Renal manifestations of spondyloarthritis are rare. The case is presented of a patient with ankylosing spondylitis of nine years of evolution. He had intense inflammation, as well as articular sequelae, with a significant deterioration in his quality of life. At the initial evaluation, the patient had a serum creatinine of 1.44mg/dL and a 24-h urine protein in the sub-nephrotic range (1.44g). Renal biopsy showed the presence of Congophilic material, confirming the diagnosis of AA amyloidosis. Treatment with a TNF blocking agent was initiated with clinical improvement, especially regarding articular disease.
Las manifestaciones renales de la espondiloartritis son poco comunes. Se presenta el caso de un paciente con espondilitis anquilosante de 9 años de evolución, con intensa inflamación y secuelas articulares y con un deterioro significativo en su calidad de vida. En la evaluación inicial, el paciente tenía una creatinina sérica de 1,44mg/dL y una proteína en orina de 24 horas en un rango subnefrótico (1,44 g). La biopsia renal mostró la presencia de material congofílico que confirmaba el diagnóstico de amiloidosis AA. Se inició tratamiento con un anti-TNF, con mejoría clínica, especialmente con respecto al componente articular.
The prevalence of ankylosing spondylitis is up to 1.5% in the general population.1 When reviewing its visceral involvement,2,3 the kidney is rarely affected. In a series of 681 patients, abnormal findings in the urinalysis were found in 8%. Six of these patients underwent renal biopsy due to 24-h urine protein greater than 1g with only one case of amyloidosis detected. After reviewing the literature, few case reports of this manifestation were found, with two case series of 13 subjects as the largest reports.4,5
Case reportPatient informationA 29-year-old man, from Northwestern Colombia, who since the age of 20 years referred inflammatory back pain, arthritis and morning stiffness in knees and ankles. The symptoms did not respond to non-steroidal anti-inflammatory drugs, and the clinical picture had been classified as rheumatoid arthritis and treated with prednisone 10–15mg/day since then. Due to deterioration in his general condition, considerable weight loss and difficulties in the control of his musculoskeletal symptoms, the patient was admitted to our hospital. Aside from his rheumatological disease, his medical, family and psychosocial history did not have any relevant findings.
Clinical findingsPhysical examination revealed an emaciated patient, with bilateral synovitis on knees and ankles. Additionally, the patient had a limitation on spinal movement with a modified Schober test of 1cm and lateral inclination of 5cm (height: 150cm).
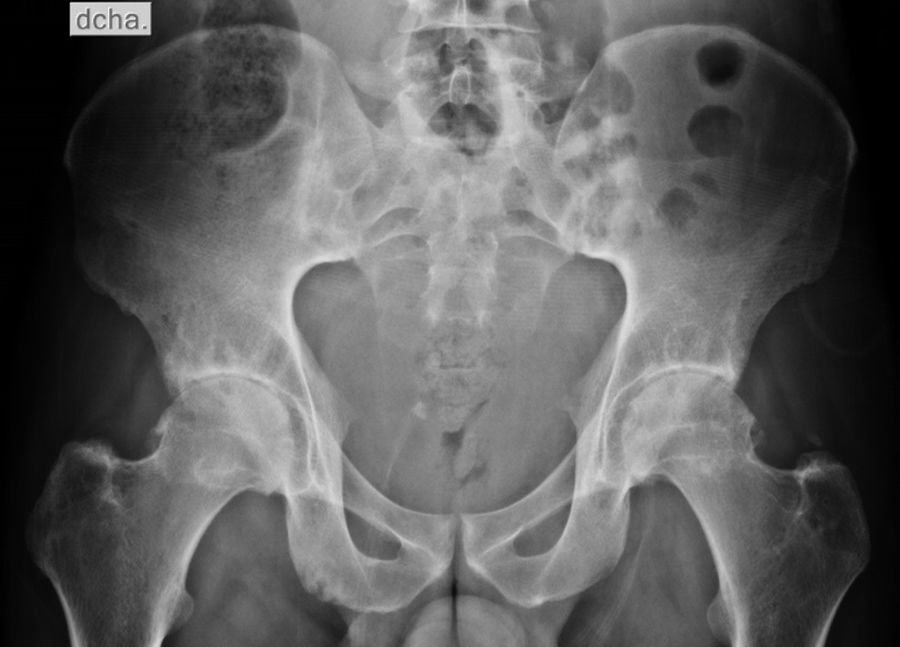
Diagnostic approachDue to inflammatory back pain, pelvic X-rays were requested, with evidence of bilateral grade III sacroiliitis (Fig. 1).
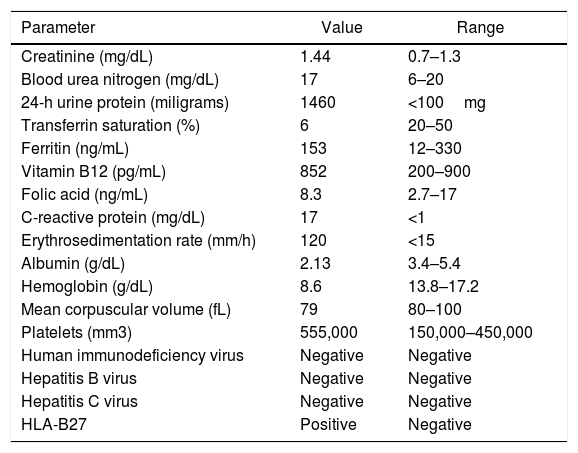
Blood chemistry evidenced systemic inflammation and creatinine elevation, of unknown duration due to the absence of previous results (Table 1). In subsequent studies, progressive deterioration in the glomerular filtration rate was evidenced (raising of serum creatine from 1.44 to 1.68mg/dL in 48h). Therefore, it was concluded that there was an acute intrarenal component with subnephrotic proteinuria and renal biopsy was obtained.
Lab tests.
| Parameter | Value | Range |
|---|---|---|
| Creatinine (mg/dL) | 1.44 | 0.7–1.3 |
| Blood urea nitrogen (mg/dL) | 17 | 6–20 |
| 24-h urine protein (miligrams) | 1460 | <100mg |
| Transferrin saturation (%) | 6 | 20–50 |
| Ferritin (ng/mL) | 153 | 12–330 |
| Vitamin B12 (pg/mL) | 852 | 200–900 |
| Folic acid (ng/mL) | 8.3 | 2.7–17 |
| C-reactive protein (mg/dL) | 17 | <1 |
| Erythrosedimentation rate (mm/h) | 120 | <15 |
| Albumin (g/dL) | 2.13 | 3.4–5.4 |
| Hemoglobin (g/dL) | 8.6 | 13.8–17.2 |
| Mean corpuscular volume (fL) | 79 | 80–100 |
| Platelets (mm3) | 555,000 | 150,000–450,000 |
| Human immunodeficiency virus | Negative | Negative |
| Hepatitis B virus | Negative | Negative |
| Hepatitis C virus | Negative | Negative |
| HLA-B27 | Positive | Negative |
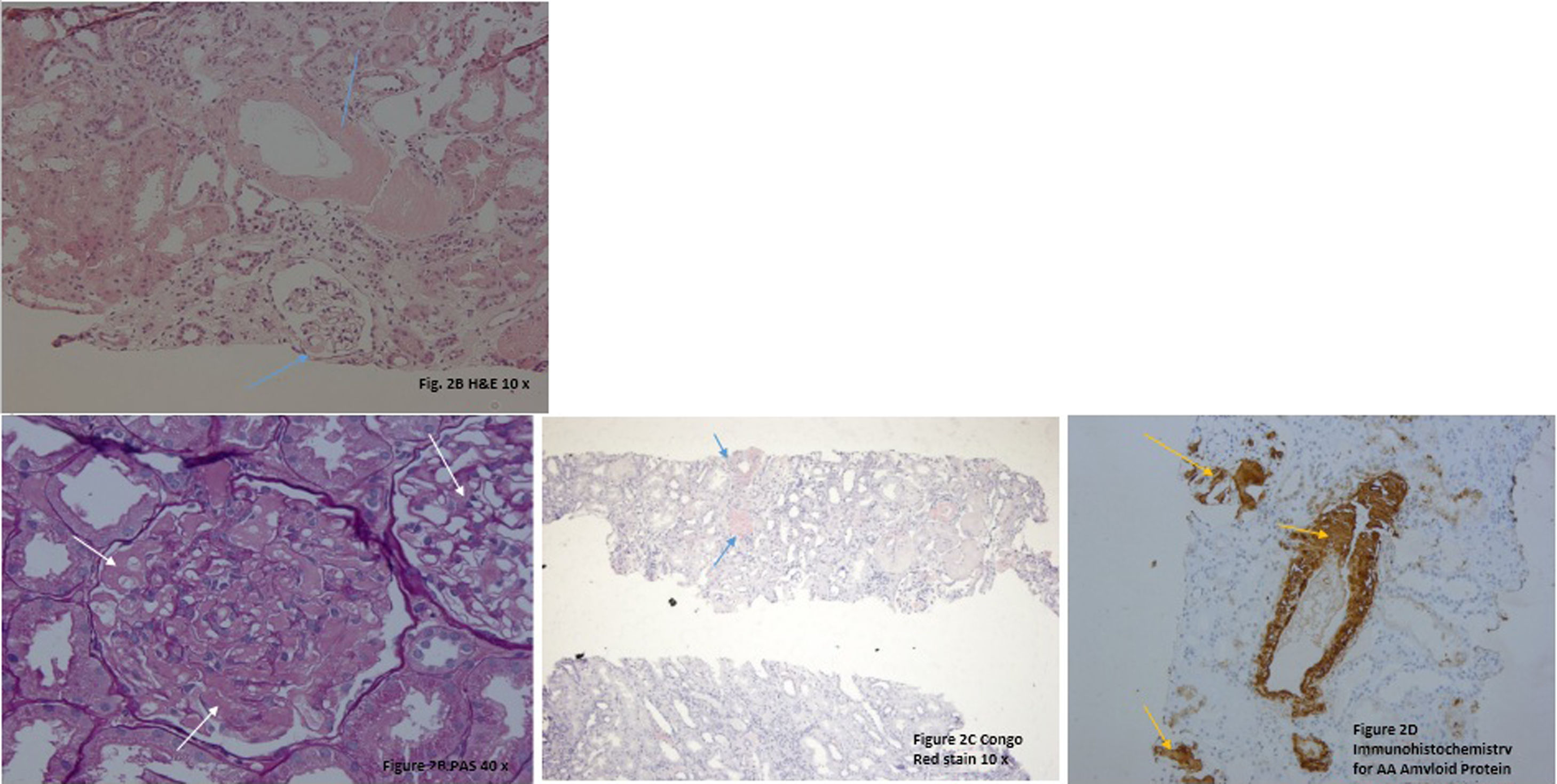
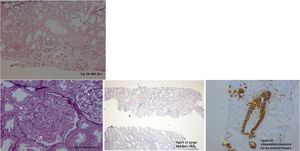
The renal biopsy showed intraglomerular congophilic deposits, in the absence of immunoglobulins or other immune deposits and the amyloid found was typified as AA, thus confirming the diagnosis of renal amyloidosis (Fig. 2A–D). Additional studies for plasma cell diseases were negative.
A. H&E 20×. There are some glomeruli and vessels with a material deposited mainly in the mesangial areas and vascular wall (blue arrows). B. PAS 40×. Note in this glomerulus the PAS negative material filling the mesangial areas and spreading tho the subendotelial aspect of the glomerular basement membranes (white arrows). C. Congo Red Stain demonstrating the congophilia of the deposited material (blue arrows). Polarization was performed, and an apple green birefringence was obtained (not shown). D. Immunohistochemistry for AA Amyloid Protein showing positivity in the glomeruli and in vascular walls (yellow arrows).
Due to axial and peripheral inflammatory involvement, adalimumab was started at a dosage of 40mg every other week, with renal function improvement at 72h (creatinine from 1.56mg% to 1.07mg%)
DiscussionRenal involvement in ankylosing spondylitis is rare and often overlooked, due to the tendency to appear late in the natural evolution of the disease and a decrease in its incidence secondary to better control of inflammation, as well as a reduction in the use of non-steroidal antinflammatory drugs (NSAIDs) after the introduction of biological therapy.6 In a cohort of 8616 patients, it was found that the risk of renal disease is 70% higher than in the general population, being much more common in individuals aged between 20 and 39 years, with a prevalence of 3.4% in men and 2.1% in women, much lower than those described in the previous literature.3 Likewise, in a cohort in whom kidney disease was actively sought, laboratory alterations were documented in 8% of the patients: proteinuria (5.9%), hematuria (2.8%), and both (0.7%).7 In Colombia, there are two published cohorts: Londoño et al.,8 and Márquez et al.9; in the first one, there is no description of extraarticular involvement8; in the second, they described five patients with renal involvement, yet it was not discriminated.9
The three etiologies to be differentiated in these subjects are: IgA nephropathy, tubulointerstitial nephritis due to NSAIDs and AA amyloidosis, entities with important differences in their management and prognosis. There are few data on their distribution due to the low prevalence. In the literature, 90% of the cases are explained by amyloidosis (60%) and IgA nephropathy (30%).10–12
It should be noted that this protein deposit does not always cause clinical manifestations, which is illustrated by Gratacos et al., in which 137 patients with a disease of more than five years of evolution underwent biopsy of subcutaneous fat with successive studies for organ involvement in those with positive Congo red stain. It was found that 7% of the subjects had deposits of amyloid material in the subcutaneous fat, yet only 20% had any clinical findings. It is unknown whether this subclinical amyloidosis is a pre-pathogenic phase, with invariably adverse outcomes, or if it corresponds, in some individuals, to a finding with no prognostic or clinical value.13
The renal lesion is triggered both by the physical distortion of the glomerular structures and by direct cytotoxicity of the amyloid precursor, after the interaction with membrane receptors and endocytosis, and is usually manifest as proteinuria, which can range from subnephrotic to massive. Kidney injury with proteinuria is the earliest manifestation of AA amyloidosis.14
The history of our patient was characterized by a progressive evolution influenced by uneven monitoring and lack of access to health services, which allowed inflammation to remain unchecked. This is compatible with what has been previously reported, in which amyloidosis is a late complication appearing as late as ten years after diagnosis, but with cases that present such short intervals as four or five years, especially in those cases with severe and persistent inflammation.15
Therapeutic strategies for AA amyloidosis are based on the management of the underlying cause; good results have been described with anti-TNF agents in the case of subjects with ankylosing spondylitis. In a case series of patients with AA amyloidosis secondary to rheumatic diseases, six individuals underwent treatment with infliximab, stabilizing creatinine and substantially reducing proteinuria in three of them in a 28-month follow-up.6 It is believed that the effect of anti-TNF agents goes beyond the suppression of inflammatory cytokines and is postulated to be secondary to a decrease in the receptor expression of advanced glycation end products (AGEs), potential mediators for cell toxicity of amyloid precursors.16–21
There are few data on the prognosis of these patients; nevertheless, Ahbap et al. reported that the mean renal survival was 64.7 months and the mortality at five years was 48.7%, indicating that a high proportion of patients will progress to end-stage renal disease if untreated. This was associated with high mortality, especially among those presenting with massive proteinuria and hypoalbuminemia.22
Funding sourceOwn resources.
Conflict of interestsAll authors declare absence of any conflict of interest.
Clínica Universitaria Bolivariana and Hospital Universitario Fundación. Santafé de Bogotá.