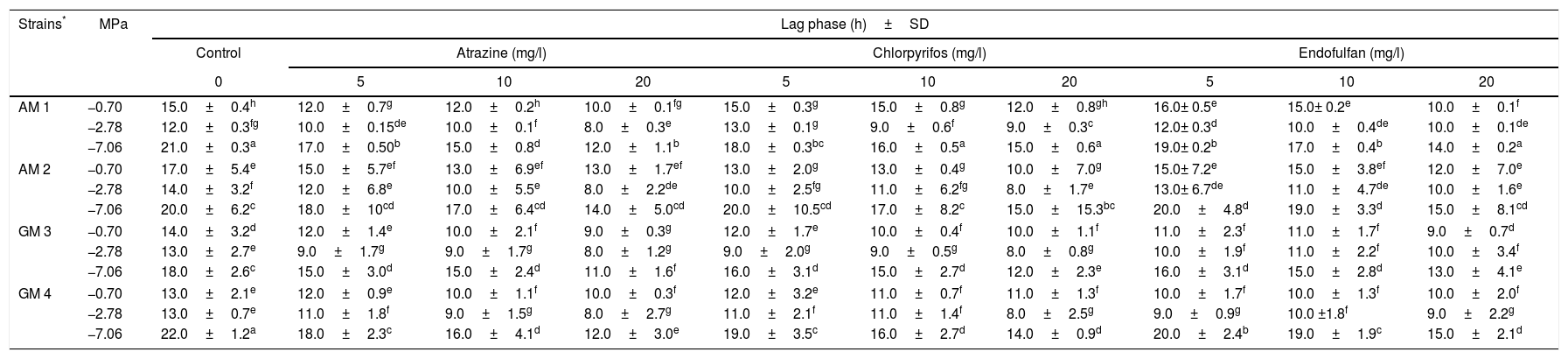
ResultsEffect of atrazine, chlorpyrifos or endosulfan on the lag phaseTable 1 shows the effect of different concentrations of three pesticides on the lag phases of four non-toxigenic Aspergillus section Flavi strains, under different MPa conditions. The longest lag phases were observed with the lowest MPa condition assayed in the control treatments. In general, all the strains showed a similar behavior under the different conditions tested. These strains showed the shortest lag phases in the pesticides treatments with respect to the control treatments. This result indicates a fast adaptation to the presence of the pesticide. Overall, the lag phases of the strains decreased as the concentration of the pesticide increased. Among these, AM 1 and GM 4 showed the highest reductions (p<0.0001) in the lag phases with 20mg/l of all pesticides and MPa conditions. At the highest osmotic stress condition (−10.0MPa) and all the conditions assayed, the strains were not able to reach the exponential phase (data not shown). Atrazine was the pesticide that most influenced (p<0.0001) the lag phases of all the strains. With 5mg/l of atrazine, there was a significant reduction in the duration of the lag phase in all strains and conditions assayed. The highest reduction (46%) was observed in GM 4 with 20mg/l atrazine and at −7.06MPa. With chlorpyrifos, the reduction in the lag phase was also noticeable with 5mg/l at −7.06MPa in all strains, except in AM 2 strains where the decrease was significant with 10mg/l. While at −0.70 and −2.78MPa this reduction was significant (p<0.0001) only with 20mg/l. There was no reduction in the lag phases of GM 3 and GM 4 strains with 10 and 20mg/l. The same was observed for AM 1 and AM 2 strains with 5 and 10mg/l of the insecticide at −0.70MPa. With respect to endosulfan, the lag phases of all the strains from 5mg/l were shorter than the respective control under all the conditions assayed, except for AM 2 at −0.70MPa where the decrease was not significant. No significant differences were observed in the treatments with 5 and 10mg/l of endosulfan at this MPa. For AM 1 and GM 4 strains the highest reductions (33%) in the lag phases were observed with 20mg/l at −0.70MPa (p<0.0001).
Pesticide effects and water potential (MPa) on the lag phase of Aspergillus section Flavi strains on a soil extract solid medium
| Strains* | MPa | Lag phase (h)±SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Atrazine (mg/l) | Chlorpyrifos (mg/l) | Endofulfan (mg/l) | ||||||||
| 0 | 5 | 10 | 20 | 5 | 10 | 20 | 5 | 10 | 20 | ||
| AM 1 | −0.70 | 15.0±0.4h | 12.0±0.7g | 12.0±0.2h | 10.0±0.1fg | 15.0±0.3g | 15.0±0.8g | 12.0±0.8gh | 16.0± 0.5e | 15.0± 0.2e | 10.0±0.1f |
| −2.78 | 12.0±0.3fg | 10.0±0.15de | 10.0±0.1f | 8.0±0.3e | 13.0±0.1g | 9.0±0.6f | 9.0±0.3c | 12.0± 0.3d | 10.0±0.4de | 10.0±0.1de | |
| −7.06 | 21.0±0.3a | 17.0±0.50b | 15.0±0.8d | 12.0±1.1b | 18.0±0.3bc | 16.0±0.5a | 15.0±0.6a | 19.0± 0.2b | 17.0±0.4b | 14.0±0.2a | |
| AM 2 | −0.70 | 17.0±5.4e | 15.0±5.7ef | 13.0±6.9ef | 13.0±1.7ef | 13.0±2.0g | 13.0±0.4g | 10.0±7.0g | 15.0± 7.2e | 15.0±3.8ef | 12.0±7.0e |
| −2.78 | 14.0±3.2f | 12.0±6.8e | 10.0±5.5e | 8.0±2.2de | 10.0±2.5fg | 11.0±6.2fg | 8.0±1.7e | 13.0± 6.7de | 11.0±4.7de | 10.0±1.6e | |
| −7.06 | 20.0±6.2c | 18.0±10cd | 17.0±6.4cd | 14.0±5.0cd | 20.0±10.5cd | 17.0±8.2c | 15.0±15.3bc | 20.0±4.8d | 19.0±3.3d | 15.0±8.1cd | |
| GM 3 | −0.70 | 14.0±3.2d | 12.0±1.4e | 10.0±2.1f | 9.0±0.3g | 12.0±1.7e | 10.0±0.4f | 10.0±1.1f | 11.0±2.3f | 11.0±1.7f | 9.0±0.7d |
| −2.78 | 13.0±2.7e | 9.0±1.7g | 9.0±1.7g | 8.0±1.2g | 9.0±2.0g | 9.0±0.5g | 8.0±0.8g | 10.0±1.9f | 11.0±2.2f | 10.0±3.4f | |
| −7.06 | 18.0±2.6c | 15.0±3.0d | 15.0±2.4d | 11.0±1.6f | 16.0±3.1d | 15.0±2.7d | 12.0±2.3e | 16.0±3.1d | 15.0±2.8d | 13.0±4.1e | |
| GM 4 | −0.70 | 13.0±2.1e | 12.0±0.9e | 10.0±1.1f | 10.0±0.3f | 12.0±3.2e | 11.0±0.7f | 11.0±1.3f | 10.0±1.7f | 10.0±1.3f | 10.0±2.0f |
| −2.78 | 13.0±0.7e | 11.0±1.8f | 9.0±1.5g | 8.0±2.7g | 11.0±2.1f | 11.0±1.4f | 8.0±2.5g | 9.0±0.9g | 10.0 ±1.8f | 9.0±2.2g | |
| −7.06 | 22.0±1.2a | 18.0±2.3c | 16.0±4.1d | 12.0±3.0e | 19.0±3.5c | 16.0±2.7d | 14.0±0.9d | 20.0±2.4b | 19.0±1.9c | 15.0±2.1d | |
Mean values are based on quadruplicated data. Means in a row with a letter in common are not significantly different according to the LSD test (p<0.0001).
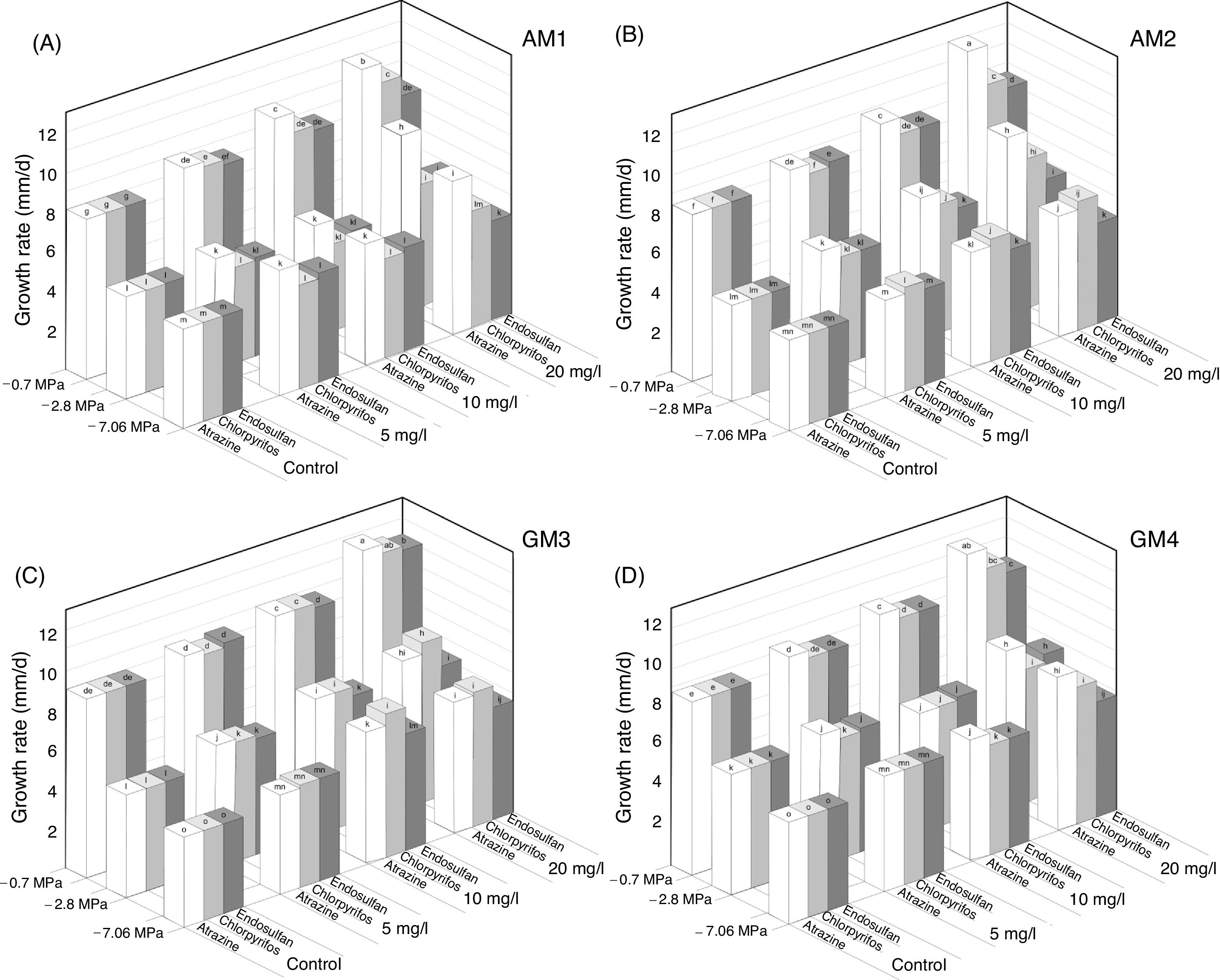
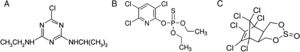
Figure 2 shows the effect of the different concentrations of the three pesticides on the growth rate of four non-toxigenic Aspergillus section Flavi strains under different MPa conditions. In the control treatments, in general, the growth rate decreased as the MPa decreased.
Atrazine, chlorpyrifos and endosulfan effects on growth rate of Aspergillus section Flavi AM 1 (A), AM 2 (B), GM 3 (C) and GM 4 (D) under different water potential (MPa) conditions on soil extract solid medium. Mean values are based on quadruplicated data. Means in a row with a letter in common are not significantly different according to the LSD test (p<0.0001).
There was a complete suppression of mycelial growth in all treatments (controls and pesticide concentrations) tested at the highest osmotic stress condition (−10.0MPa) (data not shown). These strains showed a significant increase in their growth rate in the treatments supplemented with pesticides at −0.70, −2.8 and −7.06MPa with respect to the control treatments. Atrazine was the pesticide that most influenced (p<0.0001) the growth rate of all the strains. The highest values were observed with all the concentrations of atrazine tested at −0.70 and −2.8MPa. With 20mg/l of this herbicide, the growth rate of strain AM 1 was higher (93%) than the value observed in the control treatment at −2.8MPa (Fig. 2A) (p<0.0001). Similarly, there was a significant (p<0.0001) increase in the growth rate of strains AM 1 and AM 2 with the lowest herbicide concentration (5mg/l) under all MPa conditions (Fig. 2A and B).
When A. oryzae strains grew in the presence of chlorpyrifos, an increase in growth rate was observed as the concentration of the insecticide also increased (p<0.0001). A significant (p<0.0001) increase (67%) of the growth rate of strains AM 2 and GM 3 was observed with 20mg/l of insecticide at −2.8MPa (Fig. 2B and C). Similarly, the growth rate of strain GM 4 significantly increased with 5mg/l of chlorpyrifos in all the conditions assayed (Fig. 2D).
With regard to endosulfan, all the A. section Flavi strains showed an increase in the growth rate when the pesticide concentration also increased. This fact was more noticeable at −2.8MPa. For strains GM 3, the growth rate significantly increased (p<0.0001) with 5mg/l of endosulfan in all MPa assayed (Fig. 2C).
Pesticide removal in synthetic mediumThe average recoveries of atrazine, chlorpyrifos and endosulfan in CZD medium were 86.6%±7.9, 93.0%±7.4 and 82.1±10.2%, respectively.
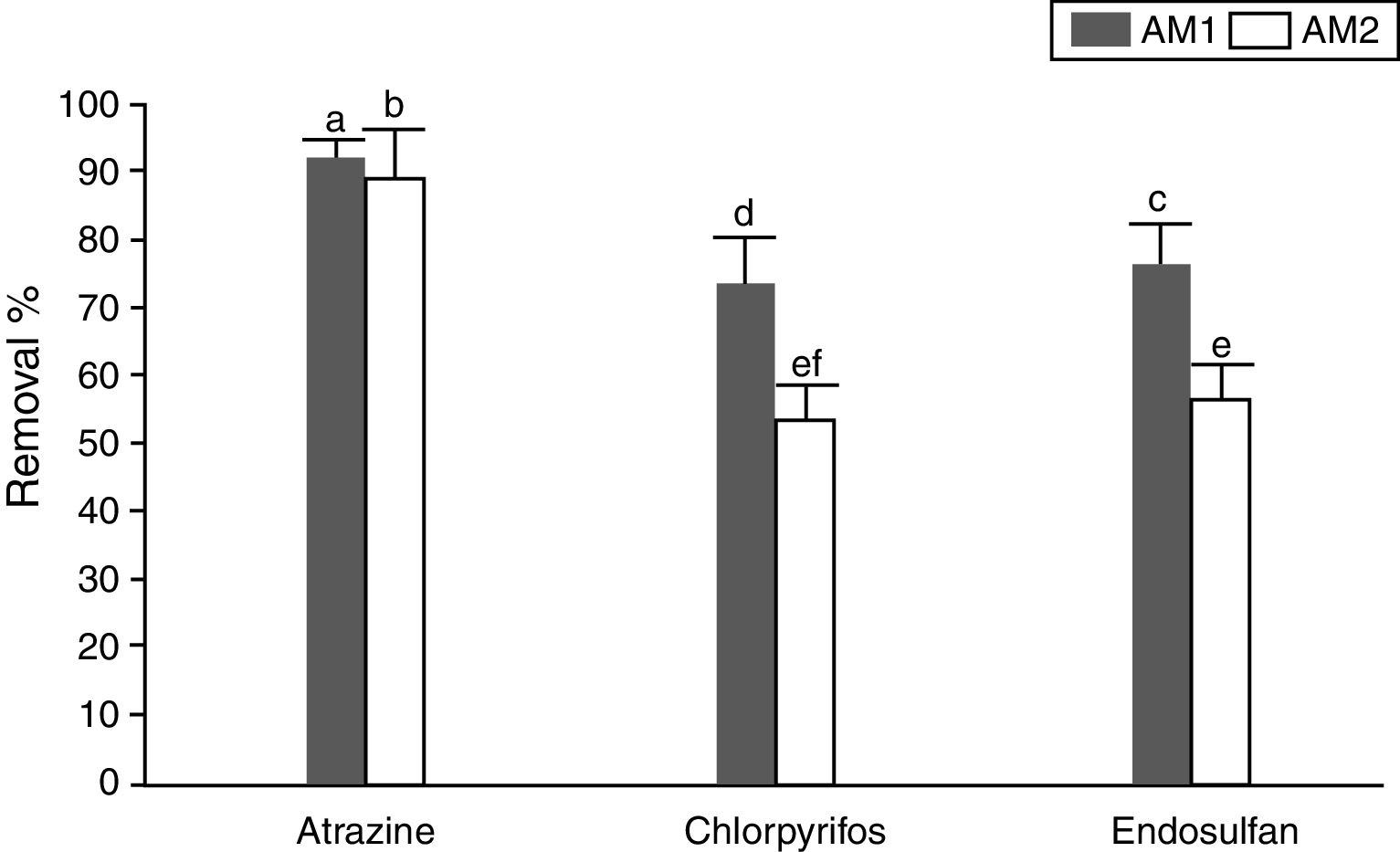
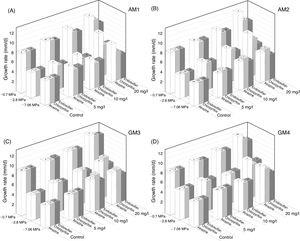
Figure 3 shows the removal percentages of the pesticides by two A. oryzae strains at optimal water potential and temperature conditions after 30 days of incubation. Both strains removed atrazine more quickly, with percentages over 90% at the first 2 days of incubation (data not shown). Conversely, chlorpyrifos removal was evident only at the end of the incubation time. A. oryzae strain AM 1 was able to remove around 73% of the pesticide, while strain AM 2 showed a removal percentage of around 50%. With regard to endosulfan, 76 and 56% removal after 30 days of incubation was observed by A. oryzae strains AM 1 and AM 2, respectively, (p<0.0001).
Atrazine, chlorpyrifos and endosulfan removal percentages by Aspergillus oryzae AM 1 and AM 2 strains at −2.78 of MPa and 25°C at 30 days of incubation. The total of pesticides recovered on day zero was taken as 100%. Values presented are the mean of three replicates. Means in a row with a letter in common are not significantly different according to the LSD test (p<0.0001).
The effect of each single variable alone (strain, MPa, pesticide and pesticide concentration), two, three and four way interaction was statistically significant (p<0.0001) in relation to lag phase and growth rate (Table 2). A significant correlation (p<0.001, r=0.974) between pesticides removal levels by A. oryzae AM 1 and AM 2 in CZD medium and growth rate on SESM medium was observed (data not shown).
Analysis of variance effect of water potential (MPa), pesticide (P), concentration of pesticide (C) and different strains (I), and their interactions on the lag phases and growth rates of non-toxigenic Aspergillus section Flavi strains
| Source of variation | Dfa | Lag phase | Growth rate | ||
|---|---|---|---|---|---|
| MSb | Fc | MSb | Fc | ||
| I | 3 | 118663.01 | 10.03* | 46.72 | 15467.59* |
| P | 2 | 60035.76 | 5.20* | 32.14 | 10671.62* |
| C | 3 | 17236623.97 | 1472.62* | 395.46 | 99999.99* |
| MPa | 2 | 1266893.00 | 109.76* | 39.57 | 13367.33* |
| I×P | 20 | 60143.68 | 5.27* | 33.09 | 10558.64* |
| I×P×C | 102 | 103999.19 | 8.62* | 0.62 | 165.79* |
| I×P×C×MPa | 278 | 17221796.97 | 1491.61* | 399.16 | 99999.99* |
The growth assays showed that the lag phase and the growth rate of non-toxigenic Aspergillus section Flavi strains growing in soil-based medium are significantly influenced by atrazine, chlorpyrifos and endosulfan concentrations, MPa conditions and their interactions. In the present study, an increase in the growth rate was observed with the highest levels of atrazine (10 and 20mg/l). These results partially agree with Carranza et al.8 who found an increase in the growth rate of Aspergillus section Nigri strains at −2.78MPa with 5 and 10mg/l of atrazine; while at −7.06MPa with 20mg/l of atrazine a decrease of 25% was observed in this parameter. This herbicide is one of the most persistent pesticides, which explains the difficulties reported for its degradation by microbial metabolism. In addition, there are few studies about the effect of different persistent pesticides on microfungi growth parameters. In this study, A. oryzae strains were able to remove 91% of the initial atrazine concentration on synthetic medium at optimal water potential after 30 days of incubation. Bastos and Magan3 found similar atrazine degradation percentages (up to 98 and 85% at −0.70 and −2.8MPa, respectively) on soil in the presence of Trametes versicolor strains. These authors reported that these white fungi are candidates for atrazine bioremediation in soil with low moisture and organic matter contents. Furthermore, Sene et al.29 showed that soil fungi such as Aspergillus spp., Rhizopus spp., Fusarium spp. and Penicillium spp. have the ability to partially degrade this herbicide. Segmental degradation was also reported with the white-rot fungus Phanerochaete chrysosporium in culture medium22. When Aspergillus section Flavi strains grew in the presence of chlorpyrifos, a significant increase in their growth rate was observed. Thus, the strains had a similar behavior in comparison to the treatments using atrazine. A. oryzae strain AM 1 showed 73% removal of chlorpyrifos after the incubation period. Several studies reported that different fungal species exhibit different abilities to degrade chlorpyrifos in liquid media and soil. Omar23 observed that Aspergillus terreus showed great potential to mineralize organic phosphorus and sulfur from chlorpyrifos in liquid media. Similar results were found by Fang et al.11 with Verticillium sp. Recently, Kulshrestha and Kumari18 have also reported a high degradation (83.9%) by Acremonium spp. Gao et al.13 obtained similar results with Cladosporium cladosporioides.
Overall, the results obtained from growth assays using endosulfan are comparable with those obtained using atrazine and chlorpyrifos. In both cases, a positive correlation between fungal growth and pesticide concentration was found. In this study, the A. oryzae strains assayed had the ability to remove 76% of the initial concentration of this insecticide in the synthetic medium after 30 days of incubation. These results partially agree with Bhalerao4, who investigated bioaugmentation with A. niger of soil containing endosulfan. These authors observed an undetectable level of the insecticide after 15 days of incubation. In a later study, this author5 showed that the same strains could tolerate 1000mg/l of endosulfan, and removal of endosulfan was observed after 168h. Another fungus, Bjerkandera adusta, was able to degrade 83% of endosulfan after 27 days. This removal percentage is comparable with those obtained in our study28. Likewise, similar results have been reported for two white-rot fungi (Trametes versicolor and Pleurotus ostreatus) and one brown-rot fungus (Gloeophyllum trabeum) in liquid cultures34. Silambarasan and Abraham31, demonstrated that Botryosphaeria laricina and Aspergillus tamarii were able to tolerate 1300mg/l of endosulfan and furthermore these strains showed an increased growth with 1000mg/l.
It is important to highlight that the levels of chemicals used in this study are higher than those detected in agricultural soils. However, those levels can be reached in fumigation or drought areas. The results obtained in the present in vitro study showed that the four non-toxigenic Aspergillus section Flavi strains isolated from soil were tolerant to atrazine, chlorpyrifos and endosulfan in levels ranging from 5 to 20mg/l, at optimal MPa conditions (−0.70 and −2.78) and even under osmotic stress condition (−7.06MPa). In addition, higher growth rates were observed when the pesticide levels were increased from 5 to 20mg/l. Moreover, such increase was more significant at −0.70 and −2.78MPa. In addition, these strains showed the ability to remove atrazine, endosulfan and chlorpyrifos from synthetic media under optimal environmental conditions (temperature and MPa) after 30 days of incubation. Among the pesticides tested, the highest removal percentages were observed with atrazine followed by endosulfan and chlorpyrifos. The increase observed in growth rate values in the presence of the pesticides suggests that these compounds can be used as carbon and energy sources under optimal and stress MPa conditions.
The results of this survey indicate that the A. oryzae strains tolerant to high levels of atrazine, chlorpyrifos and endosulfan could be considered potential bioremediation agents. Further investigation allows us to determine the potential in situ degradation of these pesticides by A. oryzae strains and to identify the degradation products.
FundingThis work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-PICT-2482/10) and Secretaría de Ciencia y Técnica, Universidad Nacional de Río Cuarto (SECYT-UNRC-18/C391).
Conflict of interestThe authors declare that they have no conflicts of interest.