to investigate the prevalence and risk factors in newborns with congenital heart defects (CHD).
Methodsthis case-control study included 234,386 births from January 2006 to June 2013 that were evaluated and registered in the Latin-American Collaborative Study of Congenital Malformations (ECLAMC) methodology, establishing the Bogota Birth Defects Surveillance and Follow-up Program (BBDSFP).
Results234,368 births were registered and 277 of them were identified to have a CHD. The most common defect among all was ventricular septal defect (13.7%) followed by atrial septal defect (10.1%). As main associations we obtained: having any type of pre-gestational diabetes mellitus had an increased risk for the development of CHD (OR 16.36 CI: 4.54–58.35). Low weight newborns (less than 2,500g) (OR: 4.13 CI: 3.13–5.44) and a gestational age lower than 36 weeks (OR: 4.92 CI: 3–5.44) were also linked to malformations.
Conclusionswomen with diabetes mellitus represent a high-risk pregnancy group, more work is needed to educate diabetic women, so CHD can be prevented and the outcomes of their pregnancy can be improved. Appropriate glycemic control before and during pregnancy may reduce CHD.
investigar la prevalencia y los factores de riesgo en recién nacidos con cardiopatías congénitas.
Métodoseste estudio caso control incluyó 234.386 nacimientos desde enero del 2006 hasta junio del 2013, los cuales fueron evaluados y registrados según la metodología del Estudio Colaborativo Latinoamericano de Malformaciones Congénitas (ECLAMC) estableciendo el programa de vigilancia de defectos congénitos de Bogotá.
Resultadosde 234.368 pacientes fueron incluidos en el estudio, 277 fueron diagnosticados con cardiopatías congénitas. El defecto cardiaco más común fue la comunicación interventricular (13.7%) seguido por la comunicación interauricular (10.1%). Al evaluar los factores de riesgo se encontró que las madres con diabetes mellitus pregestacional tuvieron mayor riesgo de tener hijos con cardiopatías congénitas (OR 16.36 IC: 4.54–58.35) y que los pacientes con bajo peso al nacer (menor de 2.500g) (OR: 4.13 IC: 3.13–5.44) y edad gestacional menor a 36 semanas (OR: 4.92 CI: 3–5.44) tenían mayor riesgo de ser diagnosticados con una cardiopatía congénita.
Conclusioneslas pacientes diabéticas en embarazo tienen mayor riesgo de que sus hijos desarrollen una cardiopatía congénita. Por lo anterior se necesita realizar un mayor trabajo tanto de educación como de seguimiento a las mujeres diabéticas, para así prevenir cardiopatías congénitas y disminuir el resigo de sus embarazos.
The most common congenital anomalies (CA) are congenital heart defects (CHD), with a prevalence of 4 to 50 per 1,000 live-births in different studies, depending on the examination age and the sensitivity of the examination technique1–6. The birth prevalence of CHD was 1.2 per 1,000 live-births in a Colombian study of 44,985 children. This was based on the pediatric cardiology examination over four years in 11 colombian hospitals, using the Latin-American Collaborative Study of Congenital Malformations (ECLAMC) methodology, establishing the Bogota Birth Defects Surveillance and Follow-up Program (BBDSFP)7.
Although the mortality associated with CHD has decreased, CHD remain one of the leading causes of infant/child mortality8. Over the past few years, there have been major breakthroughs in the understanding of genetic risk factors in CHD; however, there is relatively less information about the role of non-inherited modifiable factors in the origin of CHD and it is unknown in the majority of patients9. One of the most important risk factor is maternal diabetes mellitus, a chronic disease that is linked with difficult pregnancy and an increased prevalence of CA, perinatal morbidity and mortality in offspring. Studies show that the offspring of diabetic mothers have a five-fold increased incidence of CA compared to pregnancies in the general healthy population10–13.
This study aimed to describe the prevalence and risks factors in the origin of CHD in a colombian population. The findings could be applied to current and future cardiac and perinatal care practice and to upcoming research.
MethodsPopulationThis case-control study included 234,386 births from January 2006 to June 2013 that were evaluated and registered in the BBDSFP based in ECLAMC, an international registry of congenital malformations and an exhaustive exploration of prenatal conditions for all malformed newborns identified and for paired controls. The newborns were evaluated during the first 48hours postpartum14.
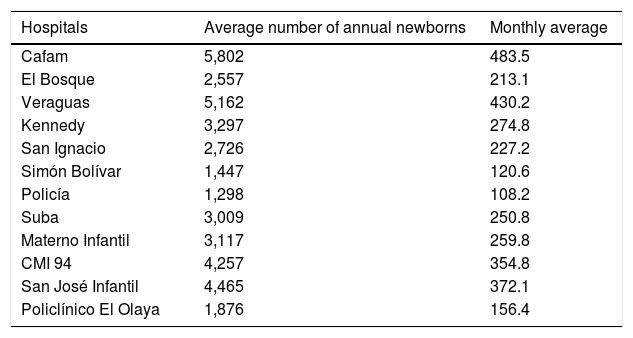
Data were collected from different hospitals having high complexity level in Bogota D.C. (Table 1): Hospital Cafam; Clínica El Bosque; Clínica Veraguas; Hospital Kennedy; Hospital Universitario San Ignacio; Hospital Simón Bolívar; Hospital Central de la Policía; Hospital de Suba; Hospital Maternoinfantil de la Victoria; Hospital San José Infantil; Policlínico del Olaya, and Clínica Maternoinfantil de Saludcoop. All these institutions gave their approval before the study was conducted.
Institutions from which data were collected.
| Hospitals | Average number of annual newborns | Monthly average |
|---|---|---|
| Cafam | 5,802 | 483.5 |
| El Bosque | 2,557 | 213.1 |
| Veraguas | 5,162 | 430.2 |
| Kennedy | 3,297 | 274.8 |
| San Ignacio | 2,726 | 227.2 |
| Simón Bolívar | 1,447 | 120.6 |
| Policía | 1,298 | 108.2 |
| Suba | 3,009 | 250.8 |
| Materno Infantil | 3,117 | 259.8 |
| CMI 94 | 4,257 | 354.8 |
| San José Infantil | 4,465 | 372.1 |
| Policlínico El Olaya | 1,876 | 156.4 |
In this study it was take into account newborns with CHD from British Pediatric Association ICD-10 codes Q20.0 to Q25.4. It was defined as cases live newborns and stillborn weighing more than 500g, who were born in one of the hospitals under observation (Table 1), and who presented with one or more of the selected CA. Multiple CA were defined as the presence of a major CA in two or more organ systems. Controls were defined as any live newborn that had been born in the same hospital during the same month as the case, and had no CA at time of birth. The controls were not sex-matched. The prevalence of birth was calculated taking into account the live newborns and the stillborn over 500g which accomplished the inclusion criteria, over the total of newborns and stillborn over 500g.
Exclusion criteriaThe following was considered as an exclusion criterion: chromosomal abnormalities.
Risk factor evaluationWe evaluated whether the following variables were risk factors or not for isolated CHD: pre-conception maternal body mass index (BMI); maternal age; gestational age; newborn weight; smoking during pregnancy; hypothyroidism; consanguinity; gestational diabetes and diabetes mellitus.
Medication reviewWe evaluated medication used by the mother's cases each trimester of pregnancy.
Ethics approval and informed consentWe declare the local ethics committee accepted this project, and informed consent was obtained from the parents of all children participating in the study.
AnalysisQuantitative variables were compared using a t-test with a confidence interval of 95% and the qualitative variables were analyzed using an odds ratio with a confidence interval of 95%. It was used Microsoft Excel 2007 and Epicalc 2000 v1.02 for data management and statistical calculations, respectively.
ResultsBaseline, socioeconomic status and other characteristics of the study populationA total of 277 cases of CHD were born in the area of study between the years 2006 and 2013 of which 77 were stillbirths, and were compared with 2,691 healthy control children, obtaining a case-control ratio of 1:9.71.
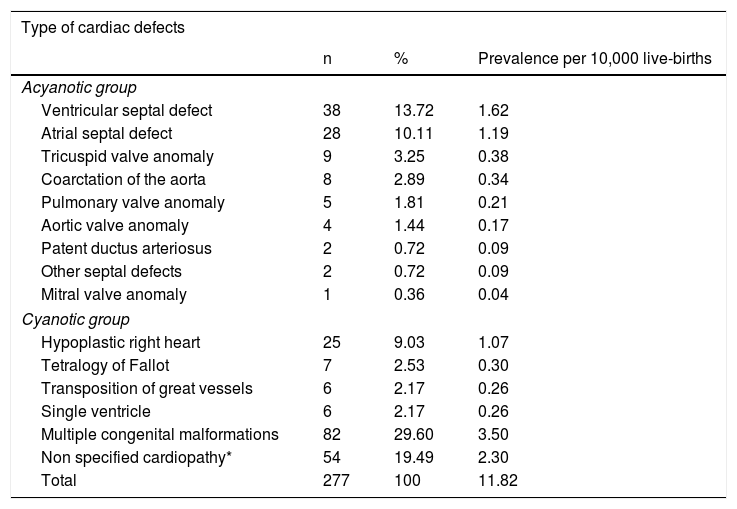
Distribution of congenital heart defectsTable 2 shows the distribution of cardiovascular malformations in cases. The prevalence of CHD was 11.82 per 10,000 live births. Of the 277 cases, the ventricular septal defect (VSD) was one of the most common malformations, followed by atrial septal defect (ASD). 29.60% of the CHD were associated with multiple congenital malformations.
Distribution of cardiovascular malformations in affected children.
| Type of cardiac defects | |||
|---|---|---|---|
| n | % | Prevalence per 10,000 live-births | |
| Acyanotic group | |||
| Ventricular septal defect | 38 | 13.72 | 1.62 |
| Atrial septal defect | 28 | 10.11 | 1.19 |
| Tricuspid valve anomaly | 9 | 3.25 | 0.38 |
| Coarctation of the aorta | 8 | 2.89 | 0.34 |
| Pulmonary valve anomaly | 5 | 1.81 | 0.21 |
| Aortic valve anomaly | 4 | 1.44 | 0.17 |
| Patent ductus arteriosus | 2 | 0.72 | 0.09 |
| Other septal defects | 2 | 0.72 | 0.09 |
| Mitral valve anomaly | 1 | 0.36 | 0.04 |
| Cyanotic group | |||
| Hypoplastic right heart | 25 | 9.03 | 1.07 |
| Tetralogy of Fallot | 7 | 2.53 | 0.30 |
| Transposition of great vessels | 6 | 2.17 | 0.26 |
| Single ventricle | 6 | 2.17 | 0.26 |
| Multiple congenital malformations | 82 | 29.60 | 3.50 |
| Non specified cardiopathy* | 54 | 19.49 | 2.30 |
| Total | 277 | 100 | 11.82 |
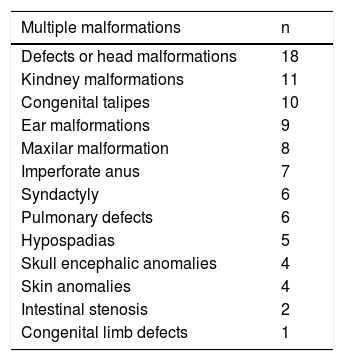
Table 3 shows the distribution of multiple congenital malformations in newborns with CHD, the most common was defects or head malformations, in which we included macrocephaly, microcephaly, holoprosencephaly, facial asymmetry, among others. Follow by kidney malformations and congenital talipes.
Distribution of multiple congenital malformations.
| Multiple malformations | n |
|---|---|
| Defects or head malformations | 18 |
| Kindney malformations | 11 |
| Congenital talipes | 10 |
| Ear malformations | 9 |
| Maxilar malformation | 8 |
| Imperforate anus | 7 |
| Syndactyly | 6 |
| Pulmonary defects | 6 |
| Hypospadias | 5 |
| Skull encephalic anomalies | 4 |
| Skin anomalies | 4 |
| Intestinal stenosis | 2 |
| Congenital limb defects | 1 |
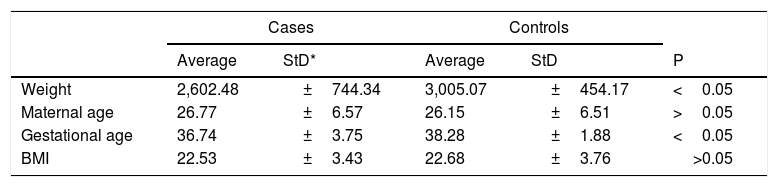
Table 4 shows the t-test results for the quantitative variables and the standard deviation in cases and controls. We found differences in weight and gestational age.
T-test results for quantitative variables.
| Cases | Controls | ||||
|---|---|---|---|---|---|
| Average | StD* | Average | StD | P | |
| Weight | 2,602.48 | ±744.34 | 3,005.07 | ±454.17 | <0.05 |
| Maternal age | 26.77 | ±6.57 | 26.15 | ±6.51 | >0.05 |
| Gestational age | 36.74 | ±3.75 | 38.28 | ±1.88 | <0.05 |
| BMI | 22.53 | ±3.43 | 22.68 | ±3.76 | >0.05 |
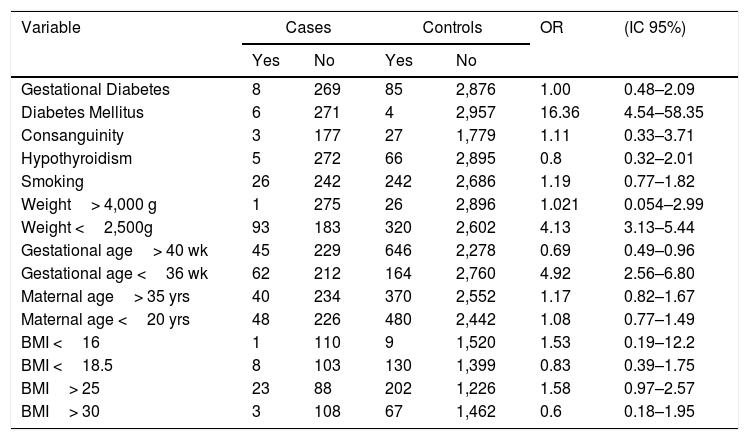
Having any type of pre-gestational diabetes mellitus had an increased risk for the development of CHD (OR 16.36 CI: 4.54–58.35). It was found that low weight newborns (less than 2,500g) (OR: 4.13 CI: 3.13–5.44) and a gestational age lower than 36 weeks
(OR: 4.92 CI: 3–5.44) increased the risk of presenting a CHD (Table 4).
However, pregnant women with a gestational age greater than 40 weeks (OR: 0.69 CI: 0.49–0.96) have lower risk of CHD presentation.
Some diseases and risk factors in pregnancy, such as passive and active smoking, hypothyroidism, maternal age, BMI, gestational diabetes and consanguinity were not associated with a higher risk of development of CHD.
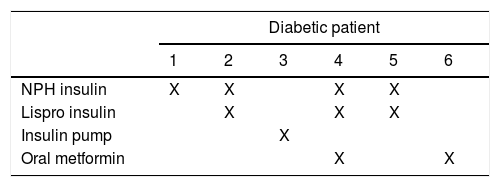
Since we found a high correlation between diabetes and CHD we searched for the medication the mothers with diabetes were taking during pregnancy; the results are shown in Table 5 and Table 6.
Risk factor comparisons between cases and controls.
| Variable | Cases | Controls | OR | (IC 95%) | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Gestational Diabetes | 8 | 269 | 85 | 2,876 | 1.00 | 0.48–2.09 |
| Diabetes Mellitus | 6 | 271 | 4 | 2,957 | 16.36 | 4.54–58.35 |
| Consanguinity | 3 | 177 | 27 | 1,779 | 1.11 | 0.33–3.71 |
| Hypothyroidism | 5 | 272 | 66 | 2,895 | 0.8 | 0.32–2.01 |
| Smoking | 26 | 242 | 242 | 2,686 | 1.19 | 0.77–1.82 |
| Weight> 4,000 g | 1 | 275 | 26 | 2,896 | 1.021 | 0.054–2.99 |
| Weight <2,500g | 93 | 183 | 320 | 2,602 | 4.13 | 3.13–5.44 |
| Gestational age> 40 wk | 45 | 229 | 646 | 2,278 | 0.69 | 0.49–0.96 |
| Gestational age <36 wk | 62 | 212 | 164 | 2,760 | 4.92 | 2.56–6.80 |
| Maternal age> 35 yrs | 40 | 234 | 370 | 2,552 | 1.17 | 0.82–1.67 |
| Maternal age <20 yrs | 48 | 226 | 480 | 2,442 | 1.08 | 0.77–1.49 |
| BMI <16 | 1 | 110 | 9 | 1,520 | 1.53 | 0.19–12.2 |
| BMI <18.5 | 8 | 103 | 130 | 1,399 | 0.83 | 0.39–1.75 |
| BMI> 25 | 23 | 88 | 202 | 1,226 | 1.58 | 0.97–2.57 |
| BMI> 30 | 3 | 108 | 67 | 1,462 | 0.6 | 0.18–1.95 |
Of the 6 newborns with CHD of the others’ studies show the same results diabetic patients, three of them had an ASD, the others had a hypoplasic right heart, an aortic valve anomaly and one of them had multiple congenital abnormalities which included transposition of great vessels with ventricular septal defect and cleft lip.
DiscussionThis case-control study aimed to identify possible associations between risk factors and CHD in a colombian population. Risk factors, such as maternal gestational age and chronic diabetes mellitus, consanguinity, hypothyroidism, smoking, weight, gestational age, maternal age, and BMI. Some associations with high risk CHD were identified; noteworthy is the suggestion of diabetes mellitus.
CHD are the most common and serious structural birth defects worldwide (9 per 1,000 live-births). Our study showed a prevalence of 11.82 per 10,000 live-births of CHD, the most frequent was VSD (13.7%), followed by ASD (10.1%). A worldwide meta-analysis of congenital heart disease showed the same results; VSD was the one with the highest prevalence (2.62 per 1,000 live-births) followed by ASD (1.64 per 1,000 live-births). However, PDA was the third most prevalent defect in the meta-analysis and, in our study, the third most prevalent was hypoplastic right heart (9%)15,16. In our study, due to the high altitude in Bogota, PDA was only considered as a CHD if it needed closure with medical management or surgery. That is the reason why the prevalence of PDA is so low compared with the worldwide meta-analysis.
Analyzing the risk factors, we found a high association between diabetes and CHD (OR 16.36 CI: 4.54–58.35); this risk in our study is consistent with other studies that compare the anomaly rate in diabetic mothers10–13,17–19. Risk factors as weight less than 2,500g, or a gestational age of less than 36 weeks, have an association with CHD; others’ studies show the same results7,20,21.
Our study did not find statically significant differences in the likelihood of CHD in some risk factors such as gestational diabetes mellitus, consanguinity, hypothyroidism, smoking, maternal age and BMI (Table 4).
The potential for the existence of cardiac abnormalities among the offspring of mothers with diabetes mellitus has been recognized for more than 50 years16. Our study finds a high correlation between pre-gestational diabetes mellitus and CHD. Although perinatal mortality has declined dramatically in diabetic pregnancies over the past years, most studies continue to show a higher mortality in these patients than in control populations22,23. studies from large regions in the United Kingdom encounter a high level of non-attendance at pre-conception care and poor glycemic control in diabetic mothers. However, the Dutch population shows that diabetic women who planned their pregnancies and have a good periconceptional glycemic control and an HbA1c of less than 7%, also have a significant increase in CA3,24. It is important to highlight that, according to the Latin American Diabetes Association (ALAD), the prevalence of diabetes in the world by 2030 is expected to be approximately 39.9 million cases, and almost 45% of those patients will ignore their condition25.
Analyzing the medication that diabetic mothers take during pregnancy, we did not find a teratogenic drug. Insulin and metformin are safe to use in the gestational period as well as cephalexin, methyldopa and folic acid. However, the use of calcium carbonate in a high dose increased the risk for conotruncal heart defects26–30.
According to the World Health Organization, diabetes mellitus prevalence in Colombia is 8,5%31. The National Health Survey32 described that 96.5% of these diabetics are treated by a general practitioner, only 75% are advised to quit smoking, lose weight and control their blood glucose. Additionally, only 20% of diabetic patients measure their blood glucose at home. These has led us to believe that colombian diabetic pregnant woman do not have the follow-up they should.
Is noteworthy to describe different risk factors for CHD that we did not analyze in our study. A recent metanalysis in China33 showed that mothers with advanced age (OR 2,6), cold or fever (OR 4,5), passive smoking (OR 2,7), noise exposure (OR 3) and radiation exposure (OR 2,9) were prone to have children with cardiac defects.
The study limitations were that newborns were only followed 48hours after delivery, that prenatal echocardiography was not available for all the patient's mothers during pregnancy, and that echocardiographic results are operator dependent.
ConclusionsWomen with diabetes mellitus represent a high-risk pregnancy group. The incidence of diabetes mellitus is increasing, especially at younger ages; therefore, the number of pregnant diabetic women will also continue to increase. Our study found a high correlation between diabetic mothers and CHD. Since 1980 the literature has described an association between poor glycemic control and high-risk pregnancies. Hence, we believe that more work is needed to educate diabetic women, so CHD can be prevented and the outcomes of their pregnancy can be improved. Diabetic women who are trying to get pregnant should have a strict glycemic control, especially in the first trimester, since pregnancies with poor first trimester control are more at risk of complex forms of CHD16,34.
Ethics approvalThis study was conducted with the approval of the Comité de Investigaciones y Ética de la Facultad de Medicina y el Hospital Universitario San Ignacio.
Competing interestsNone.
ContributorsNone.
We would like to thank all the health services that permitted us to acquire the data that made this project possible: Hospital Cafam, Clínica El Bosque, Clínica Veraguas, Hospital Kennedy, Hospital Universitario San Ignacio, Hospital Simón Bolívar, Hospital Central de la Policía, Hospital de Suba, Hospital Maternoinfantil de la Victoria, Hospital San José Infantil, Policlínico del Olaya and Clínica Maternoinfantil de Saludcoop.