Candida albicans, one of the most dreadful fungal pathogens threatening humans, could not be easily prevented. The anticandidal activity of oak gall extract, Quercus infectoria (QIE), was investigated as a potential natural alternative to synthetic and chemical fungicides. QIE anticandidal potentiality was confirmed using both qualitative and quantitative assays. Cotton textiles were treated with QIE and then evaluated as anticandidal fabrics. QIE-treated textiles had a potent anticandidal activity, which could completely inhibit the inoculated C. albicans cells. The durability of anticandidal activity in QIE-treated textiles almost completely disappeared after the fourth laundering cycle. QIE could be recommended, however, as a potent anticandidal agent for preparing antiseptic solutions and emulsions and as a finishing agent for manufacturing anticandidal disposable diapers and hygienic clothes.
Candida albicans es uno de los patógenos fúngicos más terribles que amenazan la salud humana, y su prevención no resulta sencilla. En este trabajo se investigó la actividad anticandidiásica del extracto de agallas de roble (Quercus infectoria extract; QIE) como una posible alternativa natural a los fungicidas sintéticos y químicos. El potencial anticandidiásico del QIE se confirmó mediante análisis cualitativos y cuantitativos. Se trató tejido de algodón de uso textil con QIE y se lo evaluó como tela anticandidiásica. Se verificó que dichos tejidos exhibían una potente actividad anticandidiásica y que podían inhibir completamente a células de C. albicans inoculadas. La actividad anticandidiásica, sin embargo, desapareció por completo después del cuarto ciclo de lavado. Se concluye que se podría recomendar QIE como un agente anticandidiásico potente para la preparación de soluciones antisépticas y emulsiones, y como un agente de acabado para fabricar pañales desechables y ropa de higiene con propiedades anticandidiásicas.
Candida albicans is the most common etiological agent for candidiasis, which may be the cause of most clinical mycoses11. C. albicans may be found on the human skin and mucous membranes such as the mouth, rectum or vagina. It is usually a superficial but inconvenient infection that may occur in intertriginous areas, nails and adjacent tissues26. This fungus can also pass into the bloodstream and affect the throat, intestines, bronchopulmonary system, kidneys and heart valves. Disseminated candidiasis could sometimes be a serious disease, frequently resulting in death3.
Immunocompromised patients greatly suffer from various fungal pathogen attacks; C. albicans was recorded in the majority of their systemic infections, having mortality rates ranging from 50% to 100%11.
Animal candidiasis, caused by C. albicans, has been frequently confirmed in some mammalian and avian species. The disease in poultry, chicks and other fowl is common and could have economic consequences with mortality ranging from 8% to 20%. Outbreaks have been described in several regions worldwide. Oral candidiasis was also detected in calves, lambs, colts, swine, cats, dogs, laboratory guinea pigs and mice as well as in zoo animals. Candida spp. can lead to mastitis and abortions in cattle2.
Among the causal agents of systemic mycoses and fungal opportunistic infections, C. albicans poses serious problems for physicians who are limited by a very short list of antifungal agents for its treatment22. These limitations arise mainly from the fact that both the host and fungal pathogens are eukaryotes, thus it is hard to have an antifungal agent with selective toxicity to systemic mycoses because it also binds to similar targets of host cells7. Candida infections of the skin, mouth, or vagina regularly occur for no apparent reason. The proposed common cause of infection is the excessive use of antibiotics that destroy both beneficial and harmful microorganisms in the body, and permit Candida to multiply at different body sites.
The discovery of the healing potentialities of plants dates back to prehistoric times. The Plant Kingdom has always provided lots of precious compounds and materials to humans, which were directly used for medicinal and health care purposes10. Most plant derivatives are commonly considered safe, eco-friendly and cost-effective agents compared to synthetic and chemical substances12.
Quercus infectoria Olivier (Fagaceae) is a shrub or small tree of about 2m high and mainly found in Mediterranean countries13. The galls of Q. infectoria are commonly known as oak galls and are usually found on the twigs of this tree as a result of the tree's reaction to the eggs laid by gall wasps (Cynips quercusfolii)14.
Oak galls are very rich in tannins and their main constituents are gallotannic acid, gallic acid, ellagic acid, starch and sugar8.
Q. infectoria extract (QIE) was recommended in folkloric medicine and its potentiality was documented by several researchers as a candidate curative agent for leukorrhea and other vaginal discharges, profuse menstruation, chronic diarrhea, dysentery, bleeding hemorrhoids, hemorrhages, gleet, varicose veins and long-standing gonorrhea. QIE was also indicated as a gargle and mouthwash to treat sore throat, stomatitis, pharyngitis, laryngitis, tonsillitis and also as a potent antibacterial, antiviral and larvicidal agent5,15,16,24,27,32.
It was also reported that QIE exerts a remarkable antiinflammatory activity after topical or oral administration and has the ability to prevent the formation of some inflammatory mediators19.
The present study was designed to investigate the QIE anticandidal activity and its potentiality as a finishing agent to fabricate anticandidal cotton textiles.
Materials and methodsYeast strainsCandida albicans strains, i.e., C. albicans-A (ATCC-10231), C. albicans-C from chicken combs and C. albicans-H from human vaginas were obtained from the Department of Hygiene and Preventive Medicine, Kafrelsheikh University, Egypt. The identity of the examined strains was confirmed according to the method of Barnett et al.4. Yeast strains were grown in yeast-malt extract broth (YMB, Difco, USA) and maintained on yeast-malt extract agar (YMA).
Preparation of oak gall extractGalls of Q. infectoria Olivier (Fagaceae) were kindly obtained from the Agricultural Research Centre, Cairo, Egypt. Using a mixer grinder (Spex Ind. Inc., Metuchen, NJ), the dried plant parts were powdered to get ~ 60-mesh size particles. The powder (200g) was mixed with 1 L of 70% ethanol and agitated using a rotary shaker (New Brunswick, NJ) at 320 xg for 6h. Extracts were filtered to remove the plant particles, through Whatman No. 41 filter paper in a Buchner funnel. Plant residues were re-extracted with 500ml of solvent, filtered and the extracts were pooled and evaporated at reduced pressure in a flash evaporator (Büchi, Flavil, Switzerland) at 45°C to remove almost 90% of solvent. The extract was further dried in a desiccator under vacuum until a constant weight was achieved. The final dry extract weights were recorded and then the dry matter was resuspended in a small volume of dimethyl sulfoxide (DMSO) solution.
Evaluation of anticandidal activityThe standard agar diffusion assay was firstly performed as a qualitative screening assay6. Aliquots of 100μl of each Candida strains cell suspensions were spread on YMA plates. Afterwards, 6-mm diameter filter paper disks (Whatman No. 1) were loaded with 20μl of 10mg/ml, from each of the QIE or fluconazole as a standard antifungal agent and were placed concentrically on the inoculum. YMA plates were incubated for 24h at 37°C. Following incubation, the measurements of zones of growth inhibition were assessed using a finely calibrated ruler. The growth inhibition diameter of four different areas was measured and the mean diameter was obtained for each strain.
The determination of the minimal inhibitory concentrations (MIC) of QIE towards C. albicans strains was conducted according to Tayel et al.28. Different concentrations of QIE in YM broth were prepared and serially diluted in the wells of tissue culture microtiter plates (96 wells), to obtain final concentrations in the range of 10–5000μg /ml. Yeast inoculums (100μl) were added to each well and then the plates were incubated at 37°C for 24h. The YMA plates were then inoculated with 100μl from each well at the end of the incubation period. As viable cells can only transform triphenyl tetrazolium chloride (TTC) to red color, the antimicrobial activity was confirmed by adding 20μl of 0.5% TTC aqueous solution to each well before plating and incubating for 30min. MIC was defined as the lowest concentration of QIE that completely inhibited colony growth on YMA, as confirmed by TCC staining (dead C. albicans cells could not be stained by TTC).
Treatment of cotton fabrics with chitosanScoured cotton fabrics (106g/m2 plain texture, Style S/400, TESTEX, Germany) were used in the present study. The paddry- cure method was applied in this study29. The experimental conditions were as follows: the cotton fabric was immersed in the QIE solution at pH 6.5±0.2, at its MIC level, with stirring for 2h at 60°C. Fabrics were padded and squeezed between two nips and dips to a 100% wet pick. The treated cotton was dried with hot air at 65°C for 5min, and then cured at 95°C for 30min. Later, the cotton fabric was recurrently washed with water at 40°C and dried at laboratory conditions, e.g. in a laminar flow hood at 25±2°C.
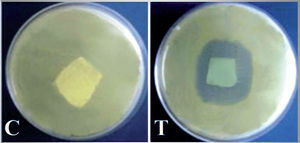
Assessment of anticandidal activity of QIE-treated fabricsThe qualitative evaluation of the anticandidal activity of QIE-treated fabrics was conducted using the growth inhibition zone test. A 2×2cm sector from control and the QIEtreated fabrics were placed on the surface of YMA plates inoculated with C. albicans and incubated at 37°C for 36h. The appearance of growth free zones around the textile indicates the anticandidal activity.
The anticandidal activity of the fabrics was also quantified according to the AATCC Test Method 100-19991. Microbial suspensions from a 36 hour old culture in YMB medium (25μl of 106cell/ml), were loaded onto 2×2cm cotton swatches. To ensure contact between the yeast cells and the fabric, a cover swatch was sandwiched over the loaded one. Samples were quenched with 5.0ml of sodium thiosulfate solution (0.02 N) after 30, 60, 90 and 120min. Serial dilutions were made using phosphate buffer pH 7, and 100μl were plated on YMA. Grown colonies were then counted after incubation at 37°C for 24h to determine the presence of viable cells. The C. albicans inhibition rate was calculated as:
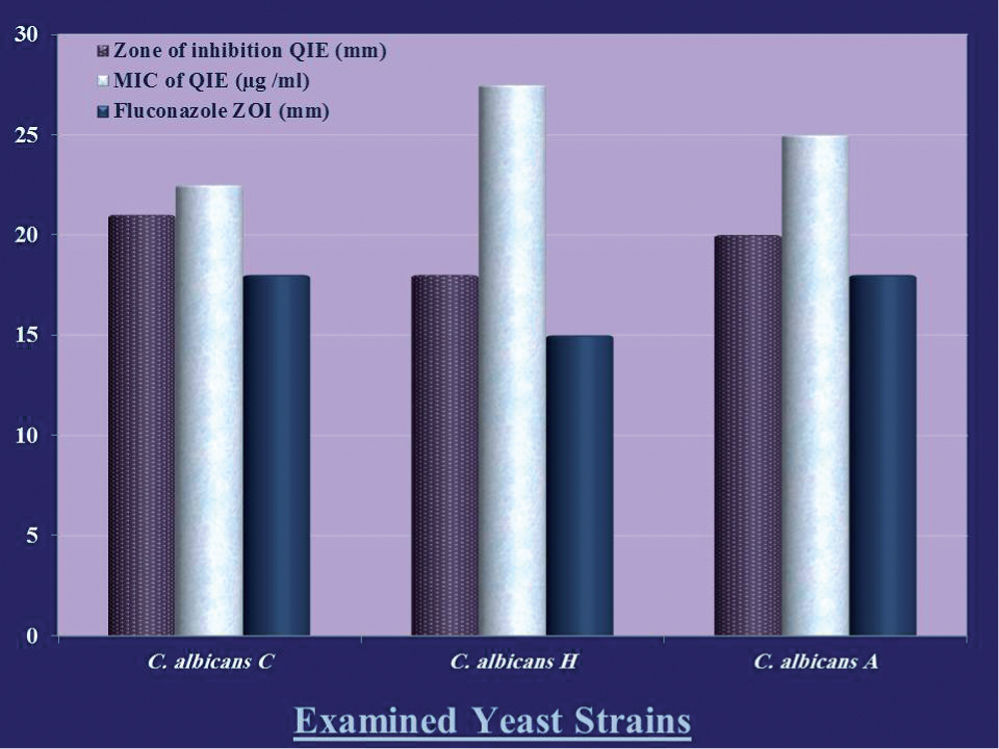
ResultsThe evaluation of QIE efficiency as an anticandidal agent against the examined C. albicans strains was conducted qualitatively and quantitatively. The results for QIE activity compared to fluconazole as standard antifungal agent are shown in figure 1.
A contradictory relationship was detected between the diameter of inhibition zones and the MIC values. C. albicans H was the most resistant strain to QIE action, as evidenced by the narrowest zone of growth inhibition of 18mm and the highest MIC value of 27.5μg /ml. Compared to fluconazole, QIE exhibited stronger anticandidal activity, which was evidenced by the wider zones of growth inhibition. C. albicans H was selected for further investigation as the most resistant examined strain.
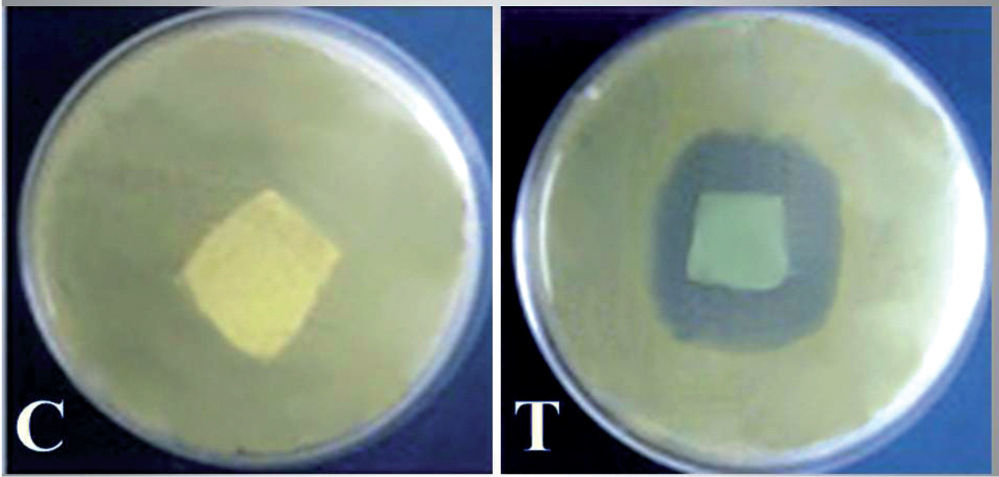
The cotton fabric was treated with a QIE solution which contained a MIC of 27.5μg/ml, and the resultant fabric exhibited a potent anticandidal activity in the inhibition zone assay (fig. 2), whereas the untreated textile did not exhibit any antimicrobial signs against C. albicans. The formed clear zone surrounding the treated textile sector, indicated the release of QIE into the agar media and the inhibition of the entire C. albicans cells without the appearance of any resistant mutants to QIE, after prolonging the incubation period for up to 7 days.
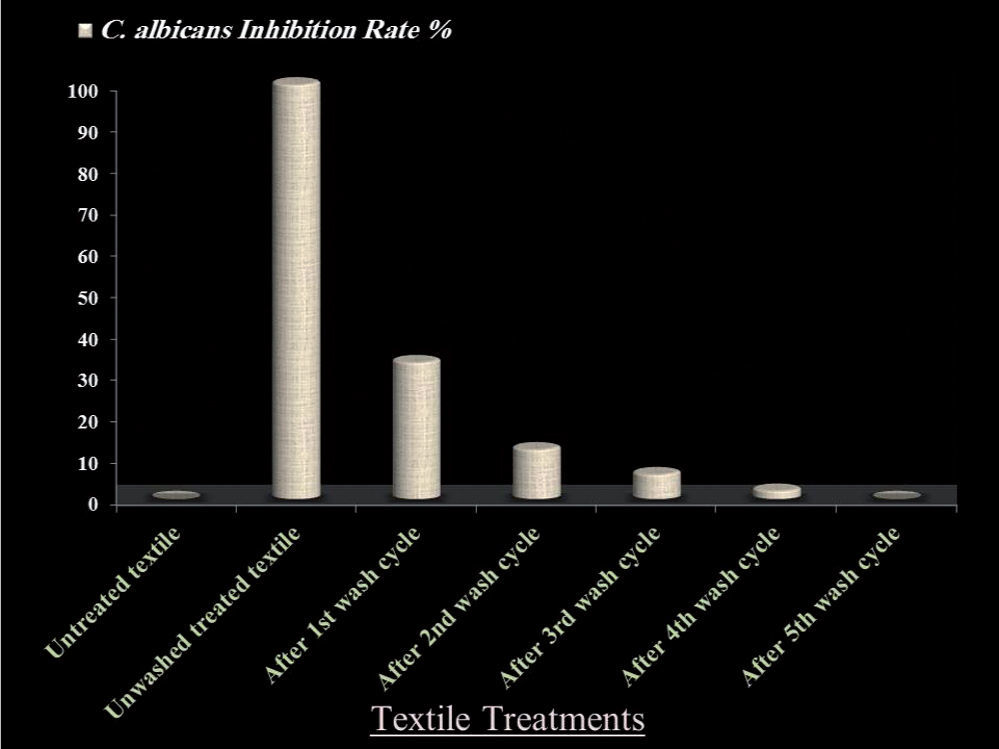
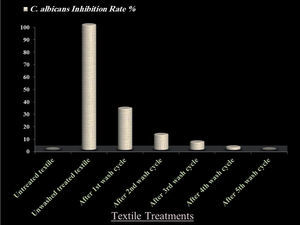
The durability test of anticandidal action on QIE treated fabrics revealed that, compared to the unwashed treated fabrics, no C. albicans inhibition could be observed in untreated textiles. The QIE- treated textiles lost their anticandidal activity subsequent to laundering cycles with percentages of 73, 88, 93, 99 and 100% after the 1st, 2nd, 3rd, 4th and 5th washing cycles, respectively (fig. 3). It was observed that the QI dye completely disappeared from the treated textiles after the 4th laundering cycle, compared to untreated textiles.
DiscussionThe ethnopharmacological use of medicinal plants and their recurrent application in medical pharmaceutical and common fields warrant their biosafety for humans9. In this study, Q. infectoria was selected for evaluation from a wide collection of examined plants (data not shown) because it exhibited a superior potentiality against C. albicans strains.
The anticandidal activity of QIE in this study was forceful and effective against all the examined strains, which could be attributed to the high QIE content from bioactive compounds. The synergism in many antimicrobial agents was stated to be beneficial in microorganism inhibition because the pathogen could not easily generate resistance against several microbicides30,31.
It was recorded that QIE contains many bioactive constituents, for e.g., a large amount of tannins and significant percentages of gallic, ellagic and syringic acids, β-sitosterol, hexamethyl ether, isocryptomerin, amentoflavone, methyl betulate, and hexagalloyl glucose17,18, the majority of these compounds have notable antimicrobial activity. Tannins, especially those obtained from plants, have been found to possess strong antimicrobial activity20. However, the high tannin content in oak galls could be considered the main responsible for their antimycotic activity.
For the accurate evaluation of QIE as an anticandidal agent, fluconazole was used as a positive control for comparison, since fluconazole was documented to be among the most commonly used antifungal agents available for treating infections caused by Candida spp.21. It was reported that repeated treatments with fluconazole and other fungicides have led to the appearance of yeast isolates resistant to this agent25, thus the presentation of a natural and effective antifungal agent such as QIE has great importance for physicians and public health overseers.
QIE in its native form could be suggested for inclusion as an active ingredient in anticandidal solutions, sprays and emulsions.
Cotton textiles were treated with QIE to investigate further potential applications of its anticandidal activity. The durability of antimicrobial activity of the treated textiles is one of the key issues to be considered in any finishing treatment15,23.
The treated cotton textiles in the present study lost most of their anticandidal activity after 4 washing cycles, which could indicate that QIE did not chemically link to the cotton fibers. However, the recommended application for QIEtreated textiles could be the fabrication of disposable diapers, gloves, hygienic bed sheets or other disposable clothes. On the other hand, it will be beneficial for QIE to be smoothly released from the cotton fibers so as to contact the infected areas with C. albicans and perform its anticandidal action. Harmonized results were reported by Gupta and Laha15, who demonstrated that the cotton textiles treated with QIE retain 50% of their antibacterial activity after the first laundering whereas this activity was completely lost after 5 washing cycles.
The application of antimicrobial agents on textiles is restricted by many limitations, as for example, these substances should be permanently effective, non-damaging to the skin as well as environmentally benign23,
In addition to the anticandidal activity of QIE-treated textiles, it will be beneficial to use them for reducing the inflammation caused by Candida infection. It was recorded that QIE has a potent antiinflammatory activity, with strong efficacy in various in vivo and in vitro experimental models of inflammation19.
However, the application of QIE could be recommended as a potent anticandidal agent for the preparation of antiseptic solutions and emulsions and as a finishing agent for the production of disposable anticandidal cotton textiles.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Always and foremost, thanks are indebted to our God ALLAH forever for his mercy in guiding and helping.