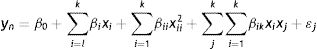Demand for fungal xylanases in industrial biotechnological processes shows a clear increase worldwide, so there is an interest in adjusting the conditions of microbial xylanases production. In this study, the ability of the fungus Fusarium solani to produce extracellular xylanases with low cellulolytic activity was optimized by Box Wilson design. The best culture conditions were determined to obtain a crude enzyme preparation with significant xylanolytic activity and little cellulolytic activity. In most treatments, the xylanolytic activity was higher than the cellulolytic activity. A negative effect on the production of endoxylanases, β-xylosidases and endocellulases was observed with the increasing of xylan concentration. Increasing the incubation time adversely affected the production of endocellulases and β-xylosidases. According to the mathematical model and experimental tests, it is possible to produce endoxylanases with minimal endocellulase activity increasing incubation time and the concentration of ammonium sulfate. The optimal culture conditions to produce a greater amount of endoxylanases (10.65U/mg) and low endocellulases from F. solani were: 2.5% (w/v) xylan, 5.0, 2.0 and 0.4g/l, of yeast extract, ammonium sulfate and urea, respectively, with 120h of incubation.
La demanda de xilanasas fúngicas en los procesos biotecnológicos industriales muestra un claro aumento en todo el mundo, por lo que hay un interés en ajustar las condiciones de producción de xilanasas microbianas. En este estudio se optimizó la capacidad del hongo Fusarium solani para producir xilanasas extracelulares con escasa actividad celulolítica mediante el diseño de Box-Wilson. Se determinaron las mejores condiciones de cultivo para obtener una preparación enzimática cruda con una actividad xilanolítica significativa y poca actividad celulolítica. En la mayoría de los tratamientos, la actividad xilanolítica fue mayor que la actividad celulolítica. Se observó un efecto negativo sobre la producción de endoxilanasas, β-xilosidasas y endocelulasas con el aumento de la concentración de xilano. El aumento del tiempo de incubación afectó adversamente la producción de endocelulasas y β-xilosidasas. De acuerdo con el modelo matemático y las pruebas experimentales, es posible producir endoxilanasas con una actividad endocelulasa mínima aumentando el tiempo de incubación y la concentración de sulfato de amonio. Las condiciones de cultivo óptimas para producir una mayor cantidad de endoxilanasas (10,65U/mg) y mínima cantidad de endocelulasas fueron 2,5% (p/v) de xilano y 5, 2 y 0,4g/l de extracto de levadura, sulfato de amonio y urea, respectivamente, con 120h de incubación.
The key enzymes involved in microbial xylanolysis are endoxylanases (1,4-β-D-xylan xylanohydrolase, EC 3.2.1.8) and β-xylosidases (1,4-β-xylan xylohydrolase, EC 3.2.1.37). The products of the catalytic reaction on xylan are xylose, xylobiose, and xylooligosaccharides33. Xylanases, cellulases, and pectinases represent about 20% of the world wide enzyme market and their use have increased exponentially in diverse biotechnological processes. In some of these processes, the products from the hydrolysis of xylan, such as xylose and xylooligosaccharides are kept as valuable chemical products. While in some biochemical processes, it is necessary to eliminate them in order to keep the cellulose fibers intact, such as in the case of the desirable cellulase-free xylanolytic complex3. These enzymes have many applications in the food, paper and cellulose pulp, textile and chemical industries (i.e. xylitol and biofuels)16,17,25,31,36. Moreover, the same enzymes are widely utilized in the treatment of lignocellulosic products from agricultural, agroindustrial and municipal residues32,47.
Industrially, xylanases are produced mainly by two microbial fermentation methods, the submerged fermentation (SmF) and the solid fermentation (SSF)42. Both processes show advantages and disadvantages. For the selection of one method over the other, it is necessary to carefully evaluate the quality of the products expected, the producing microorganism, the kind of substrates, and the technological tools available. In the last decade about 80–90% commercial xylanases were produced by SmF4. However, SSF presents many advantages compared to SmF, such as less space requirements, lower costs and the abundance of agricultural waste as a substrate for the production of enzymes. Moreover, SSF involves simplicity of the fermentation media, with fewer requirements for complex machinery, equipment or control systems. It also offers greater compactness of the fermentation vessel owing to a higher productivity per reactor volume, reduced energy demand, lower capital and low recurring costs under industrial operation. Easy scale-up processes, less downstream processing and superior yield, absence of foam build-up, and easier control of contaminants due to the low moisture levels in the system are additional advantages of the SSF2,43.
Nevertheless, the SSF is commonly used for the production of enzyme and secondary metabolites in a minor scale46. Unfortunately, at industrial scale, there are many engineering complications to control the key parameters of the SSF such as temperature, pH, O2, substrate concentration gradients, humidity and microbial inoculant.
During the enzymatic hydrolysis of lignocellulose, 50% of the total cost of the process is related to cellulase (hemicellulases and cellulases) acquisition. Therefore, the aim of this line of research is to reduce the costs to obtain these enzymes. One way to achieve this is by researching the best conditions for the production of hemicellulases, such as, the selection of the producer organism, substrates and microbial growth conditions.
Filamentous fungi are particularly interesting because they secrete cellulases into the culture media and their enzymatic activity level is remarkable in comparison with yeasts and bacteria24. Even though, the xylanases produced by Trichoderma viride or Aspergillus oryzae are commercially available, these fungal enzymatic preparations have limitations to certain applications11. To date, no natural microorganism that produces an ideal enzyme preparation for biomass hydrolysis has been discovered. Therefore, enzyme preparations must be supplemented with native or recombinant enzymes for use in particular biotechnological applications and the search for new xylanases with potential biotechnological application is ongoing. Moreover, the production of xylanases from F. solani has been recently reported by the exploration of their xylanolytic activity10 and enzyme production8,22,41.
Usually, the xylanolytic activity is adjusted by varying one environmental or nutrimental factor while other factors are kept constant. Unfortunately, this is a highly costly and inefficient strategy because it cannot yield the best global response value as it disregards the influence of the interactions between factors in the process. An interesting strategy for xylanase production is to reduce the unnecessary steps and treatments to minimize time and the process costs by experimental design methods1,44,49,51. The aim of this work was to optimize the production of extracellular xylanases with low cellulolytic activity in a wild-type F. solani isolate.
Materials and methodsBiological materialThe filamentous fungus F. solani (NCBI accession number JQ910159.1) isolated from a bean crop field was used in this study6. It was previously selected for its ability to produce extracellular xylanases and was identified by molecular tools in the Laboratory of Cellular Physiology at Instituto de Investigaciones Químico Biológicas of the Universidad Michoacana de San Nicolás de Hidalgo6. F. solani spores were preserved on silica gel at 4°C. The monosporic fungal culture was propagated by successive reseeding in potato dextrose agar (PDA).
Inoculum preparationLiquid culture medium was prepared with the following composition per liter of triple distilled water: 1.4g (NH4)2SO4, 2.0g KH2PO4, 0.1g urea, 0.3g MgSO4·7H2O, 0.3g CaCl2, 5.0mg FeSO4·7H2O, 1.56mg MnSO4·H2O, 2.0mg CoCl2, 1.4mg ZnSO4·7H2O, 1g beechwood xylan (carbon source for fungus and its production of hydrolytic enzymes according to Bailey and co-workers9), 3g dextrose, 100μl vitamins solution (MEM Vitamin Solution 1000×, Sigma Cell Culture). The pH of the culture medium was adjusted to 5.5 and was autoclaved at 121°C for 15min. Four F. solani propagules were inoculated into 40ml of culture medium, which was incubated at 150rpm at room temperature. After 48h of incubation, the mycelium was removed and washed three times with sterile deionized water. An aliquot of 500μl mycelium suspension was taken and 500μl of 0.5M NaOH was added. The mixture was incubated for 14h at 4°C to lyse the mycelium. The sample was centrifuged at 12,000rpm for 5min. The supernatant was removed to measure the content of intracellular protein.
Xylanase production by fermentationBased on the treatments listed in the experimental design shown in Table 1, the required amounts of carbon source (CS: beechwood xylan9) and the three nitrogen sources were combined. A 10x mixture of trace elements and vitamin solution was added (100μl/ml of culture medium), composed as follows per 100ml of deionized water: KH2PO4, 2.0g; MgSO4·7H2O, 0.3g; CaCl2, 0.3g; FeSO4·7H2O, 5.0mg; MnSO4·H2O, 1.56mg; CoCl2, 2.0mg; ZnSO4·7H2O, 1.4mg; vitamin solution (MEM Vitamins Solution 1000×), 100μl.
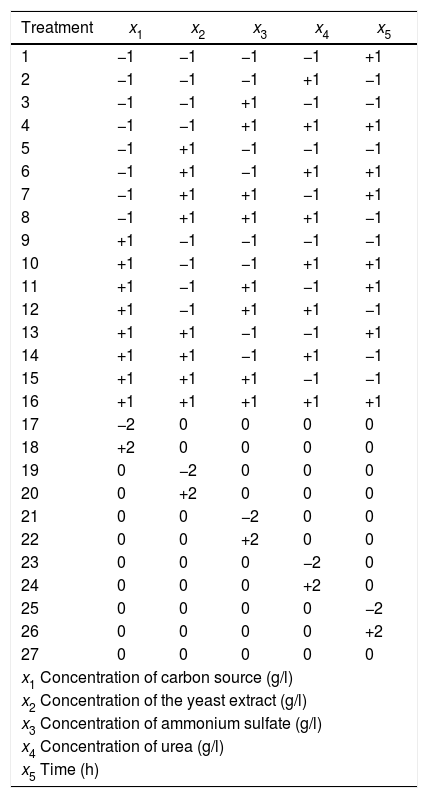
BW treatments using the codified factor levels.
| Treatment | x1 | x2 | x3 | x4 | x5 |
|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | +1 |
| 2 | −1 | −1 | −1 | +1 | −1 |
| 3 | −1 | −1 | +1 | −1 | −1 |
| 4 | −1 | −1 | +1 | +1 | +1 |
| 5 | −1 | +1 | −1 | −1 | −1 |
| 6 | −1 | +1 | −1 | +1 | +1 |
| 7 | −1 | +1 | +1 | −1 | +1 |
| 8 | −1 | +1 | +1 | +1 | −1 |
| 9 | +1 | −1 | −1 | −1 | −1 |
| 10 | +1 | −1 | −1 | +1 | +1 |
| 11 | +1 | −1 | +1 | −1 | +1 |
| 12 | +1 | −1 | +1 | +1 | −1 |
| 13 | +1 | +1 | −1 | −1 | +1 |
| 14 | +1 | +1 | −1 | +1 | −1 |
| 15 | +1 | +1 | +1 | −1 | −1 |
| 16 | +1 | +1 | +1 | +1 | +1 |
| 17 | −2 | 0 | 0 | 0 | 0 |
| 18 | +2 | 0 | 0 | 0 | 0 |
| 19 | 0 | −2 | 0 | 0 | 0 |
| 20 | 0 | +2 | 0 | 0 | 0 |
| 21 | 0 | 0 | −2 | 0 | 0 |
| 22 | 0 | 0 | +2 | 0 | 0 |
| 23 | 0 | 0 | 0 | −2 | 0 |
| 24 | 0 | 0 | 0 | +2 | 0 |
| 25 | 0 | 0 | 0 | 0 | −2 |
| 26 | 0 | 0 | 0 | 0 | +2 |
| 27 | 0 | 0 | 0 | 0 | 0 |
| x1 Concentration of carbon source (g/l) | |||||
| x2 Concentration of the yeast extract (g/l) | |||||
| x3 Concentration of ammonium sulfate (g/l) | |||||
| x4 Concentration of urea (g/l) | |||||
| x5 Time (h) | |||||
The solid support was formulated with xylan, yeast extract, ammonium sulfate or urea, moistened with phosphate buffer supplemented with a mixture of trace elements and vitamins. Fermentations were done in 200ml Erlenmeyer flasks that contained solid support and were inoculated with 500μl of F. solani mycelium suspension, which represented 10% of the culture medium volume and was equivalent to 39.5μg of intracellular protein. In all the treatments, the initial pH and fermentation volume were 5.0 and 6.0ml, respectively. During the fermentation, the pH was not controlled due to the presence of solid fermentation media and the difficulty to homogenize the buffer solution in these treatments. The inoculated flasks were incubated for the time stated in the Box-Wilson Central Composite Design (BW) at 150rpm and room temperature.
Extraction of hydrolytic enzymesFlasks were removed from incubation and 10ml of 50mM sodium citrate buffer, pH 5.0 were added to wash the solids. The supernatant was separated from the solids by filtration through filter paper and by centrifugation at 10,000rpm for 4min, and was used to quantify extracellular protein and hydrolytic enzyme activities. One unit (U) of enzyme activity is defined as the amount of enzyme that liberates 1μmol of reducing sugar per minute or p-nitrophenol under the assay conditions. Each assay was performed in triplicate.
Enzymatic assaysEndo-β-1,4-xylanase activityEndo-β-1,4-xylanase activity was measured by the method proposed by Bailey and coworkers9. The reaction mixture contained 0.6ml of 1% (w/v) birchwood xylan (Sigma-Aldrich, Co.) as substrate, suspended in 50mM sodium citrate buffer pH 5.0 and 0.6ml of enzyme properly diluted (0.2–4.5mg/ml of protein). These samples were thoroughly mixed and incubated at 50°C for 30min. The reaction was stopped by boiling the samples for 5min in a water bath, followed by centrifugation at 5000rpm for 3min. One milliliter of the reaction supernatant was extracted and the reducing sugars were quantified with xylose as standard.
Endocellulase activityThe endocellulase activity (endo-β-1,4-glucanase) is a measure of total cellulase activity and was quantified by the modified method of Ghose20. The reaction mixture contained 0.5ml of 1% (w/v) carboxymethylcellulose (CMC purchased Sigma-Aldrich Co.) as substrate prepared in 50mM sodium citrate buffer pH 5.0 and 0.5ml of enzyme properly diluted (0.2–4.5mg/ml of protein). These samples were thoroughly mixed and incubated at 50°C for 30min. The reducing sugars released were quantified with glucose as standard.
β-1,4-Xylosidase activityThe activity of β-1,4-xylosidase was determined according to the modified method of Kristufek and coworkers29. The reaction mixture contained 50μl of 10mM p-nitrophenyl β-xylopyranoside (PNPX) (Sigma) as substrate and enzyme equivalent to 0.2–4.5mg/ml of protein, properly diluted in 50mM sodium citrate buffer pH 5.0, in a total volume of 1ml. The samples were incubated at 50°C during 15min. The reaction was stopped by adding 2.0ml of 1.0M Na2CO3. The amount of p-nitrophenol released from PNPX was measured by monitoring the increase in absorbance at 410nm and interpolating the absorbance value in a standard curve of absorbance against p-nitrophenol concentration.
Quantification of reducing sugarsThe reducing sugars were quantified by the method proposed by Miller37 by adding 1ml of 3,5-dinitrosalicylic acid (DNS) reagent to 1ml of reaction mixture. Samples were incubated in a boiling water bath for 15min. The reducing sugars released were measured at 540nm in a UV-visible spectrophotometer (Varian Cary 50® Bio).
Quantification of extracellular proteinThe extracellular protein was quantified by the Lowry method with bovine serum albumin (BSA) as standard34. Each assay was performed in triplicate.
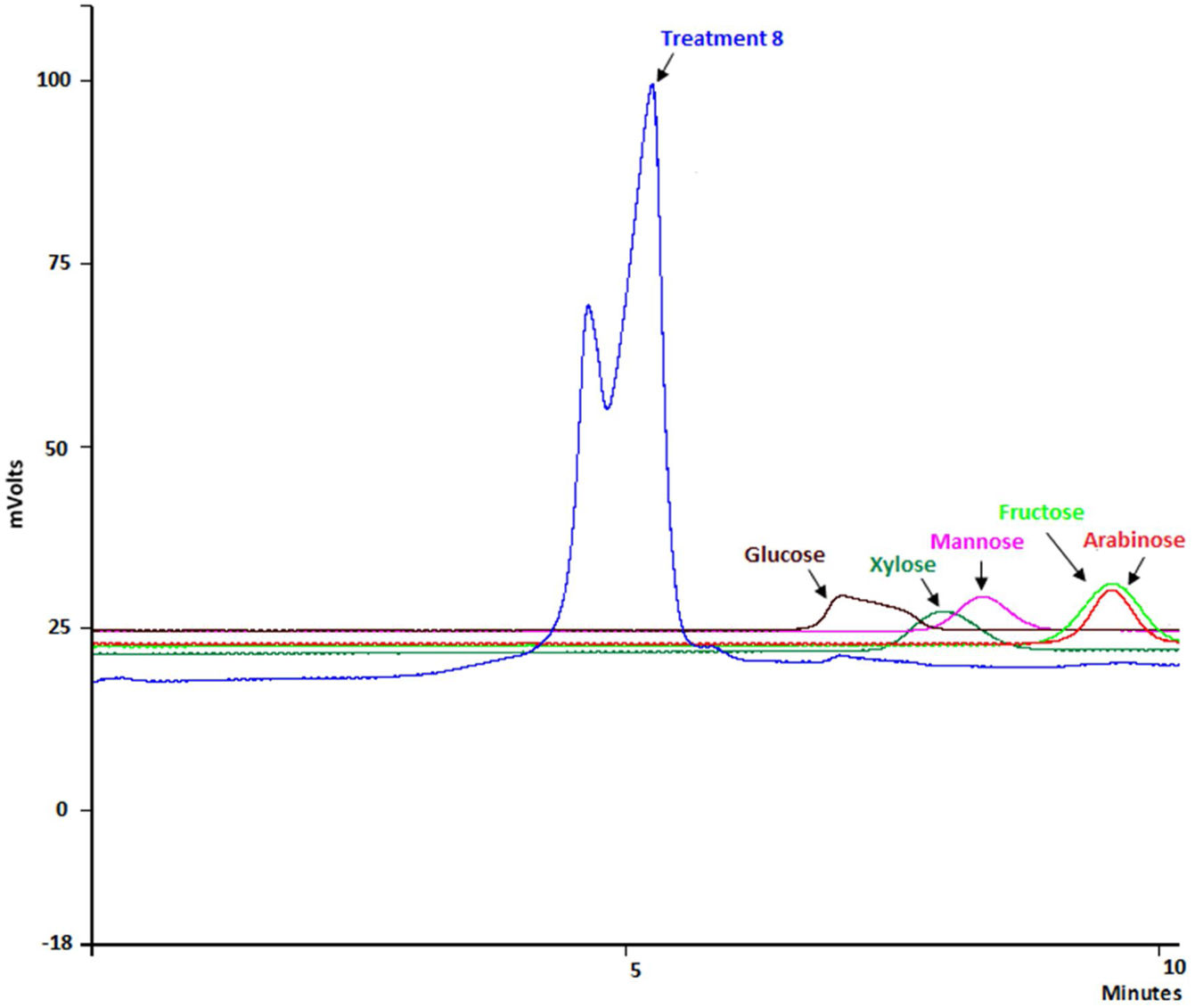
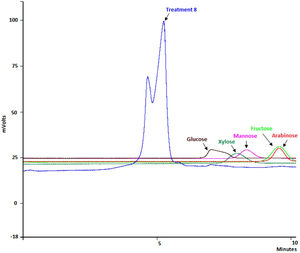
Analysis of fermentation products by high-performance liquid chromatography (HPLC)The fermentation products, such as monosaccharides and oligosaccharides, were detected from micro-filtered supernatants using HPLC, with a 300×7.8mm Metacarb 87C column, (Varian ProStar) heated at 70°C with a column oven. The monosaccharide standards used were arabinose, fructose, glucose, mannose and xylose at a concentration of 1mg/ml for the qualitative analysis of the hydrolysis products. Deionized water was used as a mobile phase with a flow rate of 1ml/min for 15min. Detection was performed with a Varian ProStar 355 refractive index (RI) detector.
Experimental designFive factors were studied for the xylanase production by fungal fermentation: fermentation time and concentrations of beechwood, yeast extract, ammonium sulfate and urea. Beechwood xylan was used as CS.
A Box-Wilson (BW) central composite design was established consisting of a fractional factorial design 2k−1 with sixteen factorial treatments, ten axial treatments and one central treatment38. Twenty-seven different treatments were generated and replicated three times (Table 1). The consideration is that a SSF is a fermentation process occurring in the absence (or near absence) of free water, with enough moisture content to support growth and metabolism of microorganisms43. After sterilization, the xylan fibers were hydrated and were swollen so that all treatments had a homogenous and uniform appearance without suspended solids.
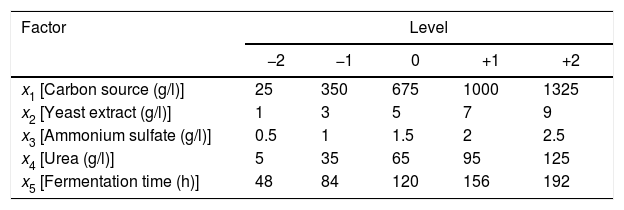
The treatments were randomized to avoid biased results in the application of design. Table 2 shows the decoded levels of each factor. Response variables measured were the activities of endo-β-1,4-xylanase, exo-β-1,4-xylosidase and endocellulase.
Adjustment of conditions for xylanase productionA general second-order model was proposed to estimate the effects of factors on the response variables. Main effects as well as interactions and quadratic effects were included in the model used, which is shown by Eq. (1):
where yn is any of the response variables, xi and xj are the k=5 factors, the β coefficients are regression parameters and ɛ experimental error.The parameters of Eq. (1) were fitted to the fermentation results and they were statistically analyzed. The desirability profile approach of Derringer and Suich19 was used in the optimization of the response variables (enzymatic activity). This approach (Eq. (1)) allows to optimize a single objective variable called desirability, which is a function of the responses in the model. This method was used to find the best conditions for hydrolytic enzyme production using the software STATISTICAv8.0® (Statsoft) and the profiler function in the Generalized linear/nonlinear result window. The solution takes into account the fact that the levels for the factors that maximize endoxylanase and β-xylosidase activities must minimize endocellulase activity. The 0 and 1 values of desirability were assigned to the minimum and maximum endoxylanase and β-xylosidase activities obtained from the treatments with the BW design, respectively. Conversely, for endocellulase activity, 0 and 1 values were assigned to the maximum and minimum enzymatic activity, respectively.
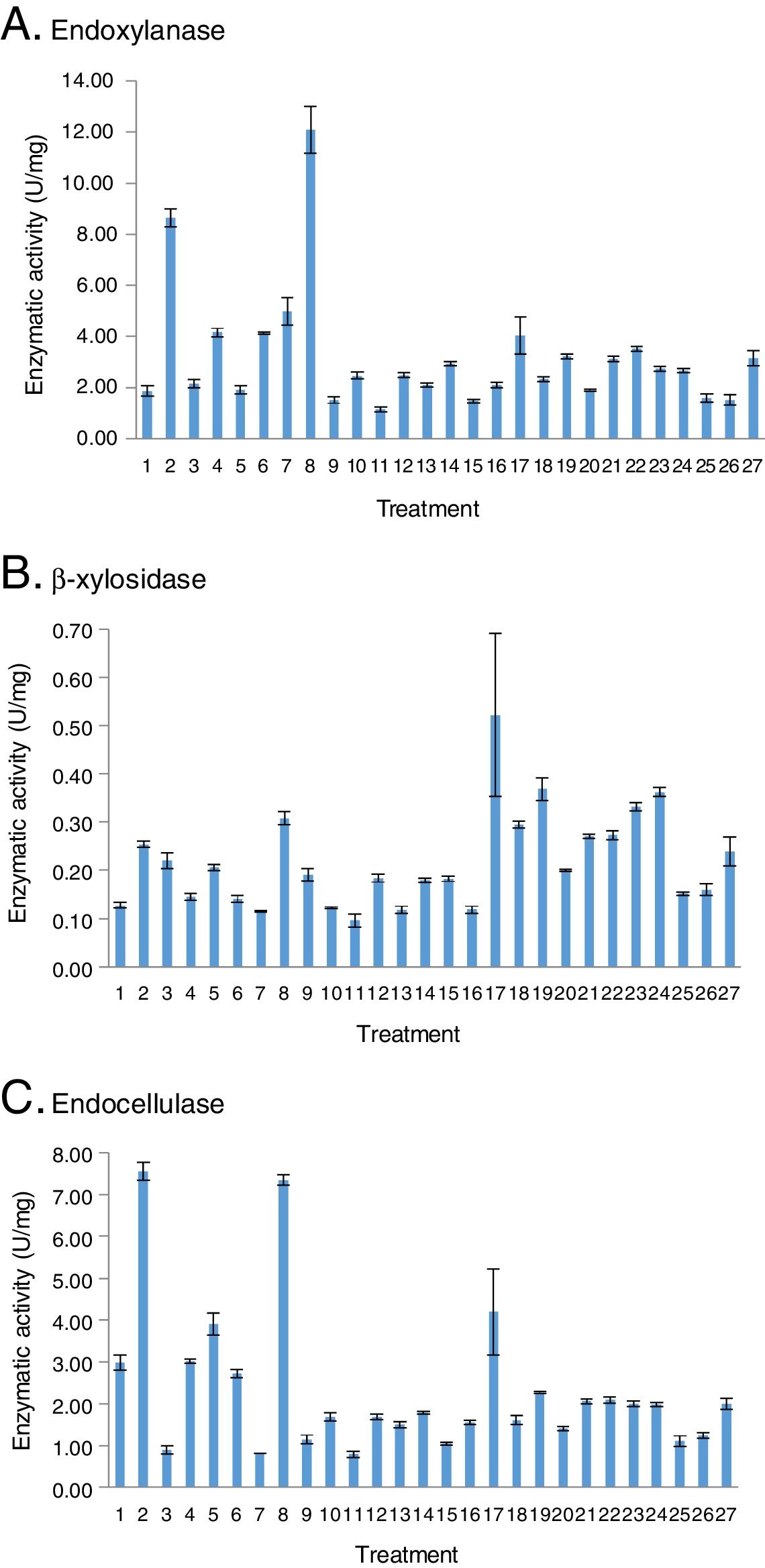
ResultsHydrolytic enzyme activity in BW treatmentsThe treatments obtained with the BW design were the following: one, in liquid medium (17, 2.5% CS), eight, in semisolid media (1–8, 35% CS), and eighteen, in solid media (19–27, 67.5% CS, 9–16, 100% CS, and 18, 132.5% CS).
The major production of endoxylases and β-xylosidases was observed in treatment 8, a solid culture (Fig. 1A) and in treatment 17, a liquid culture (Fig. 1B), respectively. On the other hand, the minor cellulolytic activity was obtained in treatment 11, a solid culture (Fig. 1C).
Interestingly, the treatments for the best endoxylanase production were also the same for the endocellulase production (2 and 8). A correlation between the xylanolytic and cellulolytic activities was confirmed (R2=0.71). On the other hand, a correlation between endoxylanases and β-xylosidases was not observed (R2=−0.41).
Model adjustment to experimental dataThrough the residual graphs for the model, adjustment and their values of the R2 correlation coefficients indicated that the adjustments made were accepted for endoxylanase (0.73) and endocellulase (0.81), but not for β-xylosidase (0.49). According to the F test, the quadratic models were fitted successfully, since the F value for the models was greater than the critical values (p<0.05): 8.15 for endoxylanase, 12.88 for endocellulase and 2.85 for β-xylosidase.
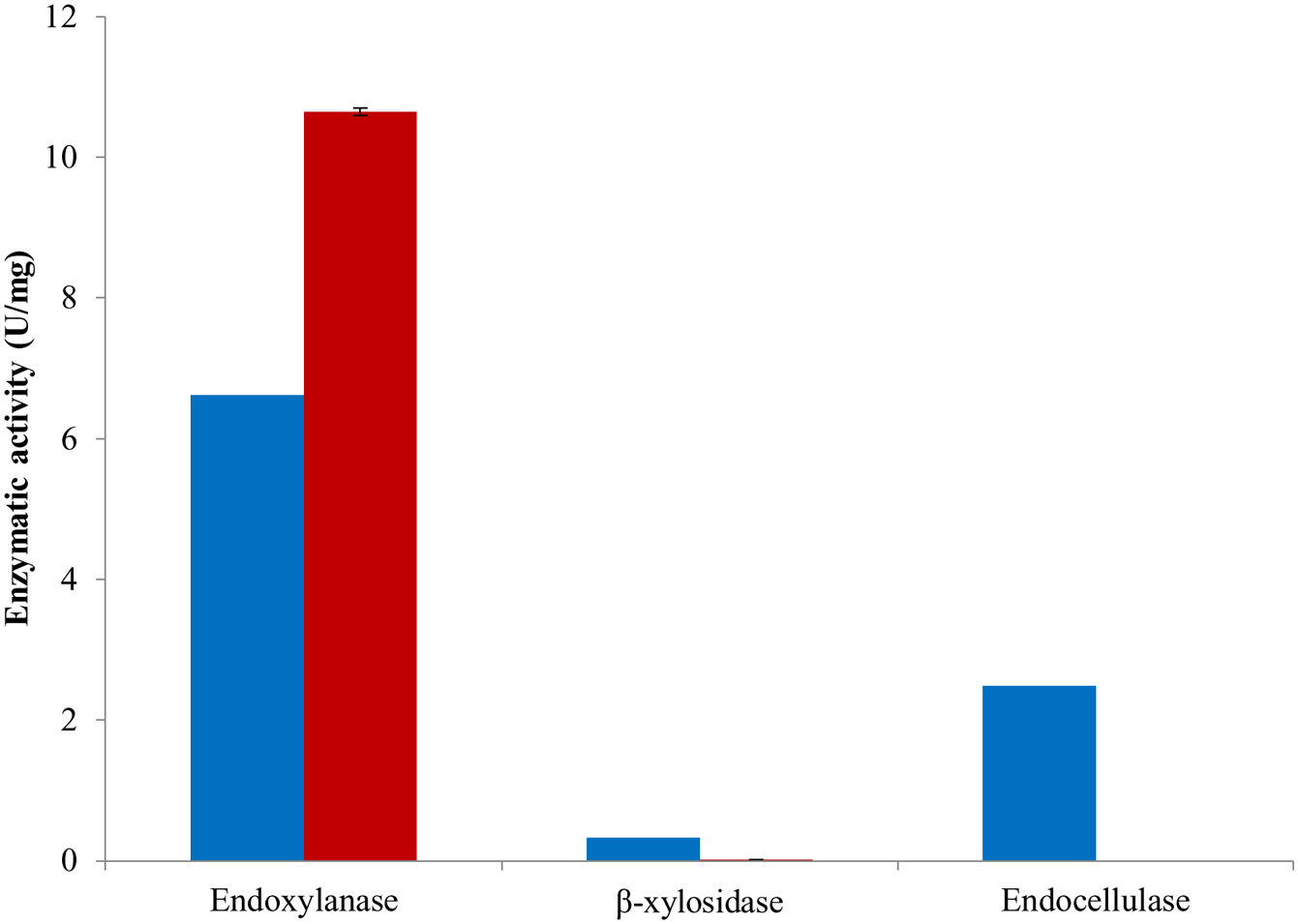
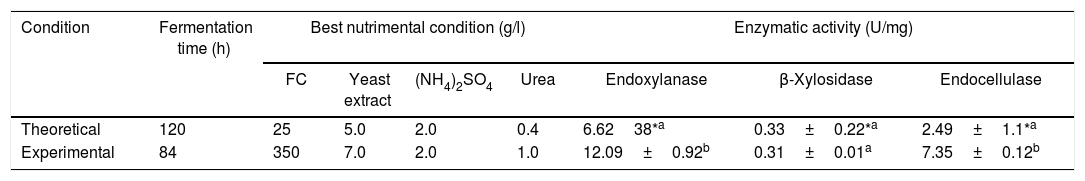
However, the best theoretical nutritional factors and the theoretical fermentation time obtained with the BW model, predicted the hydrolytic enzyme production in the following way: endoxylanases>endocellulases>xylosidases at 120h of fermentation. This theoretical result was contrasted with the best experimental treatment for the production of hydrolytic enzymes (treatment 8), see Table 3. The predicted behavior is observed with a numerical difference of two times for endoxylanases and three times for endocellulases. The major hydrolytic enzyme production was the experimental and not the theoretical.
Experimental verification of the best theoretical condition obtained with the BW model for hydrolytic enzyme production.
| Condition | Fermentation time (h) | Best nutrimental condition (g/l) | Enzymatic activity (U/mg) | |||||
|---|---|---|---|---|---|---|---|---|
| FC | Yeast extract | (NH4)2SO4 | Urea | Endoxylanase | β-Xylosidase | Endocellulase | ||
| Theoretical | 120 | 25 | 5.0 | 2.0 | 0.4 | 6.6238*a | 0.33±0.22*a | 2.49±1.1*a |
| Experimental | 84 | 350 | 7.0 | 2.0 | 1.0 | 12.09±0.92b | 0.31±0.01a | 7.35±0.12b |
Experimental data for enzymatic activity correspond to treatment 8 and they are average±SE
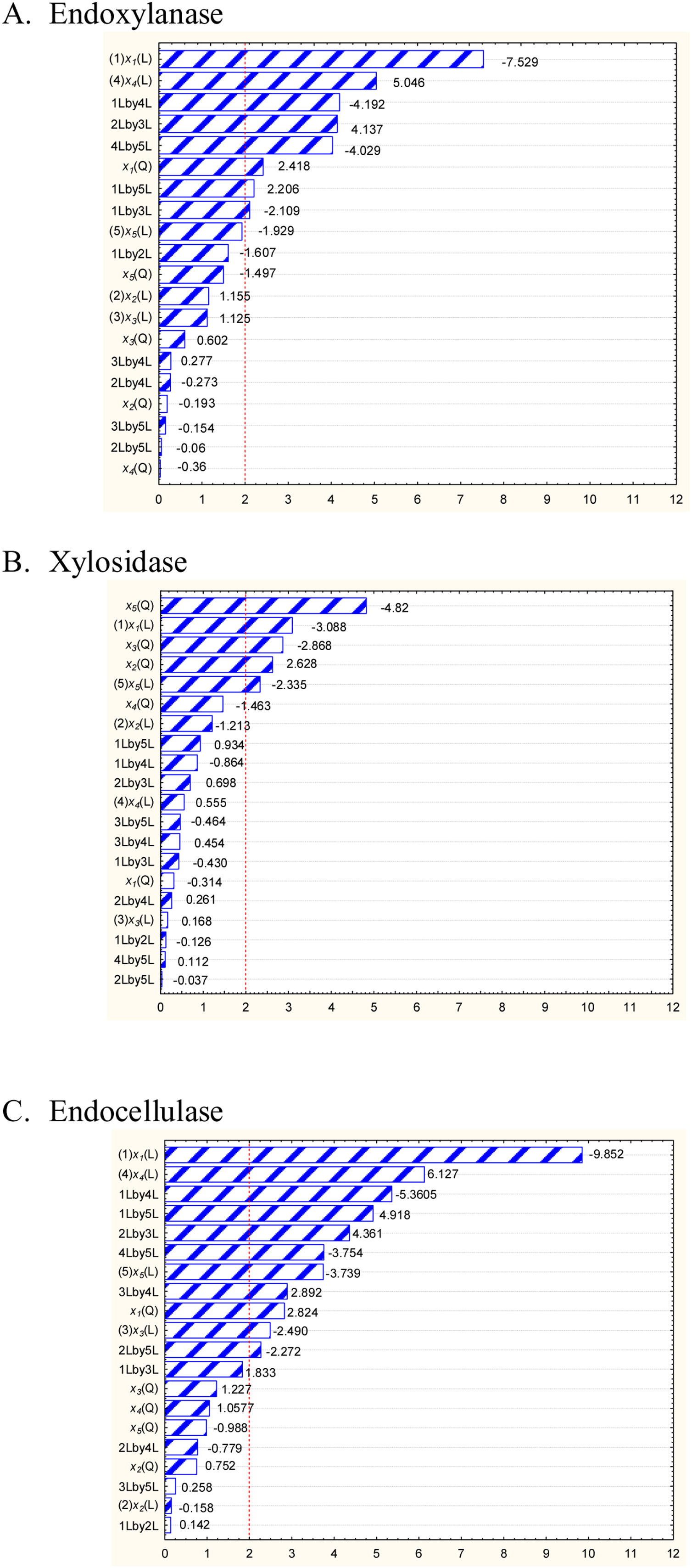
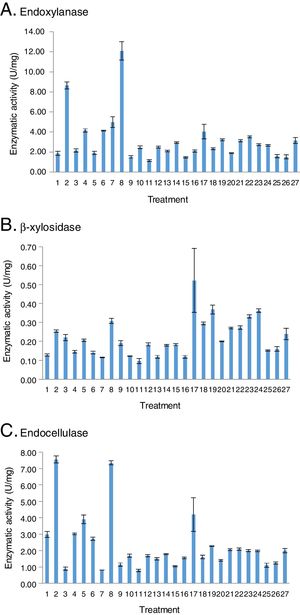
The identification of the nutrimental factors that have an effect on the hydrolytic enzyme activities was evaluated. The significance and effect of the nutrimental factors on enzyme production are shown in Figure 2. Pareto diagrams show the nutriments that were significant for endoxylanase activity with low endocellulase activity and they were xylan, yeast extract, ammonium sulfate and urea. Endoxylanase production with a minimum amount of endocellulases was possible when there was an increase in ammonium sulfate concentration as well as in the incubation time. The quadratic value of the CS concentration had a positive effect on endoxylanase and endocellulase production.
Significance and effect of the factors on the response variable: (A) endoxylanase, (B) β-xylosidase, and (C) endocellulase. (L) Linear effect of the factor, (Q) quadratic effect of the factor, (x1) concentration of carbon source (w/v,%), (x2) concentration of yeast extract (g/l), (x3) concentration of ammonium sulfate (g/l), (x4) concentration of urea (g/l), (x5) Time (h) (STATISTICAv8.0®, Statsoft).
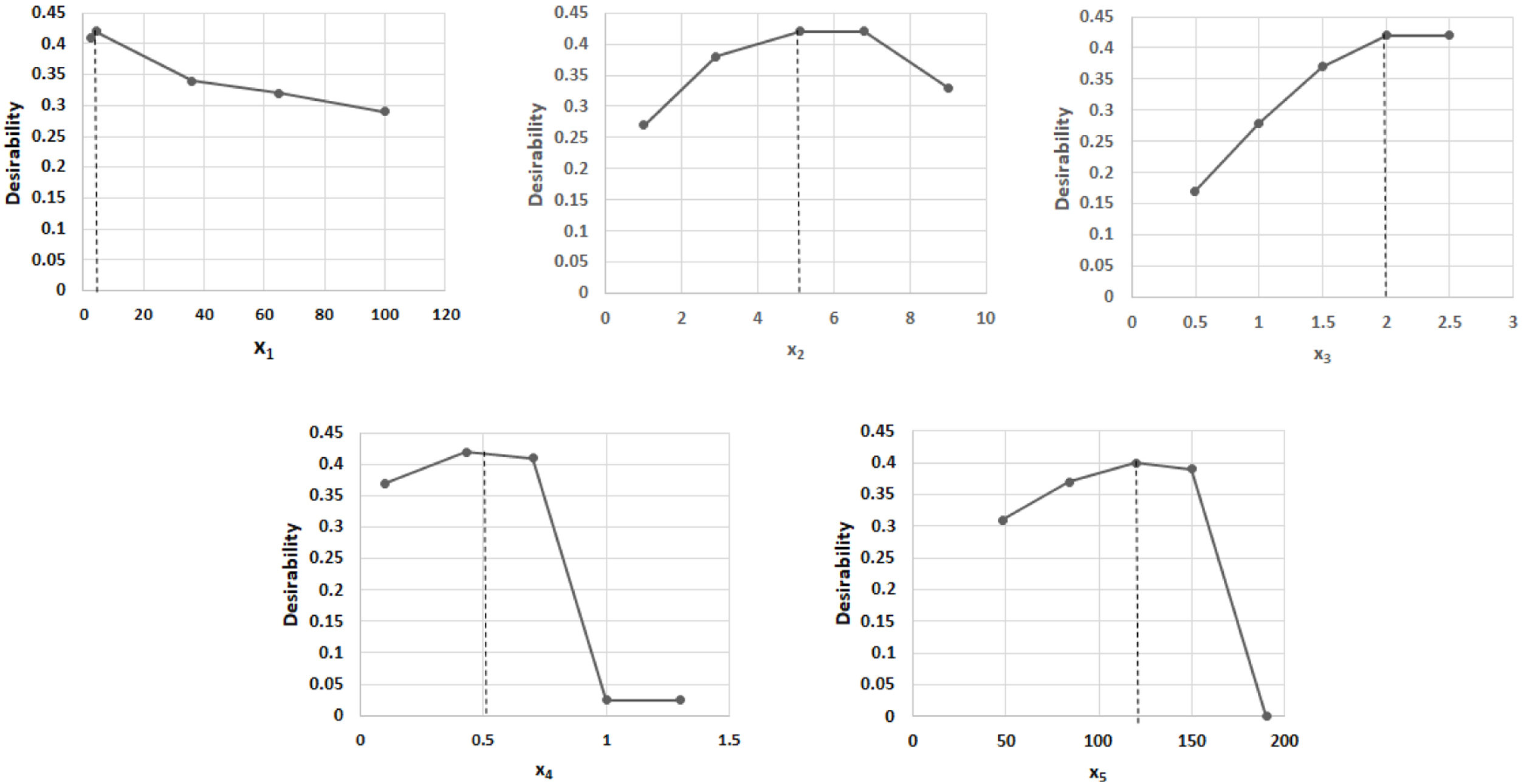
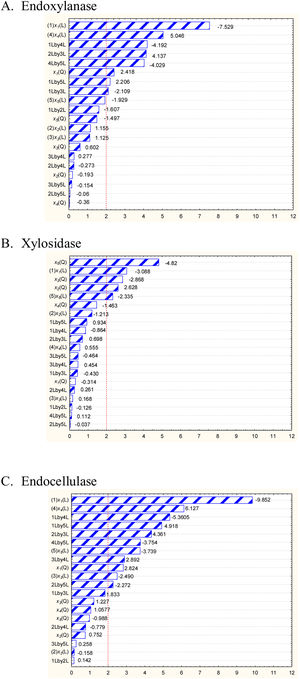
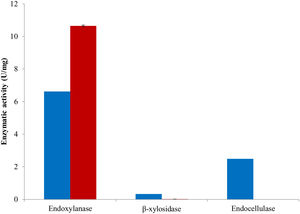
The combination of the best conditions that produce the maximum level of endoxylanase and β-xylosidase activities with the minimum level of endocellulase activity was obtained. Figure 3 shows the results of the optimization calculations based on the desirability function. The points with maximum desirability shown in the graph (x1=2.5%, x2=5g/l, x3=2g/l, x4=0.5g/l, x5=120h) were taken. Then, theoretical hydrolytic enzyme production was verified experimentally with the level of factors specified with the disability function. The endoxylanase activity obtained almost doubled the value given by the model (Fig. 4). The endoxylanase activity was of 10.65U/mg and 2.4U/mg of endocellulases. These nutrimental conditions and fermentation time were better than the conditions of treatment 8 (Fig. 1 and Table 3). According to the verification of the disability parameters (BW model), the endocellulase activity decreased more than three times. The endoxylanase activity was observed by HPLC, there was a major signal of xylooligosaccharides and free monosaccharides were not detected (Fig. 5).
Desirability profiles for hydrolytic enzyme production. Dotted line represents optimal conditions calculated for the desirability function. For calculations, the 0 and 1 values of desirability were assigned to the minimum and maximum endoxylanase and β-xylosidase activities obtained from treatments of BW design, respectively. Conversely, for endocellulase activity, 0 and 1 values were assigned to the maximum and minimum enzymatic activity, respectively (x1) concentration of carbon source (w/v,%), (x2) concentration of yeast extract (g/l), (x3) concentration of ammonium sulfate (g/l), (x4) concentration of urea (g/l), (x5) time (h).
During the xylanase production by F. solani, it was important to already know the effect of one specific nutritional variable over the hydrolytic enzyme activities when we modify several factors at once. In filamentous fungi, the production of enzymes that degrade plant polymers such as cellulases, hemicellulases, ligninases, and pectinases was regulated mainly through a coordinated pathway at a transcriptional level26. The turn on of the genes that codify these extracellular enzymes occur only when polymers, molecules from polymers, or other inductors are present52. There is a significant intercommunication between the induced expressions of the genes codifying for the different types of enzymes43. This is an important knowledge that should be considered when inducing the productions of fungal hydrolytic enzymes in any type of the well-known production processes and in any new fermentation design.
Another important aspect is that xylanolytic enzymes have already been considered a biological possibility to recover cellulose fibers from lignocellulosic materials. One characteristic of these enzymatic extracts is that they should have little or no cellulolytic activity to be used on a larger scale. For that reason and through a scrutiny a wild F. solani isolate was chosen for its interesting xylanolytic activity and the poor cellulolytic activity when grown on xylan as the sole carbon source6.
The major endoxylanase and β-xylosidase activities were obtained in different culture conditions, indicating that nutritional requirements for F. solani are specific to produce a different kind of enzymatic activity23. Such as the nutrimental behavior of Pichia pastoris observed in the heterologous expression of xylanases from Malbranchea cinnamomea genes12.
Since the treatments for the best endoxylanase production were also the same for the endocellulase production (2 and 8), a correlation between the xylanolytic and cellulolytic enzymes confirmed that the production of both enzymes is closely associated. A similar behavior was observed in the heterologous production of the endoxylanases from Pycnoporus sanguineous BAFC 212640.
In most of the treatments, the production of endoxylanases and endocellulases is associated under these culture conditions, where the endoxylanase activity is almost two times higher than the endocellulase activity. However,a lignocellulosic substrate for the production of these enzymes was not used in this work18,52. Since the fermentation media were supplemented with pure xylan, endocellulase production was also present.
This is due to the fact that the production of fungal extracellular glycohydrolases is coordinately regulated51. The presence of small oligosaccharides in the fungal cytosol elicit a high level of synthesis of glycohydrolases with different specificities1. These glycohydrolases act together in synergism for the degradation of polysaccharides7.
For instance, the same compounds may provoke expression of both cellulases and hemicellulases, albeit to different extent1. In Aspergillus niger, the transcriptional regulator XlnR that was initially identified as the transcriptional regulator of xylanase-encoding genes controls the transcription of about 20–30 genes encoding hemicellulases and cellulases. In addition, the orthologous xyr1 (xylanase regulator 1-encoding) gene product of fungus Hypocrea jecorina has a similar function as XlnR48. Some treatments did not maintain this tendency, i.e. treatment 7, in which endoxylanase production was six times higher than endocellulase.
The lack of correlation between endoxylanases and β-xylosidases can be due to the type of fungal species, the intrinsic nature of both enzymes, the kind of reaction that these enzymes catalyze, the CS used, and the fact that β-xylosidases are involved in a subsequent phase of xylan depolymerization. These results are in agreement with data already reported where the major endoxylanase and β-xylosidase production was observed at different nutritional conditions and fermentation times in a mutant strain of Aspergillus awamori 2B.361 U2/1 and in Colletotrichum graminicola21,50.
In relation to the effect of the nutrimental factors on hydrolytic enzyme production, the mathematical models indicate a negative effect in the production of endoxylanase, β-xylosidase and endocellulase when the concentration of CS increased due to the negative sign of the coefficient next to each histogram bar. The culture media that had less amount of available water limited fungus growth and enzyme production. It was not a surprising result in F. solani, since this behavior was also observed in Aspergillus spp. when it grew on different carbon sources35. This effect could be explained considering that in most SSF treatments the sterilized and hydrated xylan became a firm gel that perhaps limited nutrient diffusion and product secretion27. Consequently, the water in the medium causes a swelling of xylan fibers, and therefore, a reduction in substrate porosity and limitation in oxygen transfer. On the contrary, when the humidity level decreases, the nutrient solubility is reduced and causes a limited swelling with lower porosity. Based on these results, the catabolite xylose repression was discarded27. During the fermentation of xylan by F. solani, the highest activity of endoxylanase was observed in treatment 8, where there was no xylose in the culture medium, as can be shown in the HPLC results, since monosaccharides were not detected. It is possible that the fungus utilized the xylose at the same rate it hydrolyzed the xylan.
In treatments with low CS concentration, the amount of available water was major. This factor promotes the diffusion of chemical substances, such as the monosaccharide xylose, which could cause the repression of the endoxylanase synthesis30. On the other hand, when the CS concentration is major, the available water is minor and diffusion of xylose and xylobiose is limited; therefore, this factor restricts their transport into the cytosol, which diminishes their repressive effect in the synthesis of endoxylanases and endocellulases. These results show the influence of culture conditions over the enzymatic activity produced by the fungus. β-xylosidase and endocellulase production was negatively affected by the incubation time45. The decrease in enzyme production could occur: (1) as a consequence of the macro and micronutrients exhaustion in the culture medium during the fermentation and consequently, the disruption in the fungus physiology; therefore, the fungal response is to inhibit the secretor process of the extracellular enzymes including the lignocellulose-degrading enzymes; (2) the depolymerization of lignocellulosic materials occurs in the presence of a considerable amount of proteolytic enzymes that cause a significant reduction in xylanolytic activity as the fermentation time increases.
Based on the mathematical model, the interaction between CS with urea supplied to the fermentation media had a significant role in the production of endoxylanases and endocellulases as it can be seen in the significance (p<0.05) and effect of the factors on the response variable. Carbon and nitrogen contribute to biomolecule synthesis and carbon is essential in the production of energy. Therefore, the positive interaction between both carbon and nitrogen sources for xylanolytic enzyme production is important. However, the interaction between CS and urea for both enzymes was negative, due to the negative sign of the coefficient next to each histogram bar. Although urea individually had a positive effect on the production of xylanases and cellulases, it behaved negatively in the interaction with the carbon source, possibly due to the persistent negative effect of the carbon source, which was also observed individually.
The increment of urea concentration in the culture media favored the endoxylanase and endocellulase production, whereas β-xylosidases were not affected. The yeast extract did not produce any effect on the three enzymes. These results are in accordance with the observation that ammonium sulfate, the yeast extract, and the meat extract yielded a deficient enzyme production in F. solani F7, whereas ammonium nitrate and urea caused a considerable production of xylanases22.
The combination of the best conditions that produce the maximum level of endoxylanase and β-xylosidase activities with the minimum level of endocellulase activity was investigated. The endoxylanases and endocellulases showed a similar behavioral pattern in function of the studied factors. Therefore, in order to minimize the cellulolytic activity, in the process, the necessary adjustments were made to affect as little as possible the desirable values of endoxylanase and β-xylosidase activities.
Although the endoxylanase activity was major in the solid media with 35% CS (treatment 8) in comparison with the rest of the treatments, according to the results, the utilization of liquid media is recommendable (2.5% CS) because the β-xylosidase activity would lightly increase and the cellulolytic activity would be significantly reduced1.
During hydrolytic enzyme production, the reduction of cellulolytic activity was expected. It was inevitable that endoxylanase activity diminished since endoxylanases and endocellulases were associated, such as observed in other fungi xylanase producers5.
F. solani produced hydrolytic enzymes at 48h: however, the best fermentation time for maximum hydrolytic enzyme activities was at 120h in accordance with the adjustment. The time for maximum xylanase production varies depending on the production method: 120 and 144h for F. solani F7, from immobilized and free cells, respectively22, and the organism: 96, 192 and 336h, for Aspergillus sp. M.2.8, Aspergillus fumigatus M.7.3 and Thermomucor sp. M.7.6, respectively. In sugar cane bagasse as CS under SSF and 207.8h for C. graminicola in wheat bran as CS under SSF39,50.
After verification of conditions for hydrolytic enzyme production, it was observed that these culture conditions did not favor β-xylosidase production since enzymatic activity was not detected. Interestingly, the fermentation conditions limited endocellulase production in a favorable way, and therefore, a low endocellulase activity was detected. Different transcriptional activators for xylanolysis and cellulolysis processes, e.g. may explain this observation. The transcriptional activator XlnR (Xlr1/Xyr1) of A. niger is an important regulator in the degradation of hemicellulose and cellulose by fungi, as well as in the use of D-xylose via the catabolic pathway of the pentose. XlnR homologs are commonly found in filamentous Ascomycetes and often assumed to have the same function in different fungi. However, for Fusarium graminearum it was reported that the production of cellulases was not affected by the elimination of XlnR14,15. The presence of endocellulases was observed in F. solani as well as in F. graminerum and Magnaporthe oryzae13,28. These data suggest that F. solani has two transcriptional mechanisms, one for the degradation of cellulose and another for the degradation of hemicellulose.
The use of SSF provides to the fungi a similar environmental condition to their natural habitat (wood and organic material), and therefore it would be expected that this condition would stimulate them to have a higher production of hemicellulolytic enzymes. In the SSF, the lowest xylanase production could be due to the high CS concentration. The CS increases the viscosity of the media, and therefore, the diffusion and solubility of the media compounds as well as the transference of atmospheric gases (oxygen and nitrogen) are affected. These results are in agreement with the ones obtained in F. solani F722.
The use of model substrates, like beechwood xylan, to study the best conditions for the xylanase production by F. solani, represents an ideal approximation of lignocellulosic material degradation. In the next phase of this research, it will be interesting to study the utilization of lignocellulosic biomass or other polymers by F. solani, such as agricultural, forestal and agroindustrial subproducts or municipal residues. These polymers are rich in hemicellulose and are ideal to evaluate the hydrolytic enzyme production by F. solani. At the same time, it will be interesting to find out if by using these polymers, the enzyme production costs can be significantly reduced.
ConclusionIn the wild-type isolate of F. solani an experimental production of endoxylanases of 10.65U/mg with an absence of endocellulases was achieved based on the best prediction of the BW model. Its singular endoxylanases production and friendly manipulation of nutritional conditions are characteristics potentially useful in scaling the process to obtaining enzymes. This fungal wild strain is useful for future experiments of directed evolution to obtain strains genetically modified for overproducing endoxylanases.
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was financially supported by the UMSNH and m3P foundation. MAN held a CONACyT scholarship.