A cross-sectional study was carried out on cats attending the Small Animal Hospital at the Faculty of Veterinary Sciences of the University of Buenos Aires to assess the prevalence and associated risk factors of Feline immunodeficiency virus (FIV) and Feline leukemia virus (FeLV) in the city of Buenos Aires, Argentina. Blood samples from 255 cats with symptoms compatible with FIV or FeLV infection, collected between 2009 and 2013 were analyzed by serology (immunochromatography, IA) and by hemi-nested PCR (n-PCR). The IA and n-PCR assays showed similar percentages of positivity for FIV while the n-PCR test was more sensitive for FeLV. Differences between the diagnostic tests and their choice according to the age of the animal are discussed. The clinical histories of ninety of the 255 cats showed blood profiles similar to others previously reported and revealed a higher risk of infection in male adult cats with outdoor access.
Para determinar la prevalencia en la ciudad de Buenos Aires del virus de la inmunodeficiencia felina (FIV) y del virus de la leucemia felina (FeLV), y analizar los factores de riesgo que pudieran estar asociados a ellos, se realizó un estudio transversal en gatos atendidos en el Hospital de Pequeños Animales de la Facultad de Ciencias Veterinarias de la Universidad de Buenos Aires. Se analizaron por serología (inmunocromatografía [IA]) y por hemi-nested PCR (n-PCR) 255 muestras de sangre de gatos con síntomas compatibles con infección por FIV o FeLV. La IA y la n-PCR revelaron porcentajes similares de animales positivos para FIV, mientras que para FeLV el diagnóstico por n-PCR resultó más sensible. Se discuten las diferencias halladas entre los métodos diagnósticos y su elección según la edad del animal. Las historias clínicas de 90 de los 255 gatos mostraron perfiles sanguíneos similares a otros ya reportados y revelaron el mayor riesgo de infección con ambos virus en machos adultos con acceso al exterior.
Feline immunodeficiency virus (FIV) and Feline leukemia virus (FeLV) are retroviruses that can infect both domestic and wild cats3,7. FIV, which is a lentivirus, produces a progressive deterioration of the immune system of the animal leading to prominent secondary infections, in a very similar fashion to the Human immunodeficiency virus type 1. FeLV, which is a gammaretrovirus, is associated with proliferative, degenerative and oncogenic diseases in erythroid, myeloid and lymphoid cell lineages. Diagnosis of these infections can be performed by different methods. Detection of antibodies against structural proteins is the choice for FIV diagnosis using immunochromatography-based kits. The same kits are designed to detect FeLV antigens8. Alternatively, polymerase chain reaction (PCR) tests targeting conserved sequences such as those present within the pol region can also be used2,6.
Reports of the prevalence of these agents around the world are numerous. However, in Latin America information is scarce. There have been few reports on the FIV situation in Argentina, where specific antigens and antibodies have been detected in cats since 199413. In order to investigate the prevalence of these infections in domestic cats in the city of Buenos Aires we tested a total of 255 cats with clinical symptoms compatible with FIV or FeLV infection in the period 2009–2013. These animals were treated in the Small Animal Hospital at the Faculty of Veterinary Sciences (University of Buenos Aires) and they resided in the city of Buenos Aires and their surrounding areas. Testing, which is ordered on a regular basis by clinicians in suspected cases, was performed with a commercial immunochromatographic assay (IA) and by a nested PCR (n-PCR). These tests were performed at the Virology Department of the same faculty. Only samples from adult cats (>2 years old) were included in this study. Cats under two years old were not tested due to the possible interference of maternal antibodies. Clinical and laboratory data were also examined and compared between infected and uninfected cats as well as with data from other countries.
Blood samples (2ml) were collected in tubes containing 1% EDTA and processed during the following 12h. They were divided into two aliquots; one was centrifuged at 2000rpm for 15min and the collected serum was tested for antibodies against FIV (gp40) and antigens for FeLV (p27 group specific) with a commercial immunochromatographic assay (Speed DUO FeLV-FIV, BIO VETO Test®, BVT Virbac). The other aliquot was centrifuged with 1ml of Histopaque®-1077 (Sigma-Aldrich), at 400×g for 15min. The opaque interface containing the mononuclear cell fraction was carefully aspirated. Finally, proviral DNA extraction was performed with High Pure Viral Nucleic Acid Kit® (Roche).
A n-PCR was performed for FeLV following a previously described procedure that amplifies a 166 base-pair (bp) fragment from the ul3 gene9. For FIV, a hemi-nested PCR protocol that amplifies a 338 bp fragment from p24 of the gag gene was adapted from previous studies5.
Both PCR were carried out in a total volume of 50μl, containing 0.2–1μg of genomic DNA, 1.5mM MgCl2, 1mM each of the four deoxynucleotide triphosphates, 50pmol of each primer for FIV (for both rounds) and 3.5pmol and 15pmol for FeLV (first and second round, respectively), 1U of GoTaq polymerase and 5μl of 10X GoTaq buffer (Promega).
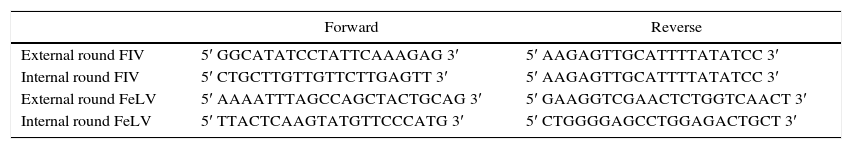
Negative and positive controls (DNA from infected cats) were included in each assay. Cycling conditions for both rounds of the FIV hemi-nested PCR were: one cycle at 94°C for 2min, 35 cycles at 94°C for 30s, 52°C for 30s and elongation at 72°C for 30s, and finally, a 10-min elongation stage at 72°C. For FeLV a previously described n-PCR method proposed by Hofmann-Lehmann et al.9 was followed. Primers for both amplifications are detailed in Table 1. Complete clinical histories were only available for 90 cats. The group analyzed was composed by female and male cats and was divided into two categories: juvenile cats (two to five years old) and elderly cats (more than five years old). To determine the association between positivity and the clinical variables an χ2 test and a two-tailed Fisher corrected test were performed using the Epi Info™ 7 (7.1.5) software. The odds ratio (OR) was calculated using the same program. The level of significance was p > or =0.05.
Primers used for FIV and FeLV n-PCRs. The reverse primer for FIV amplification was the same for both rounds
| Forward | Reverse | |
|---|---|---|
| External round FIV | 5′ GGCATATCCTATTCAAAGAG 3′ | 5′ AAGAGTTGCATTTTATATCC 3′ |
| Internal round FIV | 5′ CTGCTTGTTGTTCTTGAGTT 3′ | 5′ AAGAGTTGCATTTTATATCC 3′ |
| External round FeLV | 5′ AAAATTTAGCCAGCTACTGCAG 3′ | 5′ GAAGGTCGAACTCTGGTCAACT 3′ |
| Internal round FeLV | 5′ TTACTCAAGTATGTTCCCATG 3′ | 5′ CTGGGGAGCCTGGAGACTGCT 3′ |
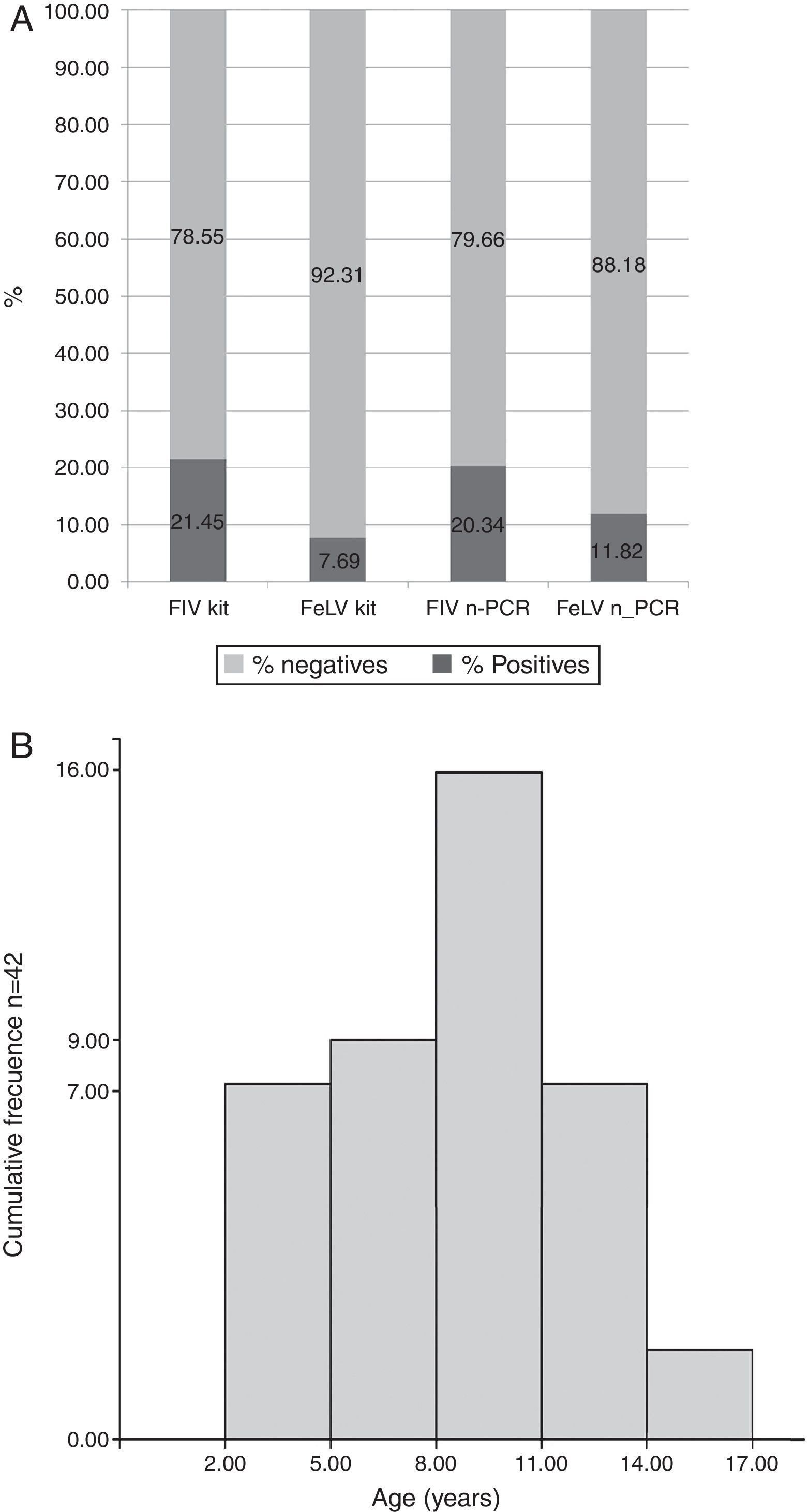
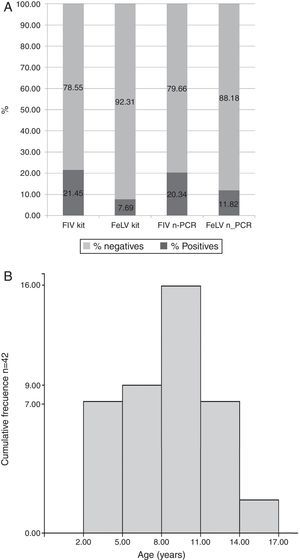
Results indicated that of the total of 255 samples, the overall prevalence by IA was 21.45% (55/255) for FIV and 7.69% (14/255) for FeLV, while 20.34% (52/255) for FIV and 11.82% (30/255) for FeLV were observed by n-PCR (Fig. 1A). Only 3 samples of the 255 (0.9%) were positive for both viruses by n-PCR. Age distribution of the infected animals showed that cats between eight and eleven years old had the highest prevalence (Fig. 1B).
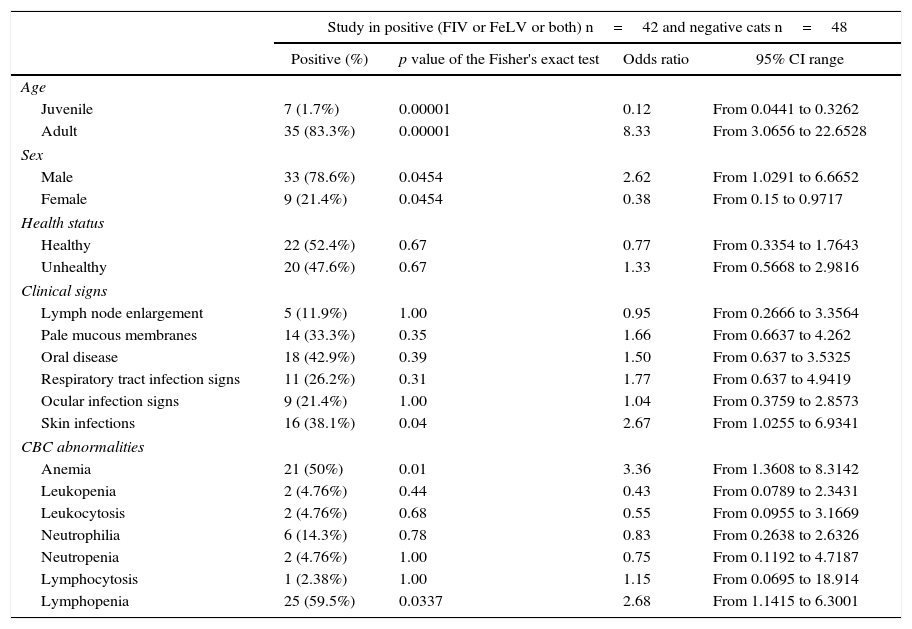
The clinical histories provided information about the clinical signs, laboratory diagnosis and anamnesis data at the moment of the veterinary consultation. From this information, it was found that 78.6% of FIV or FeLV positive cats were male while only 21.4% were female. Sixty two percent were castrated while 70% had access to the outside environment or lived with other cats. In relation to the clinical signs, 42.9% had oral lesions (ulcers or gingivitis), 41% experienced weight loss and 38.1% had skin infections. Blood cell counts and protein values data showed that 36% of the positive animals had an alteration in the ratio of total proteins versus albumin and 66% showed anemia or polymorphonuclear cell abnormalities (Table 2).
Risk variables using the Fisher's exact test for positive (FIV or FeLV) cats
| Study in positive (FIV or FeLV or both) n=42 and negative cats n=48 | ||||
|---|---|---|---|---|
| Positive (%) | p value of the Fisher's exact test | Odds ratio | 95% CI range | |
| Age | ||||
| Juvenile | 7 (1.7%) | 0.00001 | 0.12 | From 0.0441 to 0.3262 |
| Adult | 35 (83.3%) | 0.00001 | 8.33 | From 3.0656 to 22.6528 |
| Sex | ||||
| Male | 33 (78.6%) | 0.0454 | 2.62 | From 1.0291 to 6.6652 |
| Female | 9 (21.4%) | 0.0454 | 0.38 | From 0.15 to 0.9717 |
| Health status | ||||
| Healthy | 22 (52.4%) | 0.67 | 0.77 | From 0.3354 to 1.7643 |
| Unhealthy | 20 (47.6%) | 0.67 | 1.33 | From 0.5668 to 2.9816 |
| Clinical signs | ||||
| Lymph node enlargement | 5 (11.9%) | 1.00 | 0.95 | From 0.2666 to 3.3564 |
| Pale mucous membranes | 14 (33.3%) | 0.35 | 1.66 | From 0.6637 to 4.262 |
| Oral disease | 18 (42.9%) | 0.39 | 1.50 | From 0.637 to 3.5325 |
| Respiratory tract infection signs | 11 (26.2%) | 0.31 | 1.77 | From 0.637 to 4.9419 |
| Ocular infection signs | 9 (21.4%) | 1.00 | 1.04 | From 0.3759 to 2.8573 |
| Skin infections | 16 (38.1%) | 0.04 | 2.67 | From 1.0255 to 6.9341 |
| CBC abnormalities | ||||
| Anemia | 21 (50%) | 0.01 | 3.36 | From 1.3608 to 8.3142 |
| Leukopenia | 2 (4.76%) | 0.44 | 0.43 | From 0.0789 to 2.3431 |
| Leukocytosis | 2 (4.76%) | 0.68 | 0.55 | From 0.0955 to 3.1669 |
| Neutrophilia | 6 (14.3%) | 0.78 | 0.83 | From 0.2638 to 2.6326 |
| Neutropenia | 2 (4.76%) | 1.00 | 0.75 | From 0.1192 to 4.7187 |
| Lymphocytosis | 1 (2.38%) | 1.00 | 1.15 | From 0.0695 to 18.914 |
| Lymphopenia | 25 (59.5%) | 0.0337 | 2.68 | From 1.1415 to 6.3001 |
CBC: cell blood count.
FIV and FeLV are important feline pathogens because they give opportunity to other infectious agents to invade and multiply in the same host they infect. FIV and FeLV positive cats can also act as a maintenance niche for other feline pathogens such as Feline herpes virus type 1, Feline calicivirus, or zoonotic agents such as Mycoplasma haemofelis, Mycobacterium tuberculosis, Toxoplasma gondii, mycotic agents such as Cryptococcus neoformans, Malassezia pachydermatis and Microsporum canis. In addition, infected domestic cats can endanger wild feline survival because they are a permanent source of infectious viruses.
In this study we present preliminary data on the prevalence of FIV and FeLV in the city of Buenos Aires, Argentina, for a group of 255 cats obtained between 2009 and 2013 using two different kinds of tests. Traditional testing for these infections is based on antibody detection (FIV), antigen detection (FeLV) o direct detection of genomic sequences (PCR). In Argentina there are no FIV commercial vaccines available, therefore any positive serology would indicate exposure to the infectious agent or the presence of maternal antibodies.
Due to the different tests available, discrepancies can be found in the results obtained with them depending on what is detected and which kit is used. Furthermore, although several IA tests are available, the FIV antigens utilized in the solid phase of the kit can be different. In addition, the particular stage of the disease makes a test more suitable than the other (see below). These factors, as well as the specific cultural characteristics of each country, make the comparison of prevalence values very challenging. Interestingly, similar IA values have been reported from different countries utilizing comparable tests (IDEXX SNAP FIV/FeLV Combo Test Diagnostic Kit or SNAP Feline Triple Test) including: Spain 15.6% of positive cats for FeLV and 8.3% for FIV1; Mexico 5% for FeLV and 2.5% for FIV12; Poland 6.4% for FeLV and 4.3% for FIV14; Canada 6.2% for FeLV and 2.2% for FIV (Island of Newfoundland)11. However, our combined data from IA and n-PCR tests from domestic cats in Argentina indicates higher FIV rates than the ones reported in other parts of the world, although the values are similar to others informed in cities of neighboring countries. For example, 37.5% of FIV positive cats diagnosed by PCR4 was reported in Rio Grande do Sul, Brazil. In the same city but using an indirect immunofluorescence antibody assay for FeLV p27 antigen around 11% of the animals tested were found positive for FeLV. By measuring a different parameter (PCR of genomic sequences) we obtained similar values for FeLV, although the numbers are higher than those observed by IA assays (Table 1).
The FIV positive percentages found in our work were similar using both tests (IA and n-PCR) in spite of detecting different parameters. In contrast, FeLV results showed higher sensitivity with the PCR assay, as expected. It is evident that an important factor in the outcome of the test is the moment when the sample is collected. Therefore, a good anamnesis with the owner and a detailed clinical analysis are necessary to choose the most suitable diagnostic method based on the stage of the disease. For example, when a suspected FeLV positive animal has to be tested, a n-PCR assay would be the most suitable test because this method detects animals in the latent stage of infection as well. In this period, there is neither detectable viremia nor circulating antigens present and thus tests like the IA assay that detect circulating antigens would probably give a false negative result. For FIV, the few discrepancies found between the two assays could be due, according to our experience, to animals that were in a period of lymphocyte CD4+ depletion during the chronic phase of the disease when clinical signs appear. In this case, when a blood sample with a small number of or no polymorphonuclear cells present is obtained, negligible amounts of viral genomic DNA from infected lymphocytes are obtained, making the PCR detection extremely difficult. In these animals, the optimal methodology would be to perform an antibody test detection similar to the IA assay. However, in kittens where FIV maternal antibodies are still present the IA assay would result in false positive animals, and consequently, it would be necessary to retest them six months later or to perform an n-PCR assay.
With regard to the clinical history data, a higher prevalence in male than in female cats was found (Table 2). We believe that this difference is due to the characteristic male behavior in this species. Similarly to what has been described by other authors, we evidenced a higher prevalence of oral lesions in FIV infected cats in comparison to negative cats. The percentage of oral disease in positive animals was similar to that in other works recently published by Kornya et al.10 (40.6%).
With respect to complete blood count abnormalities, we observed that almost 60% of positive cats showed lymphopenia, similarly to what has been published by Spada et al.15 (58.3%) (Table 2). In spite of the presence of lymphopenia as well as lymphadenopathy, as have been previously reported by other authors, we did not consider them specific of these retroviruses. However, we found differences in the percentage rate of anemia (50% versus 91.7%).
Our findings reveal that 70% of positive cats had had contact with the outside environment or other cats, emphasizing the importance for maintaining them isolated from the contact with other animals, especially in catteries. In addition, they should be tested regularly to allow only the reproduction of negative animals. Stray cats might represent a risk factor for naïve cats. This kind of measures would also prevent infected cats from having contact with wild felines, thus preserving their health status.
Although the number of samples tested in our study is not representative of the real prevalence of these diseases in the entire country, the data obtained provides the first evidence of the circulation of both viruses in domestic cats in our territory.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that there are not conflicts of interest in this work.
We thank the Small Animal Hospital staff for providing the clinical histories utilized in this study. We also thank Dr Fiorella Kotsias for reviewing the manuscript.