Scedosporium/Lomentospora species are widely distributed in nature. They are generally saprophytes, but can cause opportunistic infections in immunocompromised patients and occasionally in immunocompetent patients that are difficult to treat due to high levels of antifungal resistance. The distribution of Scedosporium/Lomentospora species shows regional differences. Scedosporium boydii and Scedosporium apiospermum are the most frequently isolated species in our region, whereas Scedosporium aurantiacum is more common in other regions. We describe the first isolation in Argentina of S. aurantiacum in a vitreous humor infection from a previously healthy patient after traumatic injury in her left eye. Due to the suspicion of fungal endophthalmitis, a mycological study of the vitreous humor was performed. The culture allowed the isolation of S. aurantiacum. The patient was treated with voriconazole with favorable clinic evolution.
Las especies de Scedosporium/Lamentospora se encuentran ampliamente distribuidas en la naturaleza. En general son saprófitas, pero pueden causar infecciones oportunistas de difícil tratamiento debido a sus altos niveles de resistencia a los antifúngicos en individuos inmunocomprometidos y, ocasionalmente, en personas inmunocompetentes. La distribución de las especies de Scedosporium/Lamentospora muestra diferencias regionales. Scedosporium boydii y S. apiospermum son las especies más frecuentemente aisladas en nuestra región, mientras que en otras S. aurantiacum es más común. Presentamos el primer aislamiento en Argentina de S. aurantiacum de una infección de humor vítreo de un paciente previamente sano que sufrió una lesión traumática. El paciente fue tratado con voriconazol y tuvo una evolución clínica favorable.
Species of Scedosporium/Lomentospora (Lomentospora prolificans formerly Scedosporium prolificans) are widely distributed in nature. They can be found in soils, sewers, standing waters, streams, especially in areas where human activity takes place.
In general they are saprophytes but they can cause opportunistic infections in immunocompromised and occasionally in immunocompetent patients, which are difficult to treat due to high levels of resistance to antifungals11.
It is known that they cause trauma-associated infections in healthy individuals. In immunocompromised patients, the clinical manifestations range from local infection to lung colonization and severe invasive disease with mortality rates that may exceed 80%14.
Currently 11 species are acknowledged within the genus Scedosporium: Scedosporium aurantiacum, Scedosporium americanum, Scedosporium minutisporum, Scedosporium desertorum, Scedosporium cereisporum, and Scedosporium dehoogii, besides the S. apiospermum complex which comprizes Scedosporium angustum, Scedosporium apiospermum, Scedosporium boydii, Scedosporium ellipsoideum and Scedosporium fusoideum1,10,14.
Scedosporium boydii and S. apiospermum are the most frequently isolated species in our environment. They are among the most commonly recovered fungi from the respiratory secretions of patients with chronic lung diseases such as cystic fibrosis (CF). Lung colonization of patients with cystic fibrosis by Scedosporium spp. is well established and the rate ranges between 0 and 21%, being the most frequent genus after Aspergillus spp7,8.
It has been reported that the lungs, ears, and sinuses are the main sites of S. aurantiacum infection7. In Argentina, South America, two S. aurantiacum isolates recovered from the sputum of patients with CF have been reported. One isolate belonged to a patient from the city of Buenos Aires who had traveled to Egypt and Europe and the other one belonged to a patient from the province of Santa Fe, Argentina1. Moreover, in the United States of America, a single clinical case of S. aurantiacum infection in a patient with lung cancer, which was recovered from bronchoalveolar lavage fluid and lung biopsy has been reported12.
Below we present the isolation and identification of Scedosporium aurantiacum from a 4-year-old previously healthy female patient from San Antonio de Areco (Province of Buenos Aires, Argentina), who exhibited a penetrating trauma in her left eye caused by a kitchen utensil while she was playing with a friend. After being assessed by an ophthalmologist, the patient was referred to our institution. A surgery procedure was performed on the left eye for corneal trauma. The postoperative treatment included antibacterial eyedrops, intravenous treatment and prednisolone.
Ten days after the procedure, an ultrasound study was performed, confirming the presence of images compatible with inflammatory membranes and probable retinal detachment. Infectious endophthalmitis was taken into consideration.
As a result of the poor evolution of the clinical picture with a marked lack of improvement in the local and systemic treatments applied, endophthalmitis of probable mycotic origin was suspected. Vitrectomy of the left eye and intravitreal injections of amphotericin and vancomycin were the treatments performed.
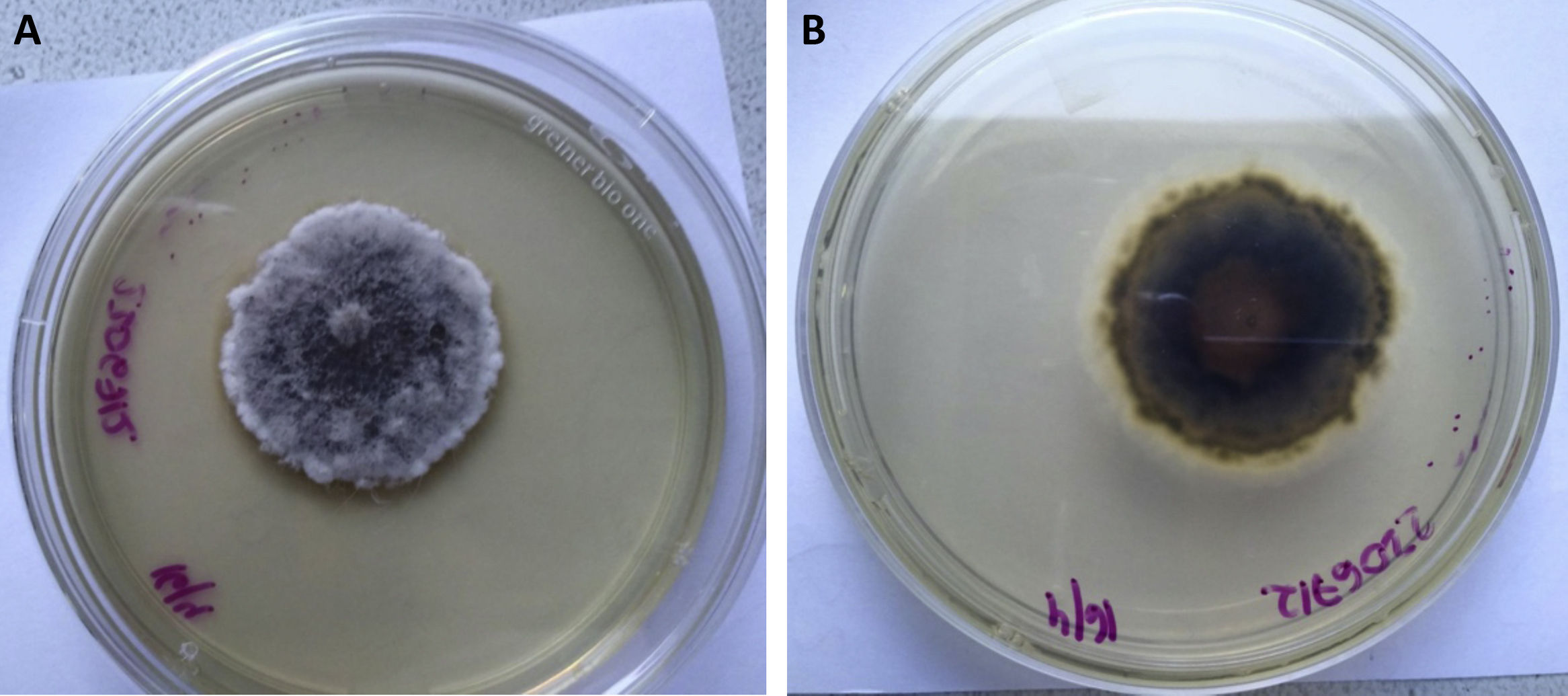
The vitreous humor was sent to our laboratory for mycological analysis. The fresh microscopic examination with potassium hydroxide (KOH) 40% was negative. The material was cultured in Sabouraud glucose agar (Britania S.A.) and incubated at 28°C. The development of a filamentous fungus that was subcultivated was obtained 48h later. After 15 days of incubation a cotton-like colony with a brown center developed. It was whitish towards the edges on the obverse and greyish brown on the reverse with a striking diffusible light yellow pigment (Figs. 1A and B).
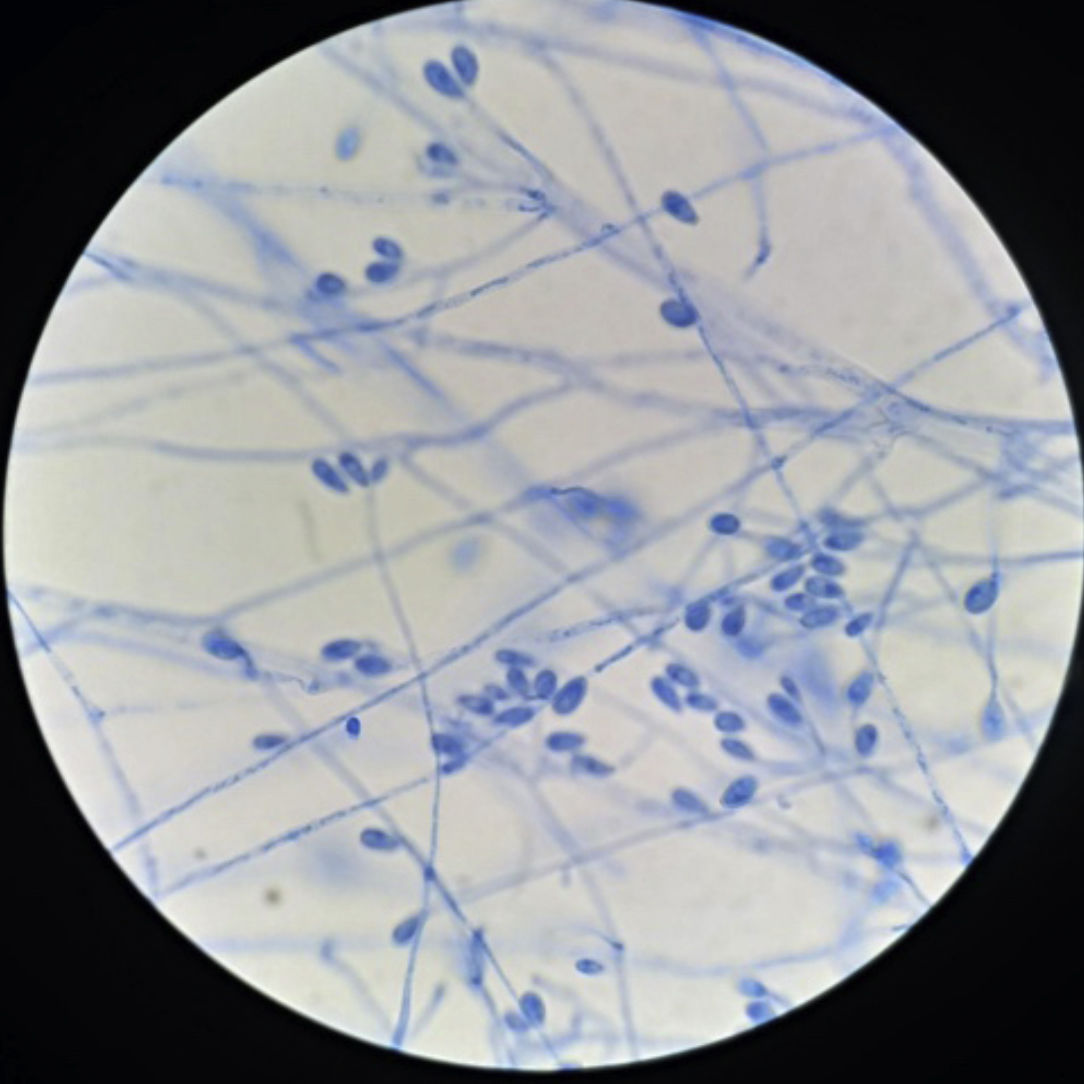
With regard to micromorphology, conidiogenic cells (annelids) emerge from undifferentiated hyphae, cylindrical to slightly flask-shaped, with single-celled conidia heads, smooth-walled, sub-hyaline, ovoids or sub-cylinders compatible in shape and size with the genus Scedosporium3 (Fig. 2), although these characteristics were not sufficient for identification at species level.
The isolates were sent to the Department of Mycology of INEI-ANLIS “Carlos G. Malbrán” to perform susceptibility tests and a molecular study. It was based on the sequence of the partial portion of the β-tubulin gene (BT2)4,5. The similarity of the sequence obtained was searched in the NCBI GenBank database using the BLAST algorithm [National Center for Biotechnology Information (NCBI) Internet homepage, [http://blast.ncbi.nlm.nih.gov/Blast.cgi] with automatically adjusted parameters.
A BLAST search using the BT2 sequence (584bp) identified our strain DMic 216397 (GenBank Accession Number OK258096) as Scedosporium aurantiacum, showing 100% similarity, 97% coverage and an E-value of 0.0 with the Scedosporium aurantiacum strain CBS 101725 (GenBank Accession Number GU1263891).
Susceptibility tests were performed using the Reference Method M38-3er Ed. of the Clinical and Laboratory Standards Institute (CLSI)2. The results of the minimum inhibitory concentrations (CIM) were the following: 8mg/l for amphotericin B, 0.5mg/l for itraconazole, 0.25mg/l for voriconazole, 0.5mg/l for posaconazole, and 2mg/l for isavuconazole.
To date, the CLSI has not yet established clinical or epidemiological cut-off points for the genus. The results obtained coincide with the data published by Heath et al.6, who studied 27 isolates of S. aurantiacum and obtained a MIC range of 2–16mg/l for amphotericin, 0.25–2mg/l for itraconazole, 0.03–0.5 for voriconazole and 0.125–1 for posaconazole.
Systemic antibiotic therapy with amphotericin B was initiated and after the confirmation of the etiological agent, it was decided to change to voriconazole, which led to a favorable clinical outcome9.
The geographical distribution of the species of the Scedosporium apiospermum complex is universal. S. aurantiacum was only reported in Australia and some European regions13. It has been reported that it colonizes the airways of patients mainly in these countries where there is a large number of patients with CF4. Another report describes the isolation of S. aurantiacum from a brain abscess in a 2011 tsunami survivor in Japan13.
In Australia, S. aurantiacum was recovered from more than 50% of the environmental isolates studied, while S. apiospermum and S. dehoogii are predominant in Austria and France, respectively.
In addition, S. aurantiacum is characteristic of agricultural areas in western France. S. boydii is the most frequent species (62%) in a French cohort, followed by S. apiospermum (24%), S. aurantiacum (10%), and S. minutisporum (4%).
In a study carried out in Germany in patients with CF, S. apiospermum was the most frequent species (49%) followed by S. boydii (29%), L. prolificans (12%), S. aurantiacum (5%) and S. minutisporum (5%).
Conversely, L. prolificans was the most frequent species isolated in patients with CF in northern Spain. In Australia, the most frequent species appears to be S. aurantiacum followed by L. prolificans and S. apiospermum.
Scedosporium species can cause keratitis in immunocompetent hosts usually after corneal trauma with a clinical presentation similar to other types of keratitis.
Endophthalmitis has also been described in immunocompetent individuals secondary to surgery, traumatic inoculation, intravenous drug addiction and contiguous extension from an adjacent site14.
Mycological diagnosis in these cases is classically based on direct microscopic examination with KOH from clinical samples and culture in appropriate media. On microscopic examination, however, the hyaline and branched hyphae are similar to those of the genera Aspergillus or Fusarium.
The identification of species of the genus Scedosporium is often difficult to carry out due to the similarity of their morphological characteristics. Currently, molecular or matrix-assisted laser identification by desorption/ionization time of flight mass spectrometry (MALDI-TOF/MS) is mandatory for reliable identification.
Nucleotide sequence-based analysis is the gold standard for the identification of these fungi. Sequencing of rDNA ITS regions allows the proper identification of the main species of Scedosporium/Lomentospora while the β-tubulin partial gene (BT2) is necessary to differentiate closely related species1.
Isolates of Scedosporium/Lomentospora present limited susceptibility to all current antifungal drugs. Species of Scedosporium are resistant to 5-flucytosine and amphotericin B, as well as to first-generation triazoles, fluconazole and itraconazole.
In addition, they have reduced susceptibility to equinocandins, in particular to caspofungin and anidulafungin. Voriconazole proved to be the most effective antifungal in the treatment against Scedosporium spp9,11 and the European guidelines recommend it as a first-line drug for treatment along with surgery, when it is possible to perform it15.
Although several cases of traumatic Scedosporium spp. infections have been documented in our region, no cases of S.aurantiacum have been reported so far1.
This is the first report in Argentina and the Americas of an isolation of S. aurantiacumin from a previously healthy patient with a traumatic infection without a history of previous trips abroad.
Conflict of interestThe authors declare that they have no conflicts of interest.