Rotator cuff tears emerge in approximately 30% of the population over 60 years of age. Arthroscopic surgical treatment of these lesions is the treatment of choice, however, despite the improved repair techniques, the rate of re-tears ranges between 11 and 94%. Therefore, researchers seek to improve the biological healing process through the use of different alternatives such as mesenchymal stem cells (MSCs). Our objective is to evaluate the efficacy of a cellular therapy drug made from allogeneic stem cells derived from adipose tissue in a rat model of chronic rotator cuff injury.
Material and methodsThe supraspinatus lesion was created in 48 rats for subsequent suturing at 4 weeks. MSCs in suspension were added to 24 animals after suturing, and HypoThermosol-FRS® (HTS) to 24 animals as a control group. Histology (Åström and Rausing scale) and the maximum load, displacement and elastic constant of the supraspinatus tendon were analysed in both groups 4 months after the repair.
ResultsNo statistically significant differences were found in the histological score comparing the tendons treated with MSCs with respect to the tendons treated with HTS (P=0.811) nor in the results of maximum load (P=0.770), displacement (P=0.852) or elastic constant (P=0.669) of the tendon in both groups.
ConclusionsThe addition of adipose-derived cells in suspension to the repair of a chronic cuff injury does not improve the histology or biomechanics of the sutured tendon.
Las roturas del manguito rotador afectan aproximadamente al 30% de la población mayor de 60 años. El tratamiento quirúrgico por vía artroscópica de estas lesiones es el tratamiento de elección, sin embargo, a pesar de las mejoras técnicas de reparación el índice de rerroturas oscila entre el 11 y el 94%. Por ello, los investigadores buscan mejorar el proceso de curación biológica mediante el uso de diferentes alternativas como las células madre mesenquimales (MSC). Nuestro objetivo es evaluar la eficacia de un medicamento de terapia celular elaborado a partir de células madre alogénicas derivadas del tejido adiposo en un modelo de lesión crónica del manguito rotador en ratas.
Material y métodoSe creó la lesión del supraespinoso en 48 ratas para su posterior sutura a las 4 semanas. A 24 animales se les añadió las MSC en suspensión tras la sutura, y a 24 animales HypoThermosol-FRS® (HTS) como grupo control. En ambos grupos se analizó la histología (escala Åström y Rausing) y la carga máxima, desplazamiento y constante elástica del tendón supraespinoso a los 4 meses de la reparación.
ResultadosNo se encontraron diferencias estadísticamente significativas en la puntuación histológica comparando los tendones tratados con MSC con respecto a los tendones tratados con HTS (p=0,811) ni tampoco en los resultados de carga máxima (p=0,770), desplazamiento (p=0,852) ni constante elástica (p=0,669) del tendón en ambos grupos.
ConclusionesLa adición en suspensión de células derivadas de tejido adiposo a la reparación de una lesión crónica de manguito no mejora las características histológicas ni biomecánicas del tendón suturado.
Rotator cuff tears affect approximately 30% of the population over 60 years of age, and are symptomatic in most cases.1 The supraspinatus tendon is the most commonly injured tendon, given its vulnerable location under the coracoaromial arch and poor vascularisation.2–5
Furthermore, the supraspinatus tendon enthesis or “footprint” is a complex structure formed by four transitional zones at its humeral insertion, tendon, non-mineralised fibrocartilage, mineralised fibrocartilage, and bone, which hinders its reproducibility after repair.6,7
Arthroscopic surgery is the treatment of choice for these lesions,8 however, despite improved repair techniques, all of the above factors contribute to the current re-tear rate of between 11% and 94%.9,10
Investigators are therefore seeking to improve the biological healing process using different alternatives such as platelet-rich plasma (PRP), growth factors, transporters, or mesenchymal stem cell (MSC) cellular therapies.
MSCs, apart from their ability to differentiate into other cell lineages, appear to have an immunomodulatory effect that could help stimulate endogenous tissue repair through their paracrine effect, thus modulating the inflammatory reaction and providing a favourable microenvironment for the formation of regenerative tissue.8 In addition, their use in suspension would avoid the problems associated with the use of certain transporters.
Our aim is to evaluate the efficacy of a cellular therapy drug made from allogeneic stem cells from adipose tissue in a rat model of chronic rotator cuff injury, using it in suspension.
Material and methodsThis project was approved by the Animal Experimentation Ethics Committee in its meeting with minutes 6.1/2018 (Internal Code: 18/002-II), and notification of the procedure was sent to the Animal Protection Section of the Department of the Environment of the Community. All surgical procedures were performed entirely in the Animal Facility of the Experimental Surgery Unit of the Hospital Clínico San Carlos, following the recommendations described by EU Directive 2010/63/EU on animal experiments and its subsequent EU regulation 2019/1010.
The sample size was calculated following the 3R principle in animal research: Replacement, Reduction, and Refinement. For this we relied on data from a previous study with the same injury and repair model where the mean maximum load before tear was 20.8N (SD: 4.4) at 4 weeks.11,12 We considered clinically significant an increase in strength of 25% between the two groups, a power of 0.8, and a significance level of 0.05. With these specifications, the calculated sample size is 24 intervened shoulders for biomechanical study (12 adding MSC+12 adding HTS) and another 24 intervened shoulders for histological study (12 adding MSC+12 adding HTS), making a total of 48 intervened shoulders or 48 rats. The statistical package “TrialSize” for equality of means of the statistical software R was used for this purpose. We calculated 10% losses, and therefore the total estimated n was 48+5, i.e., 53 animals.
A total of 49 rats (48+one loss), 8 months old, Sprague–Dawley type, and weight range between 500g and 950g, with a mean of 678g, were used. All were male and from the same laboratory (Laboratoire Janvier). All animals underwent unilateral sectioning of the supraspinatus tendon and subsequent repair of the tendon 4 weeks after injury, at which point the injury is considered chronic.13–17
Group I (n=24) was administered the cellular therapy product (adipose tissue-derived mesenchymal stem cells [ADSC]) in suspension on the tendon repair, seeking the direct local paracrine effect of the MSCs. Group II (n=24) was administered HypoThermosol-FRS® (HTS) as a control group. Euthanasia was performed 4 months after tendon repair to send half of the samples from each group for histological study (n=24, 12 from group I+12 from group II) and biomechanical study (n=24, 12 from group I+12 from group II).
The active substance of the cell therapy drug comprises mesenchymal stem cells for allogeneic use obtained from donor lipoaspirates (ADSC), obtained, and treated in the clean room of the Hospital Clínico San Carlos, according to current regulations. HypoThermosol-FRS® is the excipient that ensures correct cell viability at a concentration of 20×106cells.
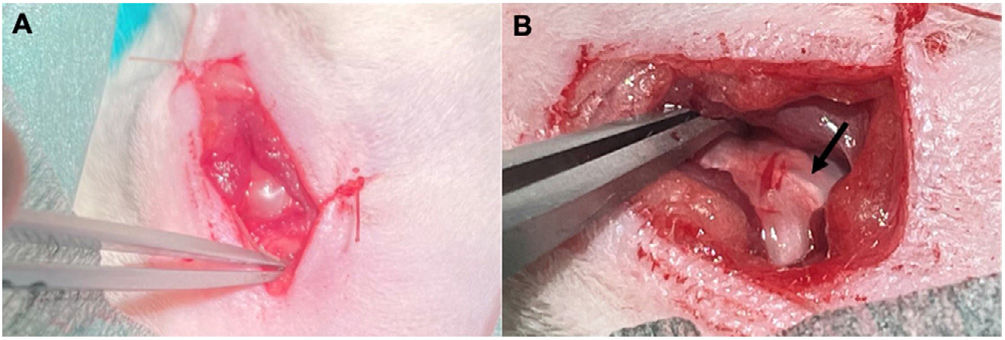
Surgical techniqueUnder sedation with isoflurane and local anaesthesia with 2% mepivacaine in skin and subcutaneous cellular tissue, a skin incision of about 2cm is made over the left shoulder with a scalpel over the superolateral part of the scapulohumeral joint. An inverted T-shaped incision is made in the deltoid muscle, allowing us to visualise the supraspinatus tendon (Fig. 1), which after its identification is marked with Prolene® 6.0, to resect it as perpendicular as possible to its major axis in the critical area of the tendon, located about 4mm from its insertion. Finally, the muscle, subcutaneous tissue, and skin are sutured with Vicryl Rapide® 4.0.
Once the intervention is completed, the gas is removed, and the animal is left on oxygen alone until it eliminates the inhaled gas.
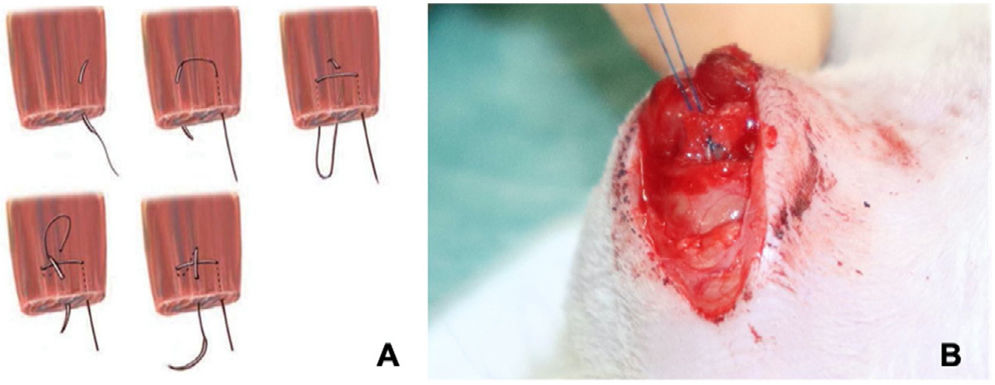
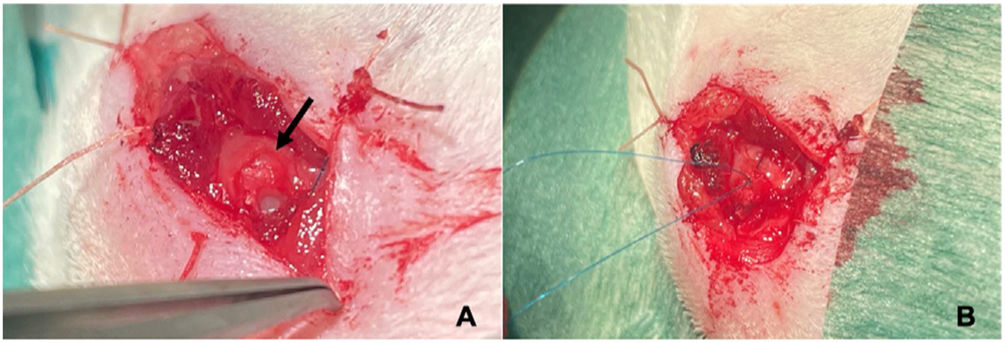
In a second operation performed under general anaesthetic 4 weeks after the first, the tendon is repaired using a modified Mason-Allen suture18 with Prolene® 6.0 (Fig. 2), after localisation of the tendon end. For fixation to the humerus, a transosseous tunnel is made through which one end of the suture is passed to bring the proximal end of the supraspinatus tendon closer to the clean region of the greater tuberosity (Fig. 3). Once the tendon has been sutured, the deltoid is closed so that the glenohumeral joint is as tight as possible.
(A) Illustration of the modified Mason–Allen suture.18 (B) Image of the surgery, modified Mason–Allen suture on the rat supraspinatus tendon.
The next step is to inject the MSC or HTS solution into the repaired tendon in suspension, depending on the study group of the animal. Finally, the subcutaneous cell tissue and skin will be sutured with Vicryl Rapide® 4.0.
During the postoperative periods following both surgeries, enrofloxacin and subcutaneous meloxicam will be injected for 3 days, for the animal's well-being.
Euthanasia is performed 4 months after the tendon repair. According to the animal protection protocol (RD 53/2013), to avoid animal suffering, the animal is given a 4% isoflurane anaesthesia mask until sedated. The animal is then placed in a hermetically sealed cabin in a CO2 atmosphere.
Histological studyAll the samples (n=24; 12 group I+12 group II) were evaluated by two investigators, double blinded. Haematoxylin–eosin was used to determine the presence of histological lesions in the tissues studied. We used the semi-quantitative assessment scale shown in Table 1, based on the Åström and Rausing scale.19 The assessments of both researchers were then pooled.
Scale and score used for histological specimens.
| −− | − | + | ++ | |||||
|---|---|---|---|---|---|---|---|---|
| Collagen matrix | Very disorganised | 2 | Disorganised | 1 | Normal, parallel fibres | 0 | ||
| Transition zone | Non-existent, bone and tendon do not touch | 3 | Disorganised | 2 | Organised, but not quite normal | 1 | Normal | 0 |
| Bursal inflammation | Absence | 0 | Presence | 1 | Moderate-severe | 2 | ||
| Granulomatous infiltrate | Absence | 0 | Presence | 1 | ||||
| Vascularisation | Irregular, perpendicular | 1 | Normal | 0 | ||||
| Tenocyte cellularity | Reduced presence | 2 | Normal | 0 | Elevated | 1 | ||
| Fatty degeneration | Absence | 0 | Presence | 1 | ||||
| Chondroid metaplasia | Absence of chondrocytes in tendon matrix | 0 | Slightly increased presence of chondrocytes in tendon matrix | 1 | Elevated presence of chondrocytes in tendon matrix | 2 |
The biomechanical study was conducted by the Department of Material Science of the Escuela Técnica Superior de Ingenieros de Caminos, Canales y Puertos, Universidad Politécnica de Madrid.
We used the Instron® tensile tester, model 4411, with a 1000N load cell and PCD 2K control software. The maximum load values (N), the degree of tendon displacement (mm), and the slope or elastic modulus of each sample (N/mm) were analysed.
We analysed both the healthy and the operated shoulder in a total of 24 animals, 12 from to group I and 12 from group II. Therefore, a total of 48 shoulders were analysed. This was because of the potential variability in size and weight from one animal to another, and thus we eliminated this bias by comparing an intervened shoulder with its contralateral shoulder.
Statistical studyContinuous variables are presented as means and standard deviations, and were analysed using the Student's t-test. Non-normal data (confirmed by the Shapiro–Wilk test), are presented as medians, using the Mann–Whitney test and the χ2 test to perform the analysis. A p-value of <0.05 was considered significant. Statistical analysis was performed with IBM® SPSS software.
ResultsThere was only one loss to follow-up. The animal belonged to group I. It died during the anaesthetic induction in the second operation without undergoing the intervention.
The total score of each group was 39 for the MSC group and 34 for the HTS group (P=0.811), with no statistically significant differences. The median of the HTS group is 1 and 2, tied at 4 samples, while that of the MSC group is 1 (Table 2). Analysing each variable separately we obtain the data shown in Table 3.
Histological results by independent variables, comparing the MSC group versus the HTS group.
| Variable | Score | MSC samples | HTS samples | χ2 test |
|---|---|---|---|---|
| Collagen matrix | 0 | 8 | 9 | 0.346 |
| 1 | 4 | 2 | ||
| 2 | 0 | 1 | ||
| Transition zone | 0 | 7 | 5 | 0.452 |
| 1 | 2 | 4 | ||
| 2 | 2 | 3 | ||
| 3 | 1 | 0 | ||
| Bursal inflammation | 0 | 9 | 9 | 0.452 |
| 1 | 2 | 3 | ||
| 2 | 1 | 0 | ||
| Granulomatous inflammation | 0 | 11 | 11 | 1 |
| 1 | 1 | 1 | ||
| Vascularisation | 0 | 9 | 12 | 0.217 |
| 1 | 3 | 0 | ||
| Tenocyte cellularity | 0 | 11 | 10 | 1 |
| 1 | 1 | 2 | ||
| 2 | 0 | 0 | ||
| Fatty degeneration | 0 | 9 | 12 | 0.217 |
| 1 | 3 | 0 | ||
| Chondroid metaplasia | 0 | 2 | 1 | 0.681 |
| 1 | 6 | 8 | ||
| 2 | 4 | 3 |
HTS: HypoThermosol; MSC: mesenchymal stem cells.
No statistically significant differences were found in any of the variables. The chondroid metaplasia variable stands out as the variable that scores highest in both groups as a pathological variable (Fig. 4).
At the biomechanical level, the results obtained are summarised in Table 4.
Mean of the biomechanical study variables in the intervened and non-intervened shoulders, compared by study groups.
| Values | MSC | HTS | Student's t |
|---|---|---|---|
| Intervened shoulders | |||
| Maximum load (N) | 50.967 (SD: 9.1095) | 51.992 (SD: 9.1296) | 0.770 |
| Tendon displacement (mm) | 5.533 (SD: 1.4405) | 5.333 (SD: 1.1927) | 0.852 |
| Slope (N/mm) | 14.85 (SD: 4.01) | 17.28 (SD: 14.96) | 0.669 |
| Non-intervened shoulders | |||
| Maximum load (N) | 55.558 (SD: 9.7207) | 58.183 (SD: 10.506) | 0.532 |
| Tendon displacement (mm) | 6.850 (SD: 2.9454) | 6.450 (SD: 1.8158) | 0.693 |
| Slope (N/mm) | 16.26 (SD: 4.89) | 17.68 (SD: 4.73) | 0.477 |
HTS: HypoThermosol; MSC: mesenchymal stem cells; SD: standard deviation.
As we can see, all the values are very similar in both samples. The average maximum load of the MSC group is 50.967N, while that of the HTS group is 51.992N, only 1 newton difference. The same occurs with the tendon displacement value, where the mean difference between the two groups is less than 1mm (5.533mm in the MSC group versus 5.333mm in the HTS group). The slope shows a greater difference between groups (14.85N/mm MSC vs. 17.28N/mm HTS), but still not enough to reach statistical significance.
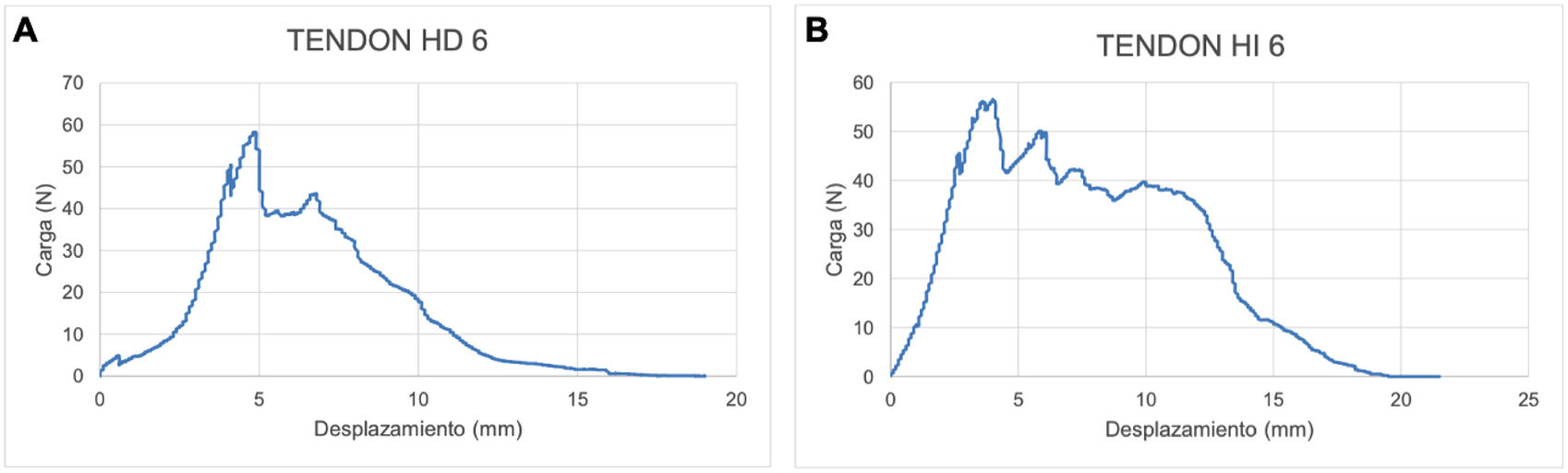
Fig. 5 shows the force–displacement curve of the healthy and intervened tendon of an animal in the MSC group. As we can see in this example, the intervened tendon displaces fewer millimetres before reaching the maximum load value of the curve (it reaches the breaking point or maximum load – 56.5N before reaching the 5mm of tendon displacement), with respect to the healthy tendon (it reaches the breaking point or maximum load – 58.2N when the tendon is displaced 5mm). The maximum load value is higher in the healthy tendon (58.2N) with respect to the intervened tendon in this case (56.5N), which means that the healthy tendon needs a higher force to cause it to tear.
DiscussionOptimising a therapy using MSCs is going to be complicated until the primary mechanisms of action exerted by the cells after administration are fully clarified. For a long time, it was thought that the therapeutic activity exerted after MSC administration depended exclusively on the ability of the cells to engraft into tissues and their ability to differentiate. However, numerous later studies began to demonstrate the effectiveness of cellular therapy despite the poor engraftment capacity of the cells, mainly when administered systemically.20 It is now believed that the mechanism of action of MSCs is associated not only with their ability to engraft and differentiate but also with their ability to release paracrine signals, although the contribution of each of these properties remains unclear.21 It is known, however, that factors associated with MSCs such as cell size, donor age, immunogenicity related to donor source (autologous or allogeneic), culture passage, as well as the number of passages, are factors associated with the distribution of the cells after administration, are important for obtaining quality cells, hence the importance of a cellular therapy product that provides this information and, therefore, this safety when they are used as a treatment option.
The most commonly used MSCs are bone marrow-derived MSCs (BMSCs) and adipose-derived MSCs (ADSCs). In our study we chose MSCs derived from adipose tissue because their main advantage is their great accessibility in daily practice, as they can be obtained through procedures such as liposuction, giving a considerably higher yield than other locations. In addition, factors such as the age of the donor, underlying diseases, or the form of culture and subsequent treatment prior to administration can be controlled. The main disadvantage is that most are used allogeneically in the recipient.
The therapeutic effects of ADSCs for rotator cuff injuries in experimental models are summarised in Table 5. As can be seen, the rat is an animal widely used in the literature for the study of rotator cuff disorders. Its ease of handling, its similarities in anatomy and mobility of the shoulder to the human, added to the previous experience of our research group with this animal, resulted in our choosing it for this study.13,22
Therapeutic effects of ADSCs on rotator cuff injuries in rat experimental models.
| Animal | Model of lesion | Type of cells | Method of delivery | Observation time | Results | |
|---|---|---|---|---|---|---|
| Mora et al. (2014) | Rat | Supraspinatus tendon acute repair | ADSC | Collagen scaffold | 2 and 4 weeks | Less inflammation, but without improving biomechanical properties |
| Barco et al. (2015) | Rat | Repair of acute injury to supraspinatus tendon | ADSC | Fibrin glue | 4 and 8 weeks | Diminished presence of neutrophils and increased presence of plasma cells without improving the histological appearance and biomechanical strength of the tendon |
| Chen et al. (2015) | Rata | Repair of acute injury to supraspinatus tendon | ADSC | One week | Diminished inflammation, improved histological appearance and maximum load at 7 days. There are no differences over more time | |
| Lipner et al. (2015) | Rat | Repair of acute injury to supraspinatus tendon | ADSC/ADSC with BMP-12 | PLGA nanofibers with gradients in mineral with fibrin hydrogel | 2, 4, and 8 weeks | ADSC transported with BMP-12 decreases mechanical properties, strength, and elasticity in the repair zone |
| Rothrauff et al. (2019) | Rat | Repair on acute supraspinatus and infraspinatus tendon injury/repair on chronic injury with intramuscular botulinum toxin A injection | ADSC | GelMA/fibrin hydrogel | 4 weeks | Increased bone mineral density of the proximal humerus in the chronic model with both groups |
| Kaizawa et al. (2019) | Rat | Repair on a chronic supraspinatus tendon injury | ADSC | Human tendon hydrogel | 8 weeks | Augmentation of the tendon with hydrogel with ADSC improves biomechanical properties and fibrocartilage area, but does not improve tendon-bone interface. |
| Shin et al. (2020) | Rat | Repair on a chronic supraspinatus tendon injury | ADSC | Sheep-derived cells | 2 and 4 weeks | Larger fibrocartilage area, higher bone volume/total volume values and biomechanical properties |
ADSC: adipose tissue-derived stem cells.
As for our histological results, none of the variables analysed in our study were statistically significant, and therefore, we were unable to demonstrate that stem cell treatment in this model of chronic injury has sufficient effect to improve the histology of the tendon. In reviewing the literature, we encountered two problems: one is that most of the studies that use MSCs do so on models of acute injury and, therefore, we cannot compare ours with these studies and the cell regeneration capacity they describe, as the healing mechanisms are not the same if the injury is acute or chronic23–27; and the other is that there is no study that focuses entirely on and assesses the paracrine effect of the cells by leaving them in suspension in the glenohumeral joint, but most use transporters or add growth factors to the samples.23,26–29
Shin et al. used adipose-derived MSCs in their chronic rat model of rotator cuff tear by placing them on a slide as a transporter. They observed at 2 and 4 weeks after repair that at histological level the cell-treated group had a statistically significantly larger fibrocartilage area.29
In terms of biomechanical results, we found no statistically significant differences to suggest that cell treatment provides a clear improvement in the biomechanical properties of the tendon.
The maximum load is the maximum force required to be applied to the tendon for it to tear; therefore, the greater the maximum load, the more resistant the tendon will be. Analysing our results, we see that the mean maximum load of the non-intervened tendons (56.871N – SD: 9.9889) is greater than the mean maximum load of the intervened tendons (51.479N – SD: 8.9344). In other words, the intervened tendons tear earlier. Similarly, by adding MSC (50.967N – SD: 9.1095) or HTS (51.479N – SD: 9.1298) over the suture, we are not able to provide that resistance that the tendon needs to increase the maximum load, and thus avoid the risk of tear more effectively.
Tendon displacement in millimetres assesses how much the tendon is able to yield before it tears at maximum load. Analysing our results, the intervened shoulder tendons withstand less tendon displacement before tear (5.433mm – SD: 1.2974) compared to healthy tendons (6.650mm – SD: 2.4016), therefore, they are stiffer. Moreover, by providing MSC (5.533mm – SD: 1.4405) or HTS (5.333mm – SD: 1.1927), we are not able to improve this biomechanical characteristic of the tendon.
Finally, the variable “slope” or also known as ‘elastic modulus’ gives an estimated evaluation of the tendon elasticity, so that the greater the slope, the less the elasticity of the tendon evaluated. Analysing our results, the slope of the healthy tendons (16.97N/mm – SD: 4.76) is very similar to the slope of the intervened tendons (16.06N/mm – SD: 4.56).
From the literature, we see that Shin et al. observed that at 4 weeks the tendon biomechanics of the cell-treated group obtained higher scores, this result being statistically significant.29 Kaizawa et al. who already had a previous study in which they implanted a tendon hydrogen (tHG), a type I collagen rich gel in a model of chronic injury, and concluded with statistically significant results an improvement in the biomechanical properties of the tendon, wanted to continue in the same line, but this time augmenting the hydrogel with ASC, to see if it further improved the scarring properties of the tendon.28 For their study they analysed 2 variables, the load to failure in newtons, and the elasticity measured in N/mm. They performed the study at 8 weeks after repair, finding that the maximum load to failure was significantly higher in the tHGASC group and the tHG group compared to the control. The same occurred with respect to stiffness, obtaining statistically significant differences in the tHGASC and tHG groups with respect to the control group. The ASCs only statistically significantly improved the bone morphometry of the insertion site, but did not improve the biomechanical or histological healing of the supraspinatus tendon.
In a previous study by our group, Tornero-Esteban et al. analysed the biomechanical impact of MSC delivery on a type I collagen transporter (OrthADAPT) in a rat model of chronic cuff tear. As in our work, they analysed the maximum load, the degree of tendon displacement, and the elastic modulus of the tendon. Their results conclude that the maximum tendon load increases over time, and with it the tendon strength (in the OrthAADAPT+MSC group). With respect to stiffness and deformity, no statistically significant differences were found.14 However, in the study later published by Lamas et al. in humans, we observed that the use of a transporter could cause adverse effects in the patient, which is why in this study we decided to dispense with the use of a transporter and take advantage of the paracrine characteristics offered by MSCs.30
The limitations we may encounter, as in most experimental studies, is the small sample size for each group. Initially, the sample size was calculated on the basis of tendon strength, and not on the basis of the histological scale of the tendon, and therefore, we cannot be sure that the lack of results on this point is because there are no statistically significant differences between the two treatments, or because the sample size is insufficient. In addition, the inability to control the degree of tendon retraction after the generation of the chronic injury could condition the rate of retear or the biological healing of the tendon. The histological assessment, although performed blind and by two investigators, has the disadvantage that certain parameters may be altered by histological variance at the tendon site of the cut performed.
ConclusionsAlthough the regulatory and mediating capacity of MSCs through their paracrine effect is increasingly evident, the addition of adipose-derived cells as a cell therapy drug in suspension on the suture did not improve the histological and biomechanical characteristics of the injured tendon in our rat model of chronic injury.
Level of evidenceLevel of evidence I.
FundingThis work was conducted thanks to the research Project funded by the Instituto Carlos III, Madrid (FIS) CIF: G83727115 File PI16/00549.
Conflict of interestsCo-author Yaiza Lópiz is a member of the editorial board of the journal, but did not participate in the blind review process or in the decision-making process of the article.
The authors have no conflict of interest to declare.