Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect any organ or system, including the central nervous system (CNS). Cerebrovascular disease is included in its spectrum of manifestations, notable for its morbidity and mortality and associated disability. However, this presentation is very rare as an initial manifestation of SLE, particularly subdural haematoma. We present the case of a 57-year-old male patient who was admitted to a primary care centre with headache accompanied by alarm signs, documenting staggered subdural collections in the right frontal region together with a pontine intraparenchymal hemorrhage on the same side, in the context of severe pancytopenia. In the course of its evolution and complementary tests in the search for the etiology of pancytopenia, after excluding the most common causes, an autoimmune origin was concluded in the context of SLE with atypical clinical presentation.
El lupus eritematoso sistémico (LES) es una enfermedad autoinmune crónica que puede comprometer cualquier órgano o sistema, incluido el sistema nervioso central (SNC). Dentro del espectro de sus manifestaciones se incluye la enfermedad cerebrovascular, en la cual destacan su morbimortalidad y su discapacidad asociada. A pesar de ello, esta presentación es muy rara como manifestación inicial del LES, en particular el hematoma subdural. A continuación, se presenta el caso de un paciente masculino de 57 años que ingresó a un centro de atención primaria con cefalea acompañada de signos de alarma, se documentaron colecciones subdurales escalonadas en la región frontal derecha, junto con una hemorragia intraparenquimatosa póntica del mismo lado, en el contexto de pancitopenia severa. En el trascurso de su evolución y con los exámenes complementarios en la búsqueda de la etiología de la pancitopenia, se concluyó, después de excluir las causas más comunes, un origen autoinmune en el contexto de un LES con presentación clínica atípica.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can involve any organ or system, including both the central nervous system (CNS) and the peripheral nervous system, with or without associated psychiatric symptoms. The incidence and prevalence regarding the neurological symptoms have had an important variation due to the diversity in their definitions. However, around 30–50% of patients with SLE may have neuropsychiatric symptoms in some point of their evolution.1,2 The distinction of whether the symptoms are caused by the disease or are due to associated comorbidities has not been clearly established. Its clinical presentation constitutes a challenge. Although many of its manifestations may be mild or self-limiting, some may be life-threatening or cause major disability, with important implications on quality of life.3
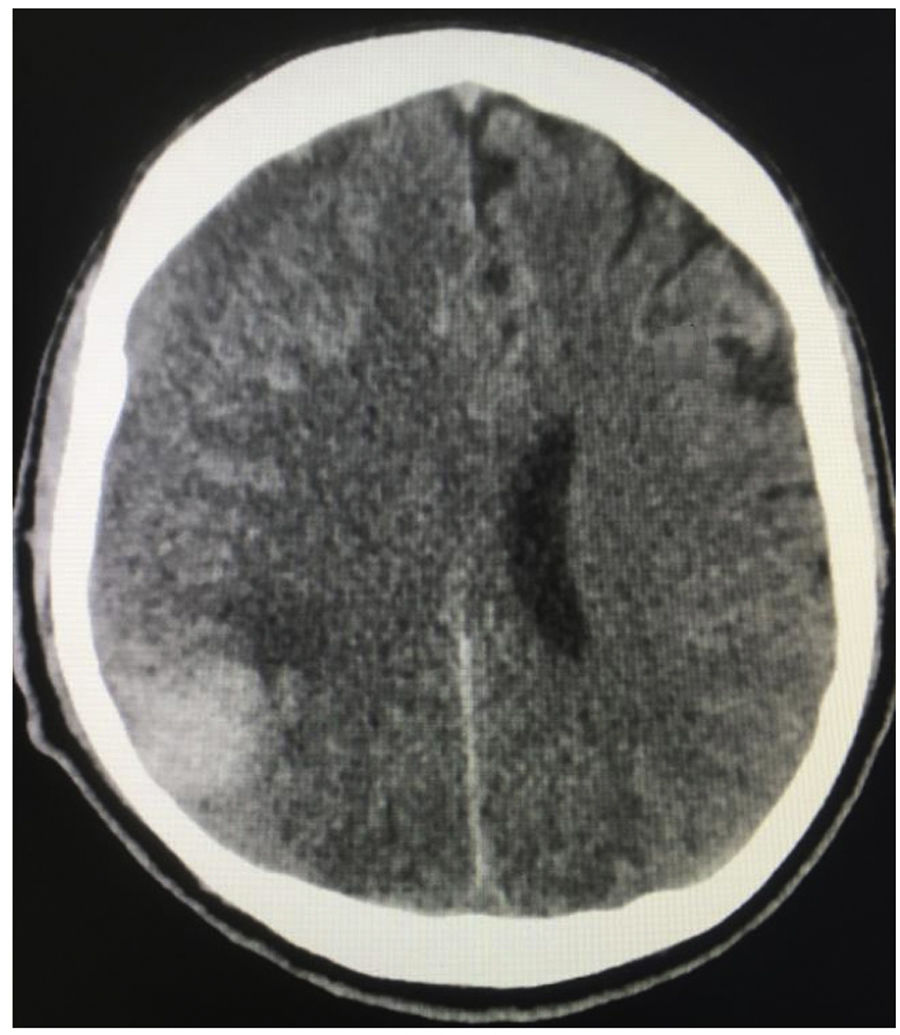
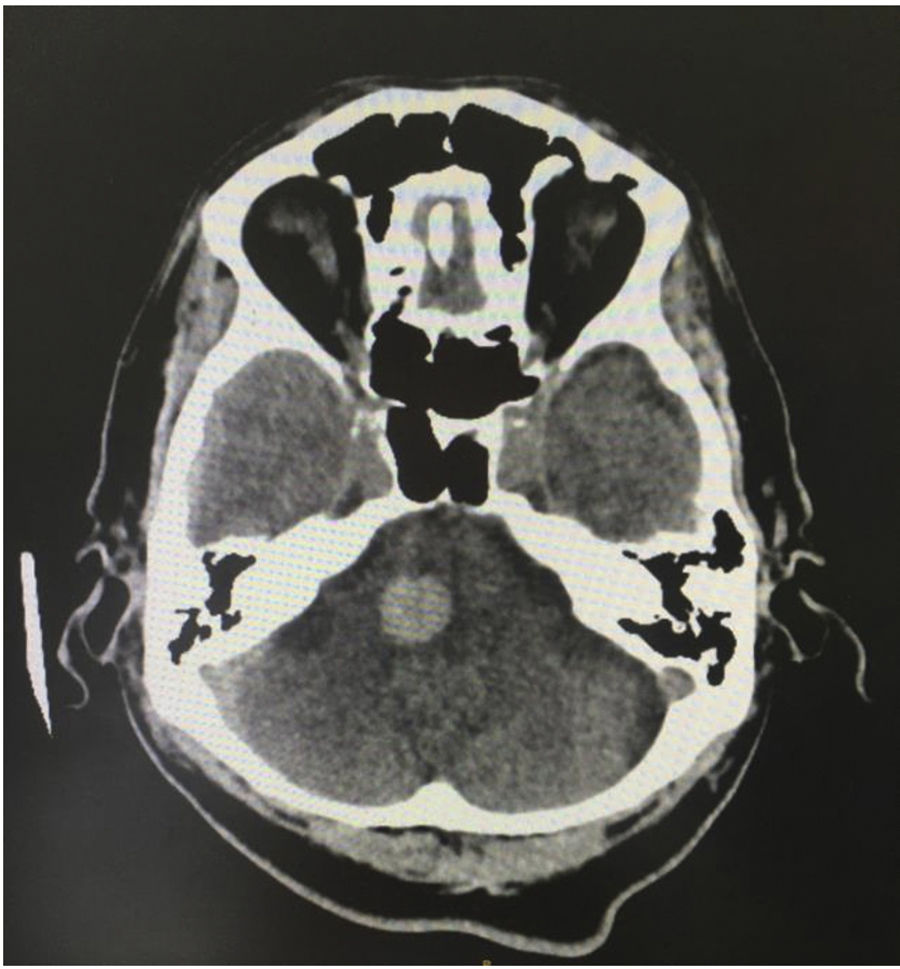
It is known that cerebrovascular accident (CVA) occurs in 19% of patients with SLE, associated with a greater decrease in life expectancy.4,5 In addition, these patients have a risk of presenting this manifestation between 1.5 and 3 times higher than the general population.6 Despite this risk, in rare occasions it is the first manifestation. This document describes the case of a male patient, in the sixth decade of life, who presented headache with alarm signs. The simple cranial tomography documented an acute right frontoparietal subdural hematoma, accompanied by pontine intraparenchymal hemorrhage in the context of severe pancytopenia.
Case reportA 57-year-old male patient referred from Buenaventura (Valle del Cauca, Colombia) due to a clinical picture of 20 days of evolution consisting in frontal throbbing headache, with a progressive increase in intensity until reaching a score of 8/10 in the visual analog pain scale. The headache increased in intensity with position changes and Valsalva maneuvers, associated with multiple emetic episodes. In the 3 days prior to the initial consultation, there was associated blurred vision and also gingivorrhagia. In the place of referral, biochemistry paraclinical tests were carried out, as a result of which the presence of severe pancytopenia was established; in the initial cranial tomography, staggered subdural collections were observed in the right frontal region, along with a right pontine intraparenchymal hemorrhage (Figs. 1 and 2); for this reason, the patient was referred to the Intensive Care Unit.
The patient had a history of primary hypothyroidism, type 2 diabetes mellitus without insulin requirement and schizophrenia of non-specific type, under treatment with levothyroxine 50 µg/day, metformin 850 mg/day and olanzapine 10 mg at night. On the review of systems he reported repeated epistaxis, along with rectal bleeding, in the last 2 months. In the neurological examination, compromise of higher mental functions (hypoprosexia, disorientation in place, alterations in the recall memory) was observed, associated with involvement of the complete right cranial nerve IV and right peripheral facial paralysis. The patient was assessed by hematology, which considered aplastic anemia as the first possibility, in relation to his occupation as a painter of ships with lead paints, and indicated transfusion support to maintain the target hemoglobin level higher than or equal to 7 g/dl and a platelet count higher than 50,000 μ/l.
The patient was evaluated by neurosurgery, which did not consider it appropriate to perform emergency surgical intervention upon admission, due to the stability of the neurological condition and the severe thrombocytopenia, which contraindicated the procedure. Treatment was started with dexamethasone 40 mg IV/day for 4 days and prednisolone at 1 mg/kg/day for 4 weeks, along with azathioprine 50 mg/day, suggested by Rheumatology. Studies were expanded to rule out other causes of pancytopenia, with reports of non-reactive serologies for hepatitis C, cytomegalovirus and Epstein-Barr virus, and Elisa for human immunodeficiency virus. Causes of deficiency origin were also ruled out. The patient had a report of positive total anticore with reactive anti-hepatitis B surface antigen antibodies, which is why infectology considered that there was a cured infection with immunological memory.
It was performed a bone marrow biopsy, which showed erythroid hyperplasia with megaloblastic changes, but with a flow cytometry without phenotypic alterations or an increase in CD34-positive precursors; these findings were considered negative for a possible hematological neoplasm. In imaging controls indicated by neurosurgery, it was considered that the right hemispheric subdural hematoma displaced the midline with scheduled surgical indication, with additional signs of neurological deterioration manifested by drowsiness associated with mild left hemiparesis, which justified the procedure. In a medical board was established the start of cyclophosphamide 500 mg/m2 associated with the glucocorticoid, and azathioprine was discontinued.
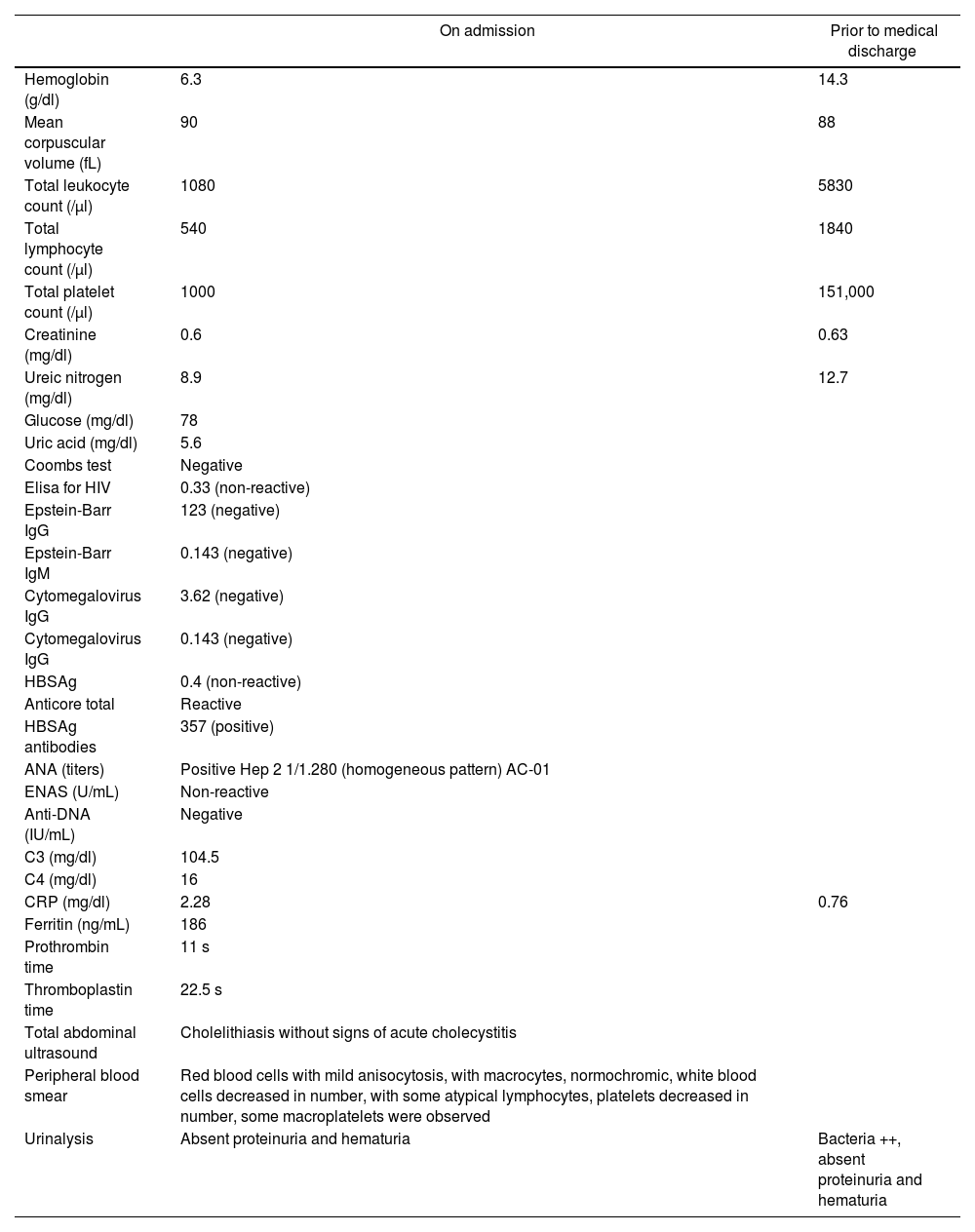
After this treatment, the normalization of the 3 cell lines was achieved and a platelet count higher than 100,000 μ/l was obtained. Given the absence of contraindication for the neurosurgical procedure, craniotomy and drainage of the right chronic subdural hematoma was performed, intervention that was accomplished without complications. Regarding the immunological profile, there was a report of ANA 1/1280 homogeneous pattern, non-reactive ENA, negative anti-DNA, negative APS profile and urinalysis without active sediment or evidence of proteinuria. An initial diagnosis of undifferentiated connective tissue disease was established, subsequently meeting criteria for SLE in a man, with atypical presentation. Table 1 shows the laboratories on admission and after treatment with dexamethasone and cyclophosphamide.
Laboratory parameters on admission and prior to medical discharge.
| On admission | Prior to medical discharge | |
|---|---|---|
| Hemoglobin (g/dl) | 6.3 | 14.3 |
| Mean corpuscular volume (fL) | 90 | 88 |
| Total leukocyte count (/μl) | 1080 | 5830 |
| Total lymphocyte count (/μl) | 540 | 1840 |
| Total platelet count (/μl) | 1000 | 151,000 |
| Creatinine (mg/dl) | 0.6 | 0.63 |
| Ureic nitrogen (mg/dl) | 8.9 | 12.7 |
| Glucose (mg/dl) | 78 | |
| Uric acid (mg/dl) | 5.6 | |
| Coombs test | Negative | |
| Elisa for HIV | 0.33 (non-reactive) | |
| Epstein-Barr IgG | 123 (negative) | |
| Epstein-Barr IgM | 0.143 (negative) | |
| Cytomegalovirus IgG | 3.62 (negative) | |
| Cytomegalovirus IgG | 0.143 (negative) | |
| HBSAg | 0.4 (non-reactive) | |
| Anticore total | Reactive | |
| HBSAg antibodies | 357 (positive) | |
| ANA (titers) | Positive Hep 2 1/1.280 (homogeneous pattern) AC-01 | |
| ENAS (U/mL) | Non-reactive | |
| Anti-DNA (IU/mL) | Negative | |
| C3 (mg/dl) | 104.5 | |
| C4 (mg/dl) | 16 | |
| CRP (mg/dl) | 2.28 | 0.76 |
| Ferritin (ng/mL) | 186 | |
| Prothrombin time | 11 s | |
| Thromboplastin time | 22.5 s | |
| Total abdominal ultrasound | Cholelithiasis without signs of acute cholecystitis | |
| Peripheral blood smear | Red blood cells with mild anisocytosis, with macrocytes, normochromic, white blood cells decreased in number, with some atypical lymphocytes, platelets decreased in number, some macroplatelets were observed | |
| Urinalysis | Absent proteinuria and hematuria | Bacteria ++, absent proteinuria and hematuria |
As previously mentioned, CVA rarely occurs as the first manifestation of SLE. Within the subtypes, it is known that the disease entails a risk 2 times higher for the ischemic CVA, 3 times higher for the hemorrhagic stroke, and almost 4 times higher for subarachnoid hemorrhage (SAH).7 On the other hand, severe thrombocytopenia could explain these hemorrhagic manifestations. Cases of SAH and intracranial hemorrhage have been described in patients with immune thrombocytopenia, but, despite this, the chronic subdural hematoma is also a rare presenting manifestation.8 In the cases described associated with thrombocytopenia, the neurological symptoms appear late in the course of the disease, because it has affected young individuals and older adults, in whom the subdural space is wider, usually accompanied by mucocutaneous hemorrhage.9
There are also published cases of subdural hematoma in patients with SLE, independently of its association with immune thrombocytopenia, without a definitive explanation for its pathophysiology. The formation of aneurysms with secondary rupture has been proposed as a possible explanation. In autopsy studies, CNS vasculitis has been documented in patients with SAH, but not in cases of subdural hematoma. Headache is usually the cardinal presenting symptom.10 A similar case has been described, of a 22-year-old female patient with characteristics of overlapping SLE, systemic sclerosis, rheumatoid arthritis and myositis, who presented progressive headache in the context of severe thrombocytopenia and anemia with subsequent description of a left temporoparietal hematoma resistant to treatment with prednisolone and azathioprine, with recurrence of the subdural hematoma, for which rescue treatment with pulses of methylprednisolone and cyclophosphamide was subsequently required.
It should be noted that this manifestation is even rarer in cases of immune thrombocytopenia secondary to a connective tissue disease.11 It is worth highlighting that pancytopenia in SLE is less common than isolated cytopenias, which makes the presentation of this case even more atypical. Other diagnostic possibilities, including bone marrow toxicity due to infections and hematological malignancy were ruled out, considering an autoimmune disease (with classification criteria for SLE) as the only etiology. The favorable response to the administration of glucocorticoids and immunosuppressants reaffirmed the diagnosis.
It is noteworthy that in immune thrombocytopenia secondary to SLE, glucocorticoids continue to be the fundamental pillar of treatment; they are able to increase the platelet count in around 2 thirds of patients, with a platelet response within 2–5 days of treatment. In addition, remission rates close to 20% have been achieved in monotherapy.12 The most commonly used treatment regimens are high doses of dexamethasone (40 mg orally or intravenously daily, for 4 days), or as an alternative, prednisone at a dose of 1 mg/kg/day for 1–2 weeks.13 The dexamethasone regimen is preferred, given that it has a faster action and avoids the toxicity inherent in the prolonged use of prednisolone. Immunoglobulin can increase the platelet count in a time lapse between 24 and 48 h, being more useful before a surgical intervention, although its effect is usually transient. Its use is also considered in patients who cannot tolerate glucocorticoids and are awaiting a second-line treatment.14
Intravenous immunoglobulin G at a dose of 1 g/kg/day is administered for 1 or 2 days. It must be taken into account that in 20–40% of cases the autoantibodies are directed against the Ibα glycoprotein and can cause thrombocytopenia through a different mechanism (independent of Fc), which explains the failure of the administration of immunoglobulin in a certain number of patients (about 25%).15 If there is no response to glucocorticoids or immunoglobulin, second-line therapies such as splenectomy, thrombopoietin receptor agonists, or cyclophosphamide are considered in some cases. The absence of positivity for antiphospholipid antibodies is striking, given its association with stroke described in patients with SLE, without it being a sine qua non condition for the definition of the syndrome.16
ConclusionsCVA is a rare manifestation as a form of presentation of SLE, particularly the subdural hematoma. In this case, immune thrombocytopenia is considered an independent risk factor for its development, but the pathophysiological mechanisms remain unclear. It is also probable that the cause is multifactorial, being involved other entities such as CNS vasculitis, rupture of aneurysms, accelerated atherosclerosis, among others. It is necessary to elucidate how the mechanisms of immune thrombocytopenia can cause vascular damage and how other association models, including antiphospholipid antibody positivity, vasculitis, atherosclerosis and non-inflammatory microangiopathy contribute to the development of the hematomas through individual or shared endothelial activation pathways.17
Ethical responsibilitiesNo research with animals or humans was performed. The right to privacy and informed consent are respected. The authors declare that no patient data appear in this document. They also declare that it does not contain personal information that allows the identification of the patient, completely preserving his anonymity (right to confidentiality). Given Resolution 8430 of 1993, research ethics committees were designated only for cases of clinical or high-risk research, which does not apply in this case.
FundingThis article has not received specific funding from the public sector, the commercial sector or non-profit entities.
Conflict of interestAs authors of this case, we certify that none of the materials in the manuscript (including tables and figures) have been previously published, nor are they included in any other manuscript.