Squamocin belongs to a group of compounds called annonaceous acetogenins. They are secondary products of Annonaceae metabolism and can be isolated from Annona cherimolia seeds. This paper deals with the stimulation of biofilm formation of Bacillus atrophaeus CN4 by employing low squamocin concentrations to increase naphthalene degradation. Bacillus atrophaeus CN4, isolated from contaminated soil, has the ability to degrade naphthalene as the only source of carbon and energy. In the absence of additional carbon sources, the strain removed 69% of the initial concentration of naphthalene (approx. 0.2mmol/l) in the first 12h of incubation. The addition of squamocin in LB medium stimulated Bacillus atrophaeus CN4 biofilm formation and enhanced naphthalene removal. Squamocin (2.5μg/ml) does not affect planktonic growth and therefore, the observed increases are solely due to the stimulation of biofilm formation.
Squamocin pertenece a un grupo de compuestos llamados acetogeninas annonáceas (ACG). Las ACG son productos secundarios del metabolismo de plantas de la familia Annonaceae y se pueden aislar a partir de semillas de Annona cherimola. Este artículo trata de la estimulación de la formación de biofilm de Bacillus atrophaeus CN4 mediante el empleo de bajas concentraciones de squamocin para optimizar la degradación de naftaleno. B. atrophaeus CN4, aislado de suelo contaminado, tiene la capacidad de emplear naftaleno como única fuente de carbono y energía. En ausencia de fuentes de carbono adicionales, la cepa degradó el 69% de la concentración inicial de naftaleno (aprox. 0,2 mmol/l) en las primeras 12h de incubación. La adición de squamocin en medio LB estimula la formación de biofilm y la remoción naftaleno de B. atrophaeus CN4. Squamocin (2,5μg/ml) no afecta al crecimiento planctónico y, por lo tanto, los incrementos observados se deben únicamente a la estimulación de la formación de biofilm.
Biofilm formation is a very common survival strategy in the bacterial world. In fact, a large number of bacteria in nature have developed the ability to adopt a protected mode of growth. Such is the case of biofilm-forming bacteria. Protected by a polymeric matrix, bacteria within biofilms can survive the harmful action of various physical and chemical agents and may also establish more effective communication mechanisms4.
With this in mind, it is easier to understand the importance of stimulating biofilm formation of bacterial populations that are forced to grow in high environmental stress conditions, for instance, polycyclic aromatic hydrocarbons (PAHs) degrading strains15–17.
Cartagena et al.2 studied a series of compounds with lactonic groups in their structures that stimulate or inhibit Pseudomonas aeruginosa biofilm formation. Among them, squamocin, even in a 0.25μg/ml concentration, was able to stimulate biofilm formation significantly. They also reported that 2.5μg/ml may cause an even higher stimulation.
Annonaceous acetogenins (ACG) such as squamocin are the secondary products of Annonaceae family plant metabolism. Several members of this plant family are common and widespread in South America. ACG constitute a family of compounds whose general structure consists of a long hydrocarbon chain containing different oxygenated moieties. Particularly, squamocin has 37 carbon atoms, 2 tetrahydrofuranic rings, a terminal lactonic group and 3 hydroxylic moieties distributed along its carbon chain7. Various biological activities of squamocin have been reported, most of them related to the inhibition of cellular respiration and its insecticidal activity19 and by its ability to stimulate biofilm production and to increase the rate of naphthalene degradation by nonspecific association with hydrophobic cell materials in a PAH-degrading strain called Pseudomonas plecoglossicida J2617. Furthermore, Parellada et al.15 described the effect of different squamocin concentrations (2.5, 25, 50 and 100μg/ml) on the biofilm formation of Pseudomonas plecoglossicida J26, a naphthalene-degrading strain. They observed that 2.5μg/ml squamocin concentrations stimulated biofilm production although the increase in concentration did not produce higher stimulations. Hence, the 2.5μg/ml concentration was chosen for the assays with Bacillus atrophaeus CN4 presented in this paper.
PAHs are highly hydrophobic and poorly biodegradable compounds consisting of fused benzene rings in different configurations. PAH volatility, carcinogenicity and capability to bioaccumulate vary depending on their molecular weight. Among them, naphthalene is ubiquitous in the home environment and several authors have proposed it as a useful model for comprehending the behavior of other PAHs9,11,20.
The strain employed for the present study (called CN4 and subsequently identified as Bacillus atrophaeus) was previously isolated from oil-contaminated soil using a PAH-enrichment technique. B. atrophaeus is a gram-positive, aerobic, endospore-forming, rod-shaped bacterium whose description is almost identical to that of Bacillus subtilis except for the production of a pigment on media containing an organic nitrogen source13. Some strains of B. atrophaeus have been reported for their ability to produce biosurfactants, i.e. B. atrophaeus ATCC 93725. Others have shown antifungal activity (B. atrophaeus CAB-1)22.
The overall purpose of the research presented in this article was to unveil the effects of squamocin on naphthalene degradation mediated by a gram-positive bacterium. To this end, it is necessary to first demonstrate the ability of the strains to use naphthalene as the sole carbon source. This study seeks to establish a relationship between naphthalene degradation and biofilm formation.
Materials and methodsSquamocin isolation, purification and characterizationThe procedures for the treatment of plant material, extraction, purification and structural elucidation of squamocin have been widely reported7,15,16. Here we present a summary of these procedures.
Squamocin was isolated from Annona cherimolia seeds obtained from “chirimoya” fruits. Dried and powdered seeds were extracted with methanol. Solvent was then evaporated and the extract partitioned first in a mixture of chloroform and H2O (1:1) and then with hexane:methanol (1:1). Squamocin was contained in this methanolic sub-extract. Water solubility of ACGs was negligible. All procedures were monitored using TLC with Kedde's reagent. The next step was squamocin isolation and purification from the methanolic sub-extract, which was accomplished by column chromatography and further purification by RP-HPLC of the obtained fractions. Structural elucidation of squamocin was assessed by spectroscopic and spectrometric techniques (IR, 1H-NMR, 13C-NMR, and MS) by comparison with data reported by Fujimoto et al.7.
Squamocin stock solutions 1mg/ml and 100μg/ml in ethanol (96%) were prepared. These solutions were used in all the assays performed in this work.
Chemicals, bacterial strain and growth conditionsThe naphthalene (>99% purity) used in the degradation experiments was purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). All chemicals used in this study were analytical grade and acquired from standard manufacturers.
B. atrophaeus CN4 was isolated from 15-day enrichment cultures of oil-contaminated soil in Argentina according to their capability to grow as pure cultures in minimal medium (MM) added with 1mmol/l naphthalene as carbon and energy source. The strain was grown at 28°C in Luria-Bertani (LB) agar medium in contact with naphthalene vapors to promote a bacterial resistance phenotype.
LB and MM media were used during microbiological determinations. LB contains (in g/l) 5 meat peptone; 2.5 yeast extract and 20 NaCl. CFU/ml were determined on solid LB agar medium containing agar (1.5% w/v). MM is composed as follows: 24.7g/l NaCl, 6.3g/l MgSO4·7H2O, 4.6g/l MgCl2·H2O, 2g/l NH4Cl, 0.7g/l KCl, 0.6g/l CaCl2, 200mg/l NaHCO3, 100mg/l K2HPO4, 20mg/l FeSO4·7 H2O and bidistilled water.
For bacterial identification, total DNA from a pure culture was extracted by the CTAB method6. DNA quality and quantity from the several sources were checked in 0.8%. Universal primers 8f and 1492r (corresponding to position 8–27 and 1541–1522, respectively in the 16S rDNA sequence of Escherichia coli) were used to amplify the 16S rDNA by PCR, as previously described21. The amplicon was sequenced by Macrogen Inc., Seoul, Korea.
Preparation of B. atrophaeus CN4 inoculaIndividual colonies were picked, inoculated in LB broth and incubated for 24h (28°C, standing incubation). A 10% (v/v) aliquot of this culture was transferred to inoculate fresh LB broth, but this time only for 14h, which was employed as B. atrophaeus CN4 inoculum. Another aliquot of the inoculum was appropriately diluted and plated on solid LB agar medium for determination of CFU/ml. The inoculum of B. atrophaeus CN4 had an OD600=0.645 corresponding to a plate count of approx. 7.53×1012CFU/ml. A similar inoculum was used in the assays described in sections ‘Squamocin effects on biofilm formation, naphthalene degradation and cell growth’ and ‘Biosurfactant/bioemulsifier production’.
An inoculum prepared as described in the preceding paragraph was centrifuged and the pellet was washed three times with sterile PBS buffer and resuspended in an equal volume of sterile MM. This cell suspension was used as the inoculum in the naphthalene removal assay in the absence of other carbon sources described in section ‘Naphthalene removal in the absence of other carbon sources’.
Naphthalene removal in the absence of other carbon sourcesThree series of screw cap tubes with MM culture broth were prepared to determine B. atrophaeus CN4 capacity to remove dissolved or suspended naphthalene in an aqueous medium free from any other carbon source. The first series contained 0.2mmol/l naphthalene (stock solutions 100mmol/l in ethanol) and was inoculated in a 10% (v/v) with a B. atrophaeus CN4 cell suspension in MM medium. The second series did not contain naphthalene, but was inoculated in the same way as the first one. The third series (sterile series) only had MM medium with naphthalene in the same concentration as in the first one. The final volume was adjusted to 3ml in all incubations; with ethanol in the series without naphthalene and with MM medium in the sterile series.
The tubes were incubated in the dark at 28°C without shaking. Cellular growth and residual naphthalene concentration were measured every 4h for 12h. The former was quantified by plate count while the latter was assessed by RP-HPLC and a calibration curve (see following section).
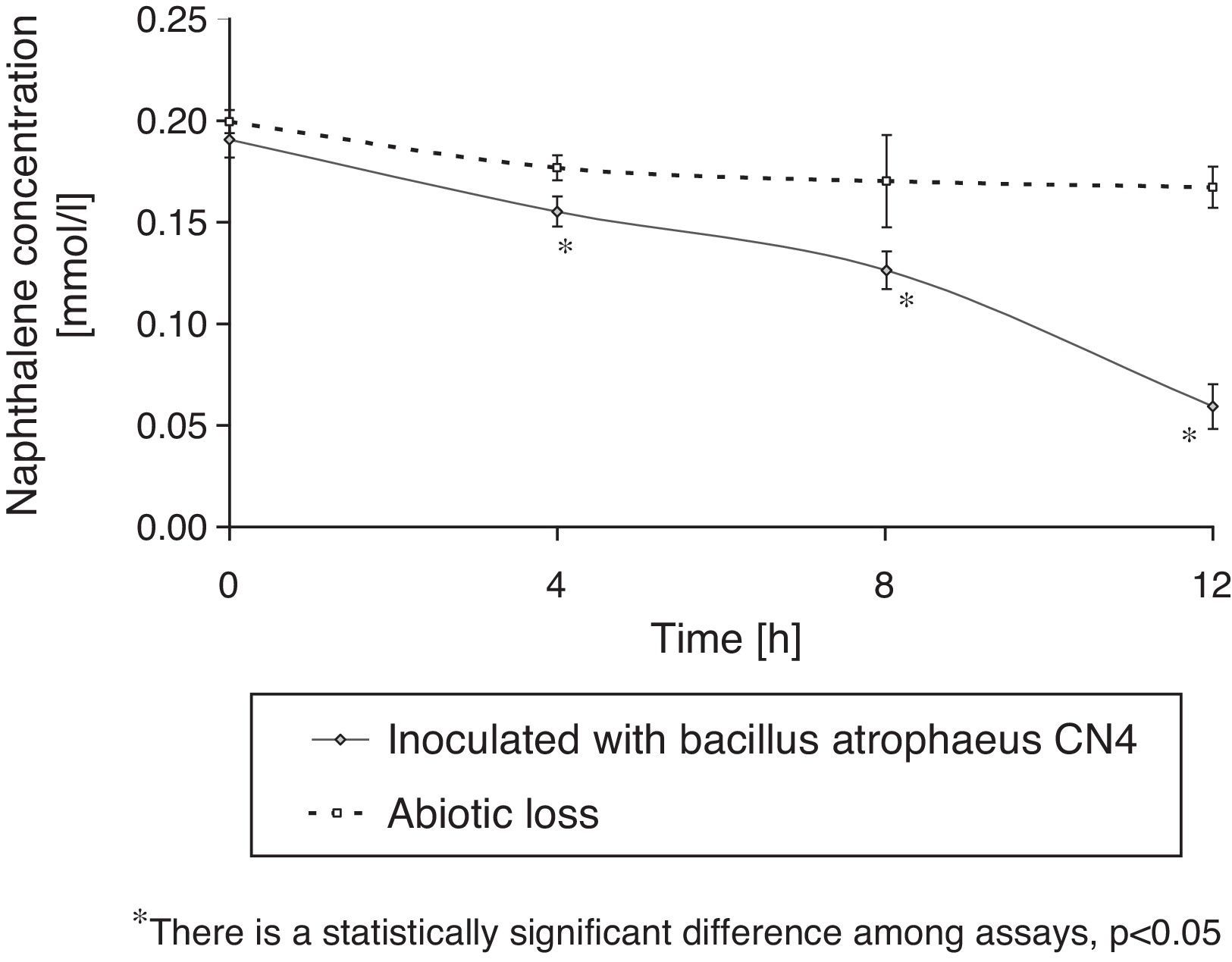
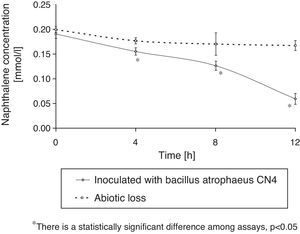
The concentration measurement of residual naphthalene in the sterile series was performed to evaluate abiotic losses. Furthermore, samples of the tubes without naphthalene were chromatographed to discard the possibility that some other compound might produce a peak with a similar retention time as that of naphthalene. Hydrocarbon removal was quantified considering the PAH loss occurred in the sterile control series. Cell growth measurements in the series without naphthalene were used to eliminate the possibility that Bacillus atropheus CN4 might use some compound of the MM medium as energy source.
Determination of residual naphthalene concentrationNaphthalene quantification was performed as follows. The tubes were vortexed for 30s and 500μl aliquots were transferred from the tubes to a 5ml volumetric flask and brought to volume with bidistilled methanol in order to stop growth and dissolve residual naphthalene. At this stage, the naphthalene that could be chemically adsorbed to cell material was transferred to the organic phase. Next, prepared samples were filtered through 0.22μm nylon filters (Microclar, Argentina) to separate cells and stored at −20°C until analysis. Naphthalene quantification was carried out by RP-HPLC (Perkin Elmer 200 series chromatograph; UV/visible spectrophotometric detector fixed at λ [nm]=276; 600 series analogical interphase). A C8 column (25×1cm i.d., 10μm particle size) was employed and a methanol–water (9:1) solution was used as mobile phase (F=1.5ml/min). Peak areas were calculated with a peak analyzer (Total Chrom analyser) and naphthalene concentrations were estimated by a calibration curve and expressed as mmol/l16.
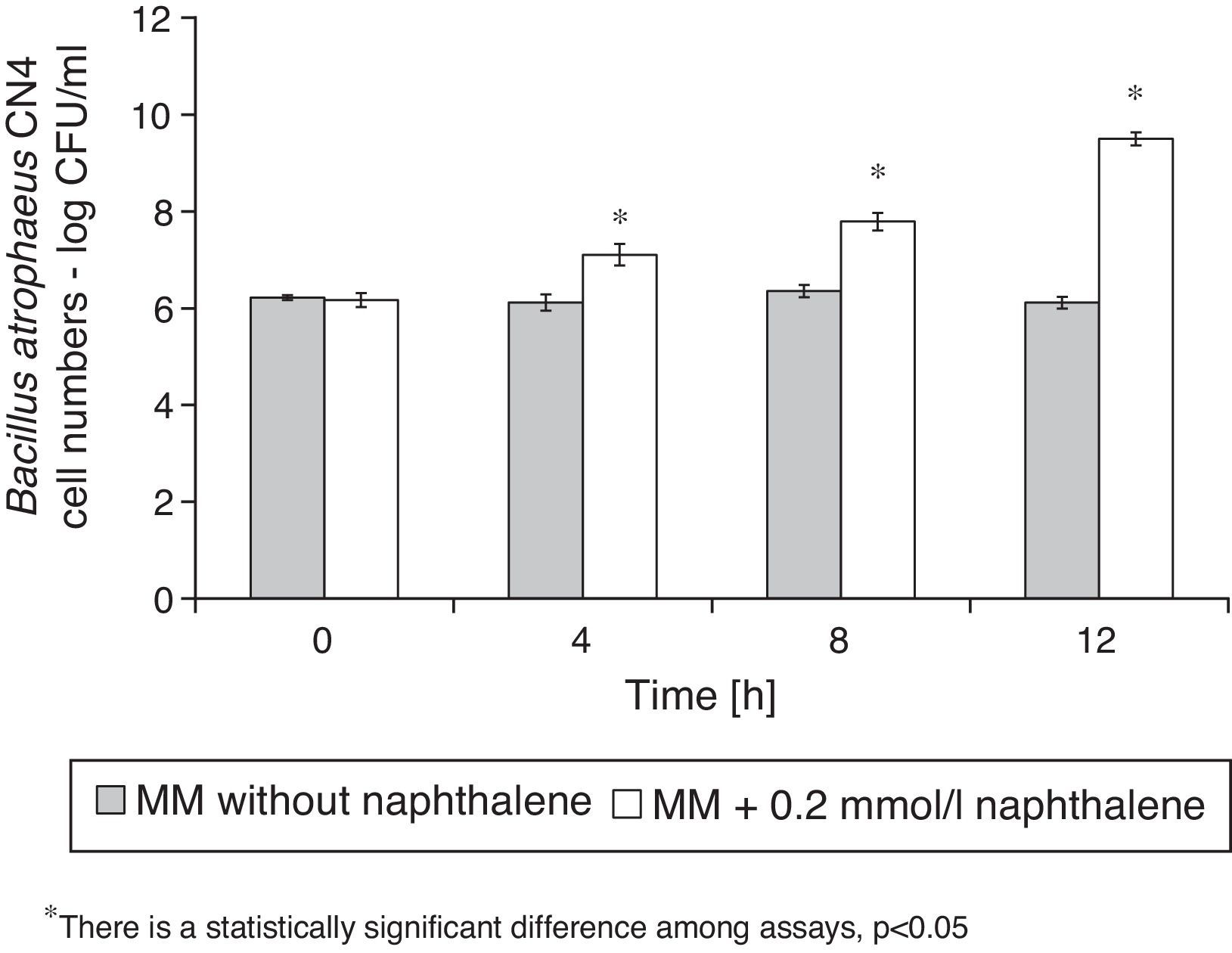
Squamocin effects on biofilm formation, naphthalene degradation and cell growthQuantification of biofilm formation on glass beadsNaphthalene and biofilm were quantified simultaneously. B. atrophaeus CN4 biofilm biomass quantification in the presence of squamocin was performed by crystal violet (CV) staining14. Measurements were performed at 0, 4, 8 and 12h of standing incubation. A series of 8ml screw cap glass tubes, 10 for each time, 5 per treatment (in the presence or absence of squamocin) were prepared. Tubes contained: 3g of 4mm diameter glass beads to serve as an abiotic surface to support biofilm formation, 3500μl of LB medium, 10μl of a squamocin ethanolic solution (1mg/ml) (final concentration 2.5μg/ml; equivalent quantities of EtOH were placed in control assays) and 40μl of a naphthalene ethanolic solution (100mmol/l) (final concentration approx. 1mmol/l). The distance between the beads and the liquid–air interface is very short so oxygen may adequately diffuse. Tubes were later inoculated with 400μl (10%, v/v) of a 14h LB cell culture (OD600nm=0.6; 1012CFU/ml approx.) of B. atrophaeus CN4. Non-inoculated tubes were used as abiotic control. Once inoculated, the glass tubes were incubated without shaking at 28°C for 0, 4, 8 and 12h and aliquots of 500μl were taken from the corresponding tubes for naphthalene quantification assays (see section ‘Naphthalene degradation in the presence of squamocin’). Then, 100μl of crystal violet solution (1%) were added to each tube and incubated at room temperature for 15min. Next, beads were thoroughly rinsed in the tube with water to remove non-adherent cells and excess dye. The dyed biomass was redissolved with the addition of 4000μl of 96% ethanol. Absorbance of the resulting solution was measured at 560nm in a spectrophotometer (Shimadzu A160 UV-Vis). The measurements taken at t=0 are representative of any tinction that might occur before biofilm formation begins on any support surface or by non-specific adhesion to any component of the culture medium.
Control represents 100% of biofilm production and 0% of stimulation of biofilm formation. This correlates with the production of biofilm in the presence of ethanol, the solvent employed in the squamocin solution. The changes in biofilm production may be attributed to squamocin since control also contains ethanol.
A nutritive culture medium such as LB was chosen for these assays to ensure good cell growth, especially at the beginning, because of the large amounts of naphthalene employed.
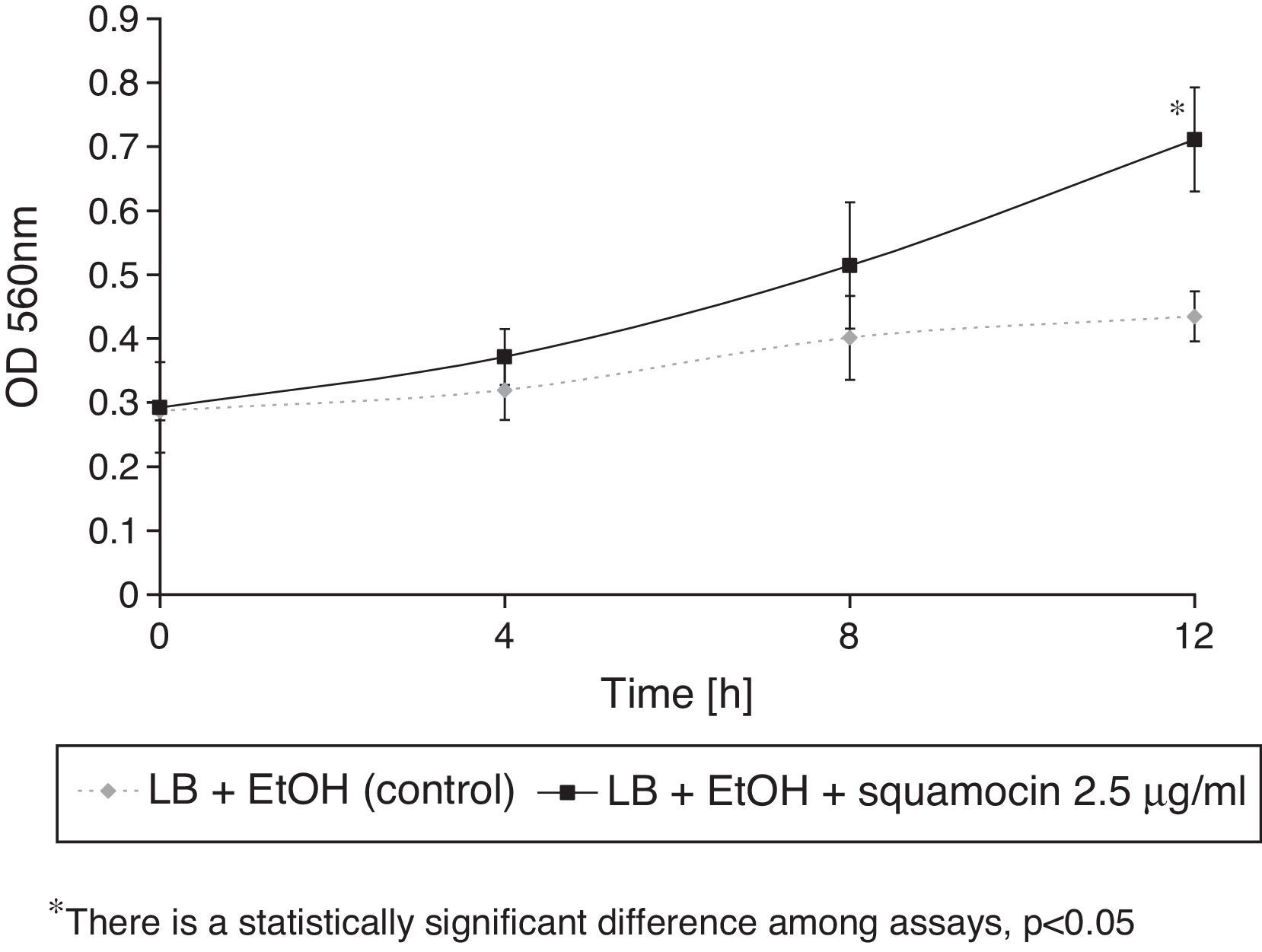
Naphthalene degradation in the presence of squamocinIn this assay, the concentration of naphthalene was determined in batch cultures containing glass beads as support for biofilm formation. Naphthalene degradation in these systems occurred due to the combined action of planktonic and biofilm cells. The quantification of naphthalene degradation was done as described above.
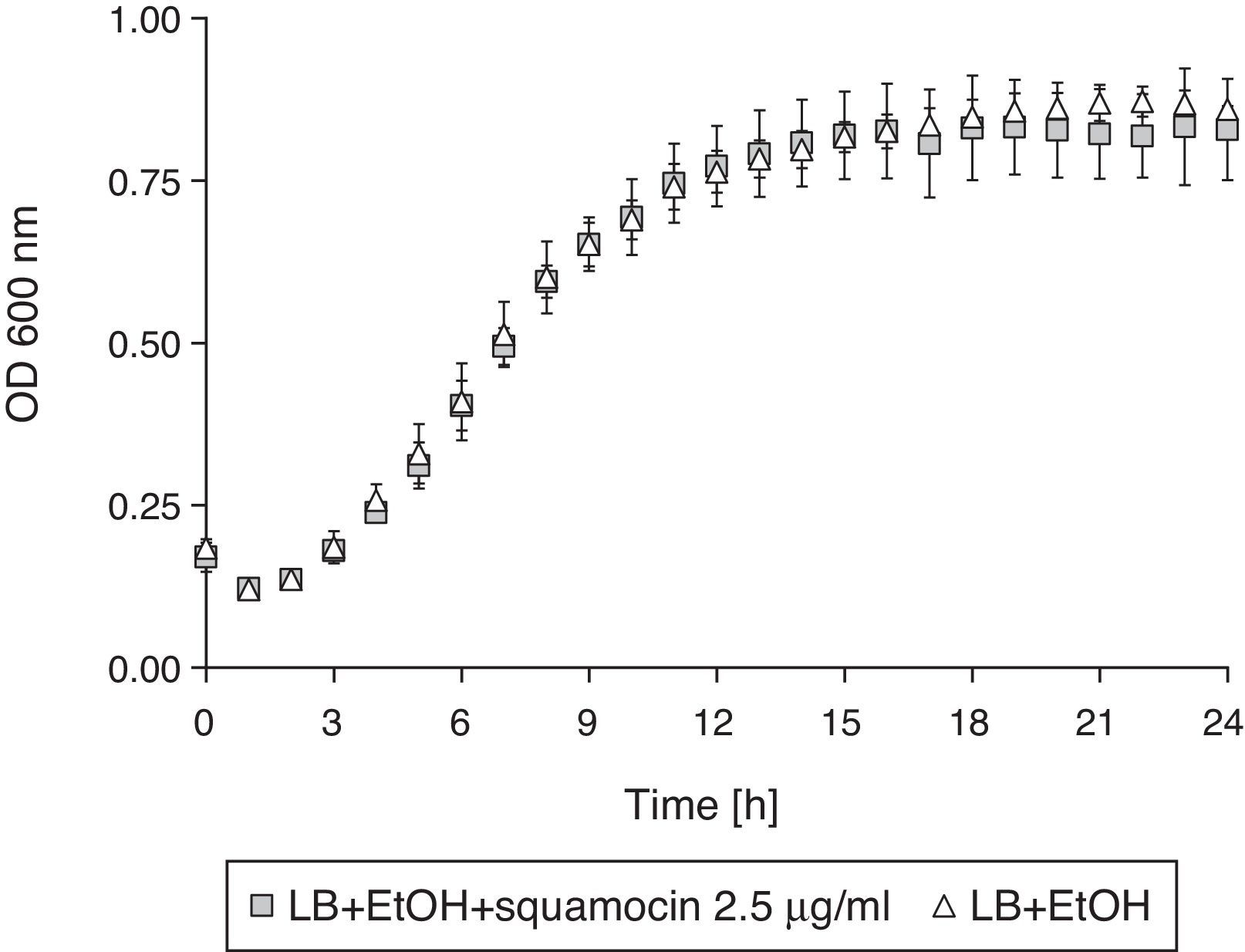
Squamocin effect on B. atrophaeus CN4 cell growthThis study was conducted in a 96 well-microtiter plate to unveil the effects of squamocin 2.5μg/ml on B. atrophaeus CN4 growth. LB broth in the wells was supplemented with a squamocin solution (5μl of a 100μg/ml ethanol stock solution, final concentration 2.5μg/ml, assay A). Since ethanol was used as squamocin solvent, it was added in equivalent amounts to the control assays so that the ethanol concentration was the same (2.5%) in both cases (assay B). Then, the wells were inoculated with 10% (v/v) of a 14h cell culture of B. atrophaeus CN4 obtained according to section ‘preparation of B. atrophaeus CN4 inocula’. Abiotic controls were also prepared (inoculation was substituted by an equivalent volume of LB culture medium). Comparison between assays A and B revealed the effects of squamocin on bacterial growth. Final volume in the 96 well-microtiter plate was set at 200μl. Then OD600nm was measured every hour in a spectrophotometer (Biotek-Power Wave XS2 with GEN5 data analysis software) during a 24h (28°C) incubation period. Assays were conducted in octuplicate.
Biosurfactant/bioemulsifier productionBioavailability of poorly water soluble compounds such as naphthalene can be increased by the presence of compounds that reduce surface tension or stabilize emulsions8. Some authors have reported that some strains of B. atrophaeus can produce biosurfactants5.
The ability of the strain to form compounds capable of reducing surface tension was analyzed by the drop collapse method. Five μl of mineral oil were placed in each well of the lid of a 96-well-microplate. The surfaces thus created were allowed to stabilize for 1h at room temperature. Next, 12μl of either a B. atrophaeus CN4 cell culture supernatant, a B. atrophaeus CN4 cell culture supernatant grown in the presence of 2.5μg/ml squamocin (12, 24 or 48h of incubation at 28°C), LB medium (with or without squamocin) or an aqueous solution of Tween 80 (positive control) were added in quintuplicate. Once the phases came into contact, the shape of the drop on the oil surface was inspected. Biosurfactant-producing cultures yielded flat drops within 1min12.
For emulsification index determination (EI24), four ml of a supernatant of a B. atrophaeus CN4 cell culture grown in the presence or absence of squamocin (12, 24 and 48h of incubation at 28°C in LB, pH=7.0) and 6ml of hexane or kerosene were vortexed at high speed (2min) to form an emulsion. Cell culture supernatants were obtained by centrifugation at 8000rpm for 15min at 25°C and then sterilized with 0.22μm Millipore filters. Control assays were prepared with LB medium and LB medium with squamocin to establish their possible emulsioning capacity. The emulsification index (EI 24) was calculated as the height of the emulsion layer, divided by the total height. This result was multiplied by 100 to obtain a measure in percentage. The measurements were made after 24h at room temperature3.
Statistical analysisThe differences in absorbance mean values (Abs±SD) were evaluated by variance analysis (ANOVA). The Tukey's test was used for all pair wise multiple comparisons of groups. In all statistical analyses, values of p>0.05 were considered not significant (Statistix 7.1, 2000).
ResultsBacterial identificationAccording to the databank of 16S rRNA sequences of the EzTaxon-e server10 (http://eztaxon-e.ezbiocloud.net/) the strain was partially identified as Bacillus sp., closely related to B. atrophaeus (99.9% identity with B. atrophaeus JCM 9070(T)). Biochemical characterization of Bacillus sp. CN4 was performed and it agreed with that reported by Nakamura for B. atrophaeus13.
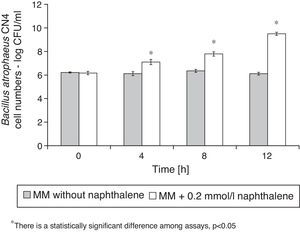
Naphthalene removalB. atrophaeus CN4 was able to grow with naphthalene suspended and/or dissolved in an aqueous medium as the sole carbon and energy source (Fig. 1). No evidence of bacterial growth was observed in the sterile controls. After 12h of incubation, naphthalene concentration was reduced by 69% in the assays containing cells and by 16% in the abiotic controls (Fig. 2). The decrease in naphthalene concentration in the abiotic controls was attributed to volatilization losses. The chromatograms of the samples from tubes without naphthalene did not contain any peaks with similar retention time to that of naphthalene.
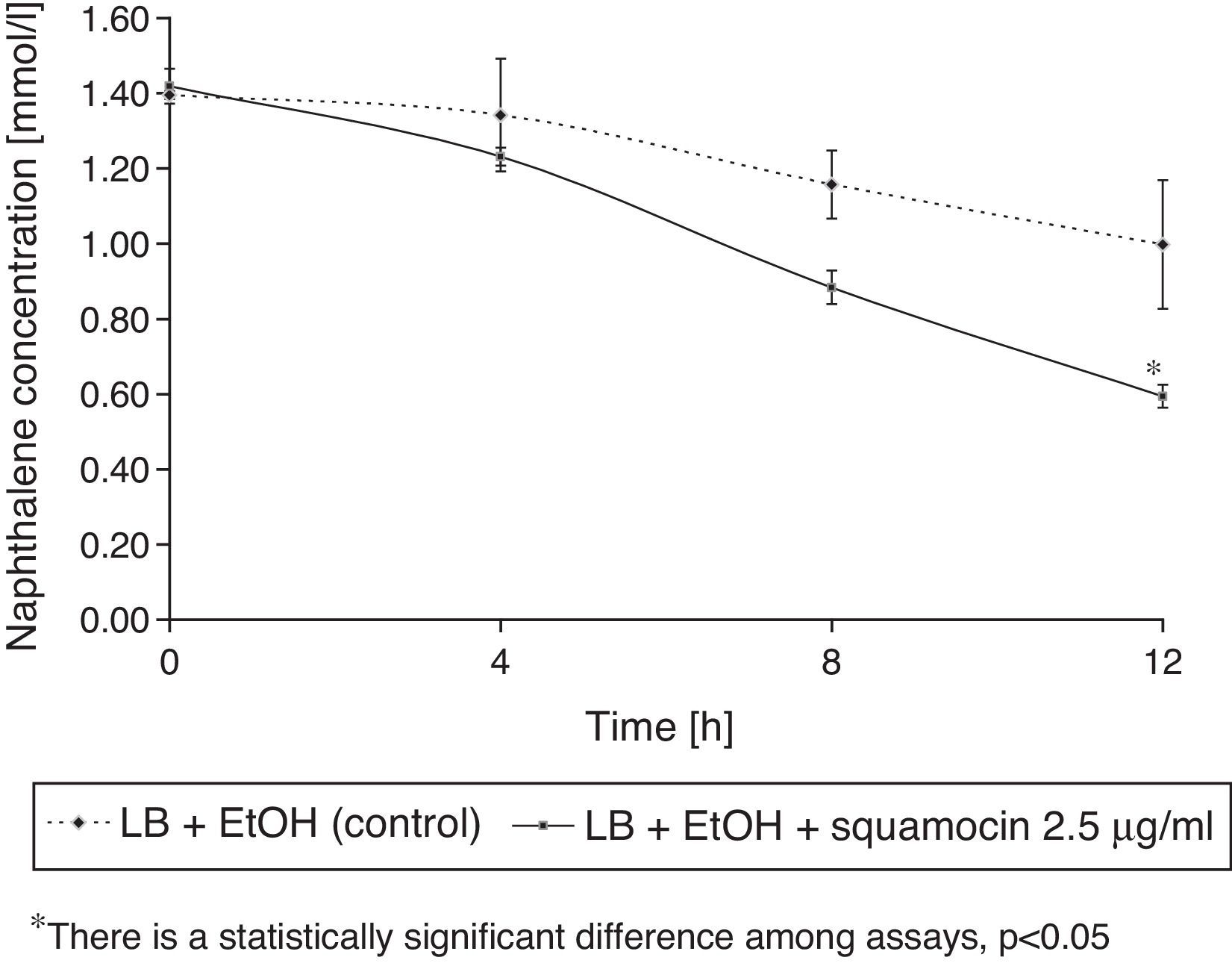
B. atrophaeus CN4 biofilm formation on the surface of glass beads in the presence or absence of 2.5μg/ml squamocin is presented in Figure 3. B. atrophaeus CN4 can form biofilm in the presence or absence of squamocin. After 12h, the beads exposed to the squamocin medium contained more adsorbed cells (OD560nm was 64% higher) than the beads exposed to media without squamocin suggesting stimulation of biofilm formation.
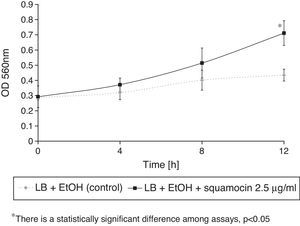
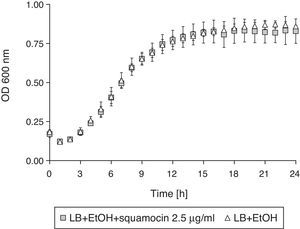
Squamocin effect on B. atrophaeus CN4 cell growthThe addition of squamocin to the medium had no statistically significant effect on the growth of B. atrophaeus CN4 in the planktonic phase (Fig. 4).
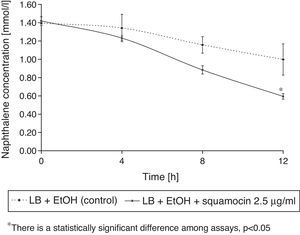
Squamocin effects on naphthalene removalB. atrophaeus CN4 had the ability to remove naphthalene (dissolved and/or suspended in LB broth). A sustained decrease of naphthalene concentration was observed over time. After 12h of incubation, naphthalene concentration was reduced by 58% in the assays containing squamocin and by 25% in the assays without squamocin (Fig. 5). Naphthalene concentrations in sterile controls did not statistically differ (p>0.05) after 12h.
Biosurfactant and bioemulsifier productionNeither drop collapse method nor EI24 determination gave positive results for the production of surface active/emulsifying agents. No stable emulsions could be formed with hexane or kerosene. No flat drops could be observed in the presence of any of the B. atrophaeus CN4 cell culture supernatants. The presence of squamocin did not alter any of these behaviors.
DiscussionSquamocin stimulates B. atrophaeus CN4 biofilm formation and finally increases its naphthalene degradation capabilities. Although being gram negative organisms, a similar behavior has been observed for strains Pseudomonas aeruginosa PA1002 and Pseudomonas plecoglossicida J2615, which suggests that the effect of squamocin does not necessarily depend on the bacterial cell wall structure.
Regarding the effect of plant compounds on biofilm stimulation, Beauregard et al.1, found that plant polysaccharides can enhance Bacillus subtilis biofilm formation; however, in this case the authors suggested that these plant polysaccharides can serve as a carbon source used to produce the extracellular matrix. Nevertheless, the use of squamocin as an additional source of carbon may not be the cause of the increments observed in this study because its concentration is very low and in previous studies it was discovered that it remained without changes associated to cell material17.
It is general understanding that biofilms are a dormant and not active stage of cells, resulting from their high tolerance against environmental stresses. In order to associate biofilm formation and better naphthalene degrading activity of the cells, Shimada et al.18 proposed that the increase in naphthalene degradation of a Pseudomonas strain was not due to biofilm cells, but to superactivated cells that detached from it. However, it could be inferred that biofilm formation is prior to the release of these cells and thus the squamocin effect would be prior to this phenomenon.
This led us to conclude that the effect of this ACG is circumscribed to the process of biofilm formation. Results suggest that this ACG may also stimulate biofilm formation in a gram-positive bacterium, which contributes to the knowledge of the activity of these compounds.
das Neves et al.,5 who worked on another strain of B. atrophaeus, have reported on its biosurfactant production and culture conditions that might improve it. Hence, we do not discard the possibility that the strain may form biosurfactants in other culture conditions.
The results of biosurfactant and/or bioemulsifier assays and the non-significant effects of 2.5μg/ml of squamocin on B. atrophaeus CN4 cell growth lead us to conclude that the increase in naphthalene degradation is due to the stimulation of biofilm formation.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), National Research Council of Argentina (CONICET) and Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT - Proyect code PIUNT D552). EAP is recipient of a fellowship from CONICET.