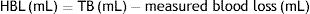Bone as a material varies its composition and mechanical properties throughout life. Although these variations are better understood in adulthood, there is little experimental information on the variation of these properties in early stages of development. The objective of this study is to analyze the mechanical behavior and chemical properties of cortical bone tissue from two animal species in these earliest stages.
Material and methodologyTwenty specimens of cortical bone were manufactured from bovine and ovine species that were in different stages of development (feeding exclusively on breast milk, in the transition period to feed or pasture, and young animals but on a solid food diet). The specimens were subjected to tensile tests, recorded with a high-speed camera to obtain deformation maps. Measurements of the tensile force until the specimen broke were also carried out. A fractographic study was carried out with a scanning electron microscope to analyze the fracture surface and an analysis of the amount of calcium in each of the specimens using X-ray dispersion spectroscopy.
ResultsA statistically significant and positive correlation was found between the elastic modulus of the specimens and their calcium content. A trend towards more rigid behavior with age was observed.
ConclusionsYoung bone tissue tends to stiffen with age as the calcium content increases with an increase in elastic modulus.
El hueso como material varía su composición y sus propiedades mecánicas a lo largo de la vida. Aunque estas variaciones se conocen mejor en la edad adulta, existe poca información experimental sobre la variación de estas propiedades en los estados tempranos de desarrollo. El objetivo de este estudio es analizar el comportamiento mecánico y las propiedades químicas de tejido óseo cortical de 2 especies animales en estas etapas más tempranas.
Material y metodologíaSe fabricaron 20 probetas de hueso cortical de especies bovina y ovina que se encontraban en diferentes etapas de desarrollo (alimentación exclusiva de leche materna, en período de transición a pienso o pasto y animales jóvenes, pero con dieta de alimentos sólidos). Las probetas fueron sometidas a ensayos de tracción, grabados con una cámara de alta velocidad para obtener mapas de deformación. También se realizaron medidas de la fuerza de tracción hasta la rotura de la probeta. Se efectuó un estudio fractográfico con microscopio electrónico de barrido para analizar la superficie de fractura y un análisis de la cantidad de calcio en cada una de las probetas mediante espectroscopía de dispersión de rayos-X.
ResultadosSe encontró una correlación estadísticamente significativa y positiva entre el módulo de elasticidad de las probetas y el contenido en calcio de estas. Se observó una tendencia a un comportamiento más rígido con la edad.
ConclusionesEl tejido óseo joven tiende a rigidizarse con la edad al aumentar el contenido en calcio con un aumento del módulo elástico.
It is essential that the relationship between the biology and mechanics of bone tissue be ascertained, inasmuch as a mechanically unsuitable bone cannot fulfil its purpose. The connection between the biology of the bone, its composition, and its mechanical function is conceptualized as a functional adaptation. Studying, analysing, and systematising bone as a tissue and as an organ helps to understand the correlation that exists between structure and function, given that the shape of the bone must reflect a balanced morphology of the various stresses to which it is subjected.1,2
The bones of the skeleton not only withstand the demands and accidental impacts, but, above all, they suffer repetitive and cyclical loads, as well as loads of varying magnitude that occur in everyday activity. Each bone maintains a functional competence; that is to say, that it adapts to any number of possibilities and situations, which Biewener3 called «safety factors», very common in engineering design, that are defined as the rate of failure resistance of a structure to the highest stress that can be expected during its use.4
Seventy percent (70%) of bone content consists of minerals (calcium, phosphorous, magnesium...), oxygen, and hydrogen, in proportions that correspond to hydroxyapatite. The remaining 30% is made up of collagen. Small crystals of hydroxyapatite attach to the collagen fibres. From a mechanical point of view, it behaves as a heterogeneous and anisotropic material. The bonding of collagen to hydroxyapatite is what lends the bone its exceptional mechanical properties. The less the skeleton weighs, the lower the cost and the more economical it is, thereby increasing efficacy. Thus, long bones are designed as hollow tubes with a greater thickness of the bone wall in the middle portion than at the ends, where tensile stresses and compression are greater.5
The mineral properties of bone are highly sensitive to the degree of mineralization of the bone matrix, finding exponential growth in bone rigidity as mineralization increases.6–9 In fact, Currey et al.7 demonstrated that the modulus of elasticity and calcium content were very closely related to each other. Nevertheless, other publications have failed to detect a relationship between mineralization and the strength of the material and, in fact, have even observed a negative correlation.
The purpose of this project is to develop a technique by which to analyse the mechanical behaviour of cortical bone tissue and its relationship with its chemical composition in early stages of development. Our objective is to determine the changes in the mechanical properties at different time points of bone development in early ages in two different animal species (bovine and ovine) and to determine whether this change correlates with the amount of calcium in a similar way in both species.
Materials and methodologyLong bones (tibias and femurs) from two mammalian species (bovine and ovine) were obtained through local suppliers, taking care that the bones were within the appropriate age groups: specimens fed solely on maternal mild (suckled), transitioning to solid food, and mature (fed exclusively on solid food).
The suckled specimens included calves of up to 12 months of age and sheep of up to 4 months of age; the group of animals transitioning to solid food included those that had begun to graze; that is to say, between 12 and 18 months for the bovine subset and between four and 12 months for the sheep; while the mature individuals ranged from 18 months and 12 months and older for cows and sheep, respectively.
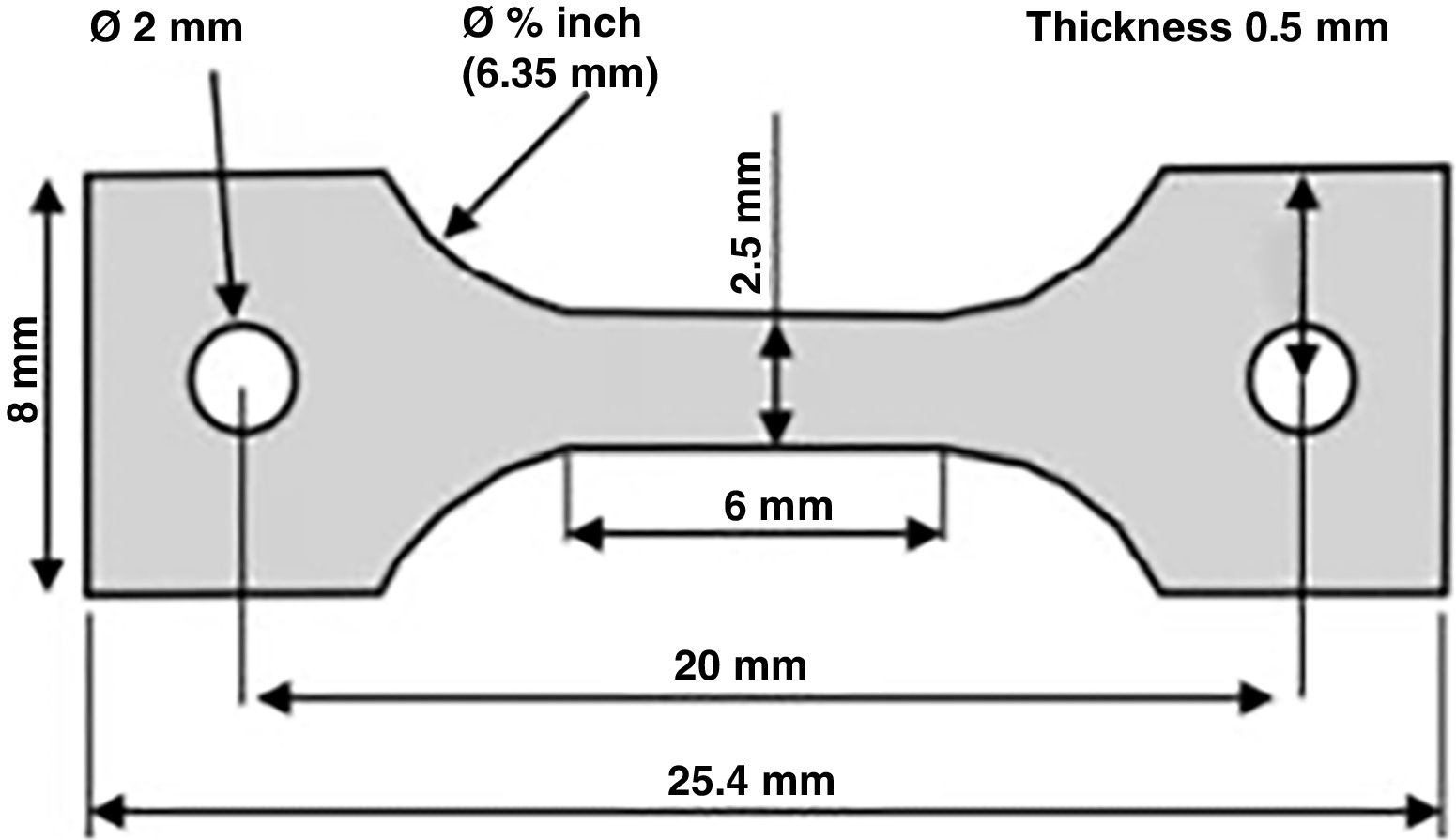
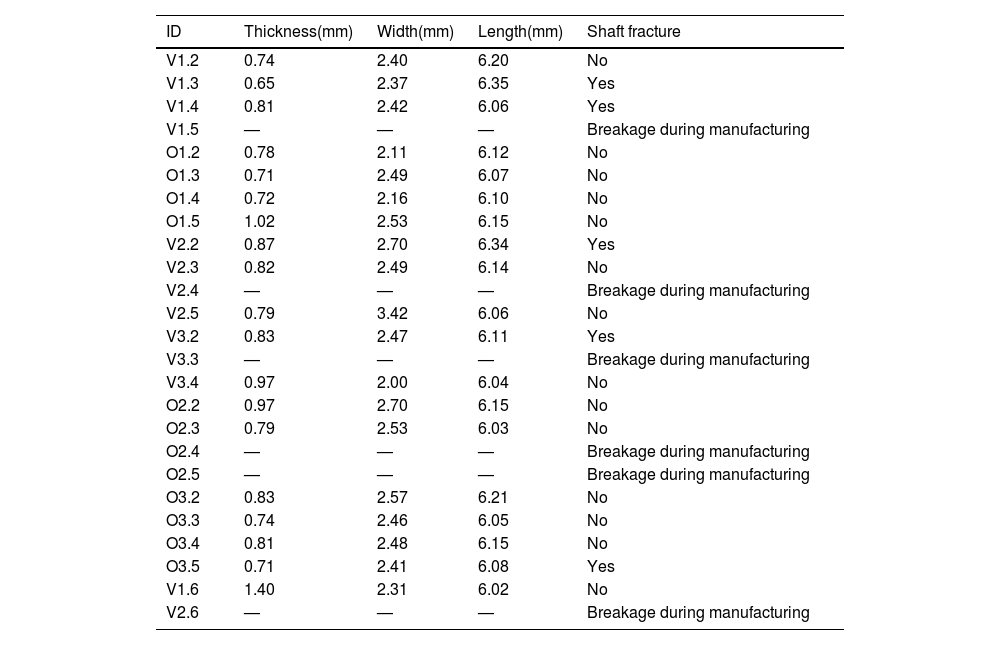
Specimens were created from bone tissue taken from the diaphysis of the bone, obtaining longitudinal shavings that were 0.8mm thick, using a 1mm thick circular blade saw with refrigeration (Remet Micromet Evolution 8502, Bologna, Italy). These shavings were machined to attain dog bone-shaped specimens (having a nominal size of 6×25mm)10 (Table 1 and Fig. 1).
Dimensions and characteristics of the specimens tested.
| ID | Thickness(mm) | Width(mm) | Length(mm) | Shaft fracture |
|---|---|---|---|---|
| V1.2 | 0.74 | 2.40 | 6.20 | No |
| V1.3 | 0.65 | 2.37 | 6.35 | Yes |
| V1.4 | 0.81 | 2.42 | 6.06 | Yes |
| V1.5 | — | — | — | Breakage during manufacturing |
| O1.2 | 0.78 | 2.11 | 6.12 | No |
| O1.3 | 0.71 | 2.49 | 6.07 | No |
| O1.4 | 0.72 | 2.16 | 6.10 | No |
| O1.5 | 1.02 | 2.53 | 6.15 | No |
| V2.2 | 0.87 | 2.70 | 6.34 | Yes |
| V2.3 | 0.82 | 2.49 | 6.14 | No |
| V2.4 | — | — | — | Breakage during manufacturing |
| V2.5 | 0.79 | 3.42 | 6.06 | No |
| V3.2 | 0.83 | 2.47 | 6.11 | Yes |
| V3.3 | — | — | — | Breakage during manufacturing |
| V3.4 | 0.97 | 2.00 | 6.04 | No |
| O2.2 | 0.97 | 2.70 | 6.15 | No |
| O2.3 | 0.79 | 2.53 | 6.03 | No |
| O2.4 | — | — | — | Breakage during manufacturing |
| O2.5 | — | — | — | Breakage during manufacturing |
| O3.2 | 0.83 | 2.57 | 6.21 | No |
| O3.3 | 0.74 | 2.46 | 6.05 | No |
| O3.4 | 0.81 | 2.48 | 6.15 | No |
| O3.5 | 0.71 | 2.41 | 6.08 | Yes |
| V1.6 | 1.40 | 2.31 | 6.02 | No |
| V2.6 | — | — | — | Breakage during manufacturing |
Twenty-five specimens were created that were categorised into six different groups based on the animal species and age group. The specimens were identified according to the stage of development of the animals’ diet and the group to which they belonged. V1 and O1 were used to refer to specimens that were still being suckled (bovine and ovine, respectively), V2 and O2 for animals transitioning to solid food, and V3 and O3 for the more mature mammals. Six of these specimens were damaged during the manufacturing process and could not be used in the experimental testing. Consequently, the calculations were made on 19 specimens.
The specimens attained (n=19) were kept saturated in physiological saline solution. Before carrying out the experiments, the surfaces of the samples were assessed using a high-resolution digital microscope (Olympus DSX10-UZH, Tokyo, Japan).
Mechanical tensile tests were performed on a universal tensile testing machine (Ibertest IBTH-5/500, Spain), with an aluminium clamping system specifically designed to ensure no misalignment between the upper and lower ends of the specimens and to guarantee the axial loading of the specimen.
The tests were performed in a quasi-static mode at a strain rate of 50mm/min and with a preliminary load of 2.2N. The strength data were obtained using a 6-axis load cell and a DTS Slice Micro data acquisition system. The strain was calculated as the tensile strength divided by the initial cross-section in the middle of the reference area, using the formula:
In which σ represents stress; F, the tensile load applied, and A, the cross section of the specimen measured in the middle of the area of the shaft. Deformation was calculated using the displacement measurements provided by the tensile testing machine and applying the following equation:
In which ɛ denotes deformation; Δl is the difference between the final length and the initial length, and l0 represents the initial length. We regard the initial length to be 6mm for the specimens that broke in the central area and 20mm in the case of those specimens that broke in the area where they were clamped, said area comprising the distance between the bores in the aluminium bars. The tests were recorded at 100Hz using a high-speed camera (iXCamera) synchronised with the data acquisition system.
The modulus of elasticity of the samples was estimated by developing a proprietary code in the MATLAB software programme (R2023a Update 1, The Mathworks Inc).
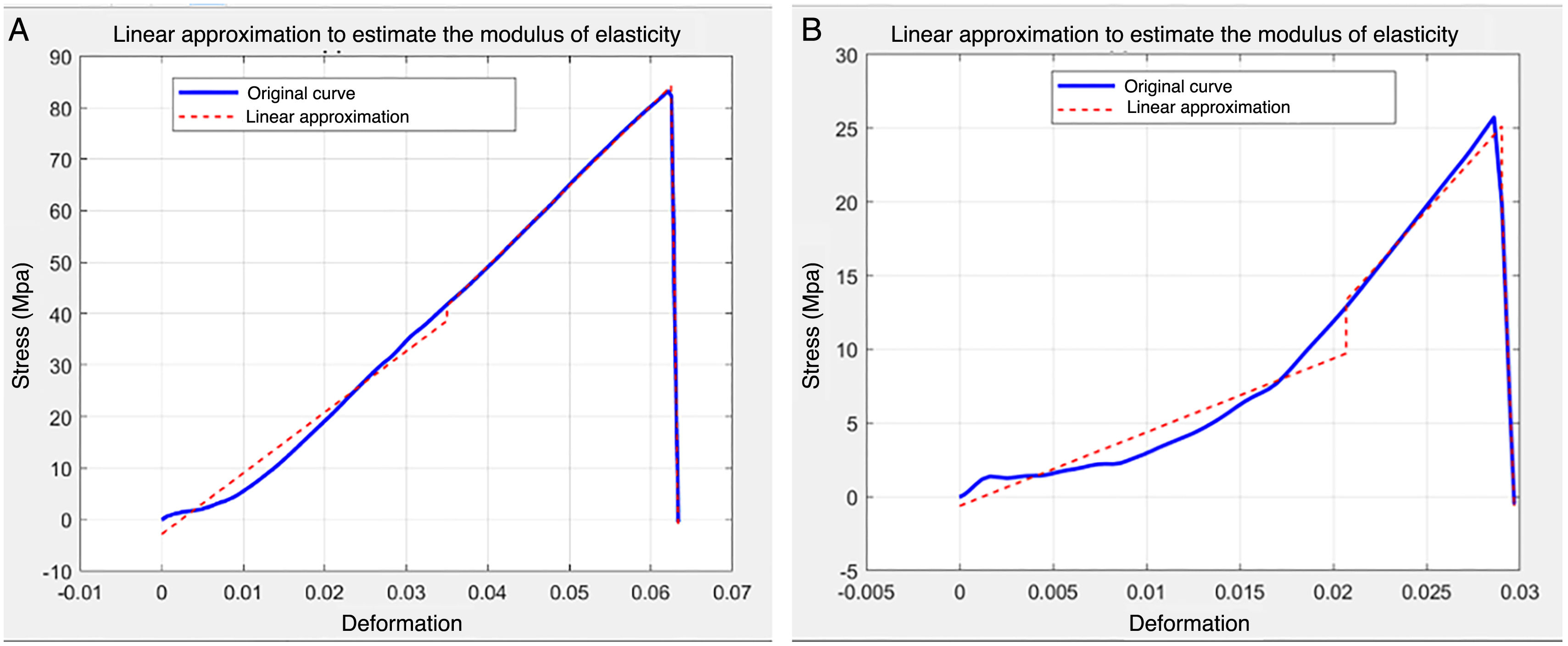
Each of the stress/strain curves was simulated by a linear curve in segments. The number of segments of the curve was obtained by iterations in which the RMS error of each section was minimised. The final number of spans was selected visually by finding the number of spans that minimised the error for the different linear segments. Finally, the modulus of elasticity was chosen as the slope corresponding to the second linear section of the estimation, inasmuch as the first linear section generally corresponded to a section with less slope that was likely to be related to an alignment of the heads of the tensile machine during the first instants of the test.
In addition, the morphology of the fracture surface and the amount of calcium of each specimen was evaluated by performing a fractographic and compositional analysis under a scanning electron microscope (SEM) (Zeiss Auriga Gemini). As bones are not a conductive material, the specimens underwent a metallisation process prior to analysis with a conductive carbon tape. A carbon layer of 12.67nm was then deposited on each specimen using a Leica EM ACE600 metalliser. The metallisation vacuum pressure was set at 10-4mbar and 26 pulses were required to deposit the appropriate layer thickness. For the fractographic study, the working voltage was 15kV and the only fractures inspected were the surface fractures of those specimens that broke in the shaft. As for the compositional analysis, the QUANTAX program (Bruker AXS) was used, based on energy-dispersive X-ray (EDX) microanalysis. One of the specimens could not be correctly metallised and, as a result, the compositional analysis was limited to the 18 remaining specimens.
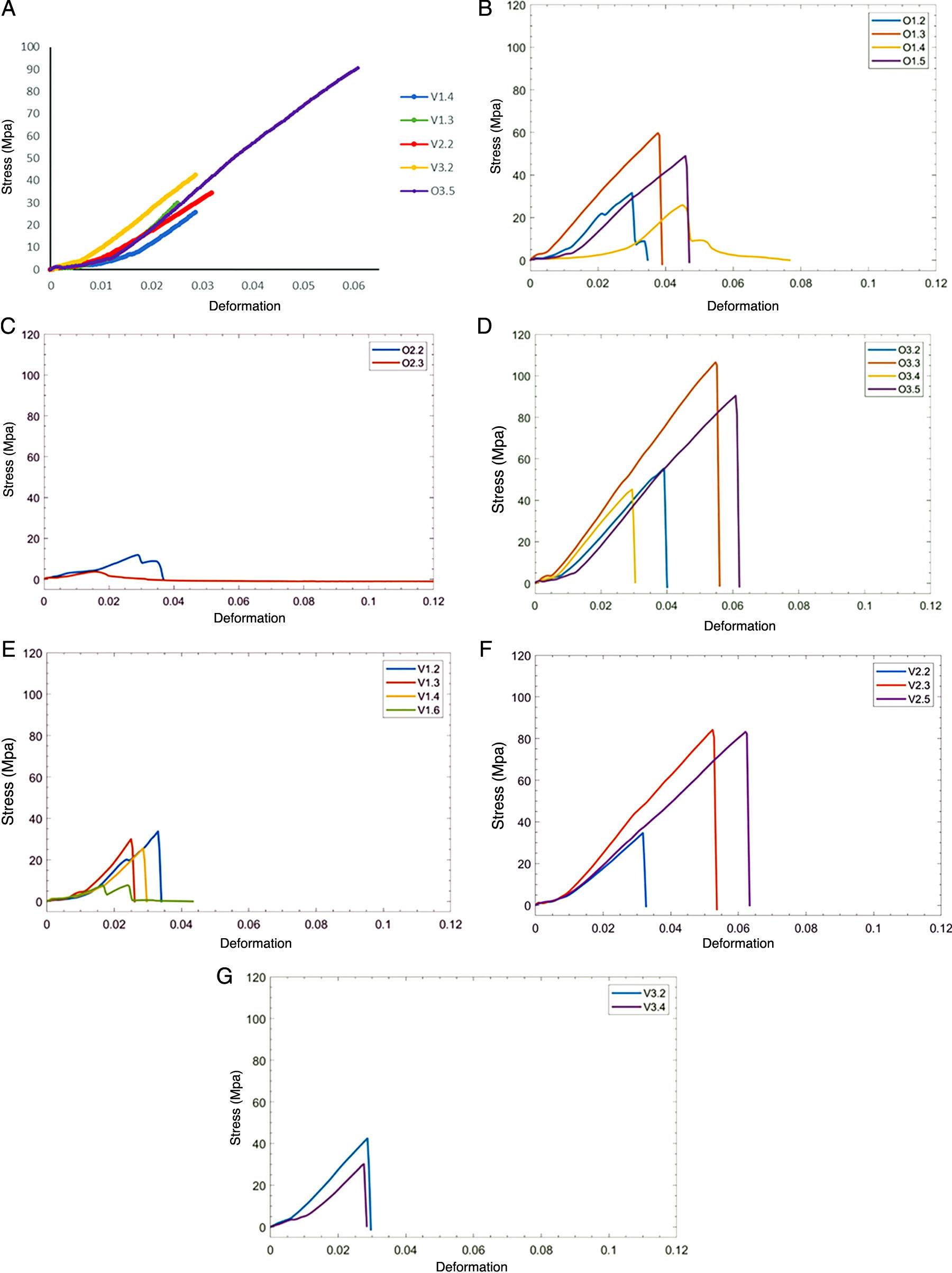
It should be noted that the calculation of the modulus of elasticity is only meaningful for those specimens that broke in the area of the shaft (Fig. 2), although stress-strain curves were plotted for all specimens tested (Fig. 3). Likewise, it is important to point out that the calculation of the modulus of elasticity only makes sense for those specimens that broke in the shaft zone, although the stress-strain curves were plotted for all the specimens tested (Fig. 3).
A) Stress-deformation curves of specimens that failed in the area of the shaft. B-D) Stress- deformation curves for the ovine specimens as a function of age group: suckling individuals (top), individuals in transition (middle), mature individuals (bottom). E-G) Stress- deformation curves of bovine specimens as a function of age group: suckling individuals (top), individuals in transition (middle), mature individuals (bottom).
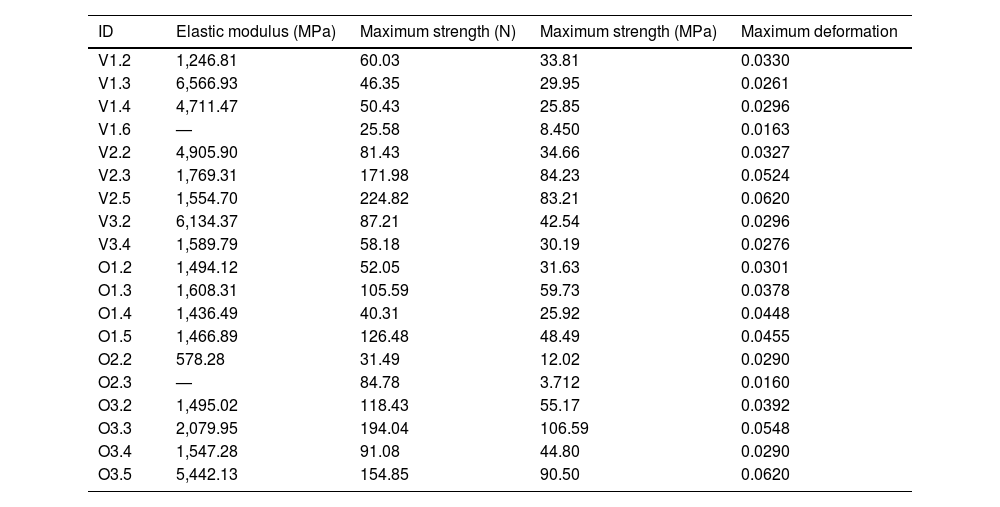
To calculate the modulus of elasticity, the load curve of the specimen prior to fracture and an initial length of l0=6mm, which corresponds to the length of the specimen shaft, were analysed. Table 2 displays the values of the modulus of elasticity, strength, stress, and maximum deformation obtained for these specimens (in the shaded cells).
Values of maximum strength, maximum stress, and maximum deformation. The shaded specimens correspond to the specimens that broke in the area of the shaft; consequently, the value of the elastic modulus corrected by the length of the shaft (6mm) is provided.
| ID | Elastic modulus (MPa) | Maximum strength (N) | Maximum strength (MPa) | Maximum deformation |
|---|---|---|---|---|
| V1.2 | 1,246.81 | 60.03 | 33.81 | 0.0330 |
| V1.3 | 6,566.93 | 46.35 | 29.95 | 0.0261 |
| V1.4 | 4,711.47 | 50.43 | 25.85 | 0.0296 |
| V1.6 | — | 25.58 | 8.450 | 0.0163 |
| V2.2 | 4,905.90 | 81.43 | 34.66 | 0.0327 |
| V2.3 | 1,769.31 | 171.98 | 84.23 | 0.0524 |
| V2.5 | 1,554.70 | 224.82 | 83.21 | 0.0620 |
| V3.2 | 6,134.37 | 87.21 | 42.54 | 0.0296 |
| V3.4 | 1,589.79 | 58.18 | 30.19 | 0.0276 |
| O1.2 | 1,494.12 | 52.05 | 31.63 | 0.0301 |
| O1.3 | 1,608.31 | 105.59 | 59.73 | 0.0378 |
| O1.4 | 1,436.49 | 40.31 | 25.92 | 0.0448 |
| O1.5 | 1,466.89 | 126.48 | 48.49 | 0.0455 |
| O2.2 | 578.28 | 31.49 | 12.02 | 0.0290 |
| O2.3 | — | 84.78 | 3.712 | 0.0160 |
| O3.2 | 1,495.02 | 118.43 | 55.17 | 0.0392 |
| O3.3 | 2,079.95 | 194.04 | 106.59 | 0.0548 |
| O3.4 | 1,547.28 | 91.08 | 44.80 | 0.0290 |
| O3.5 | 5,442.13 | 154.85 | 90.50 | 0.0620 |
MPa: Megapascals.
To complete the analysis, the process of calculating the modulus of elasticity was repeated for all the specimens tested, but in the case where the fracture occurred outside the central area, the initial length between the machine fastening holes was used in the calculation of the deformation (l0=20mm).
Finally, non-parametric tests (Spearman's correlation) were carried out to verify whether there was a relationship between the modulus of elasticity, the maximum strength, maximum stress and maximum deformation obtained in the tensile tests and the amount of calcium quantified in the specimen. The level of statistical significance was set at p<0.05.
ResultsTensile testOnly 5 specimens broke in the area of the shaft, while the others fractured at one of the heads of the specimen. In several of these samples, initial defects were observed in the bone as a result of machining, which probably played a part in the location of the fracture of the specimen.
Judging from the stress/deformation curves obtained, the specimens exhibited a linear elastic behaviour, which led to them behaving like a brittle fracture.
Both peak stress and peak force increased with the developmental stage of the sample, achieving values of 29.95 megapascals (MPa) and 25.85MPa for the suckling species samples, around 35MPa for the bovine specimen transitioning to solid food, and 42.54 and 90.5MPa for mature bovine and ovine samples (Table 2).
Because of the small sample size, it proved difficult to determine the trend of variation of the peak stress or deformation at break as a function of the stage of development of the bones of each species. We observed a tendency towards more rigid behaviour of the curves (steeper slope) with increasing age of the sample, although this should be interpreted with caution due to the variability within each age group.
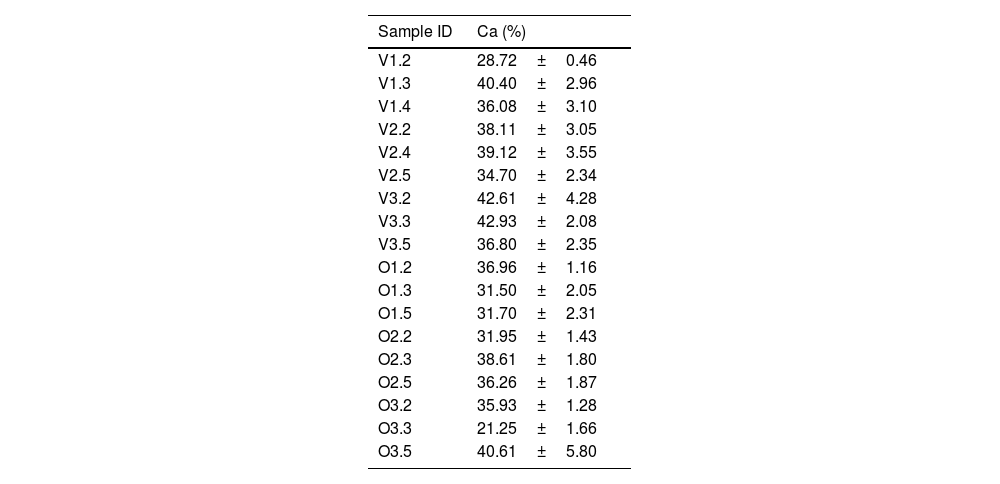
Compositional analysisThe percentage of calcium detected in the specimens and analysed using the EDX technique (Table 3) illustrated a great deal of dispersion.
Compositional analysis (%) obtained in the 18 samples used EDX (X±DE).
| Sample ID | Ca (%) |
|---|---|
| V1.2 | 28.72±0.46 |
| V1.3 | 40.40±2.96 |
| V1.4 | 36.08±3.10 |
| V2.2 | 38.11±3.05 |
| V2.4 | 39.12±3.55 |
| V2.5 | 34.70±2.34 |
| V3.2 | 42.61±4.28 |
| V3.3 | 42.93±2.08 |
| V3.5 | 36.80±2.35 |
| O1.2 | 36.96±1.16 |
| O1.3 | 31.50±2.05 |
| O1.5 | 31.70±2.31 |
| O2.2 | 31.95±1.43 |
| O2.3 | 38.61±1.80 |
| O2.5 | 36.26±1.87 |
| O3.2 | 35.93±1.28 |
| O3.3 | 21.25±1.66 |
| O3.5 | 40.61±5.80 |
EDX: Energy-dispersive X-ray.
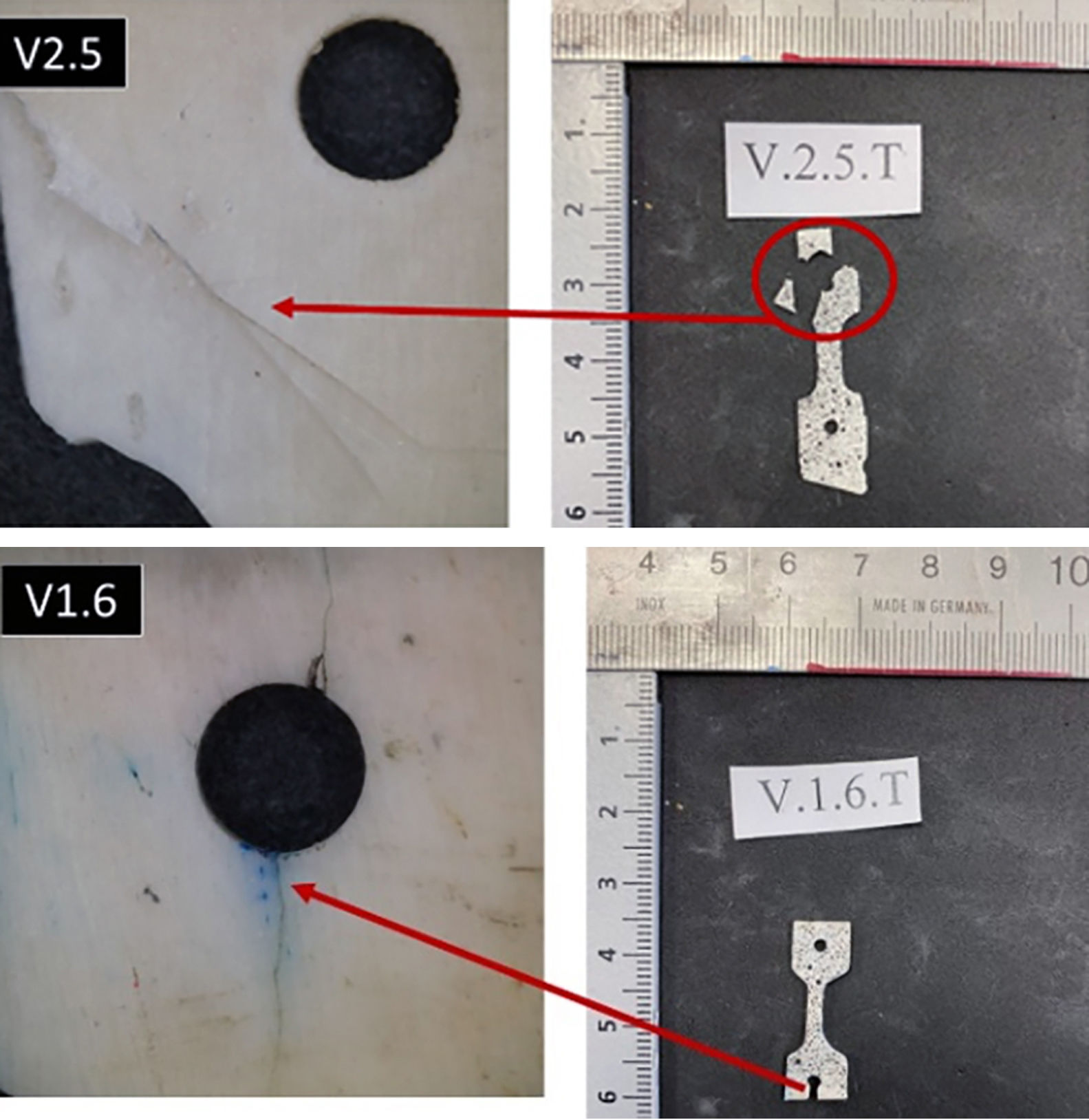
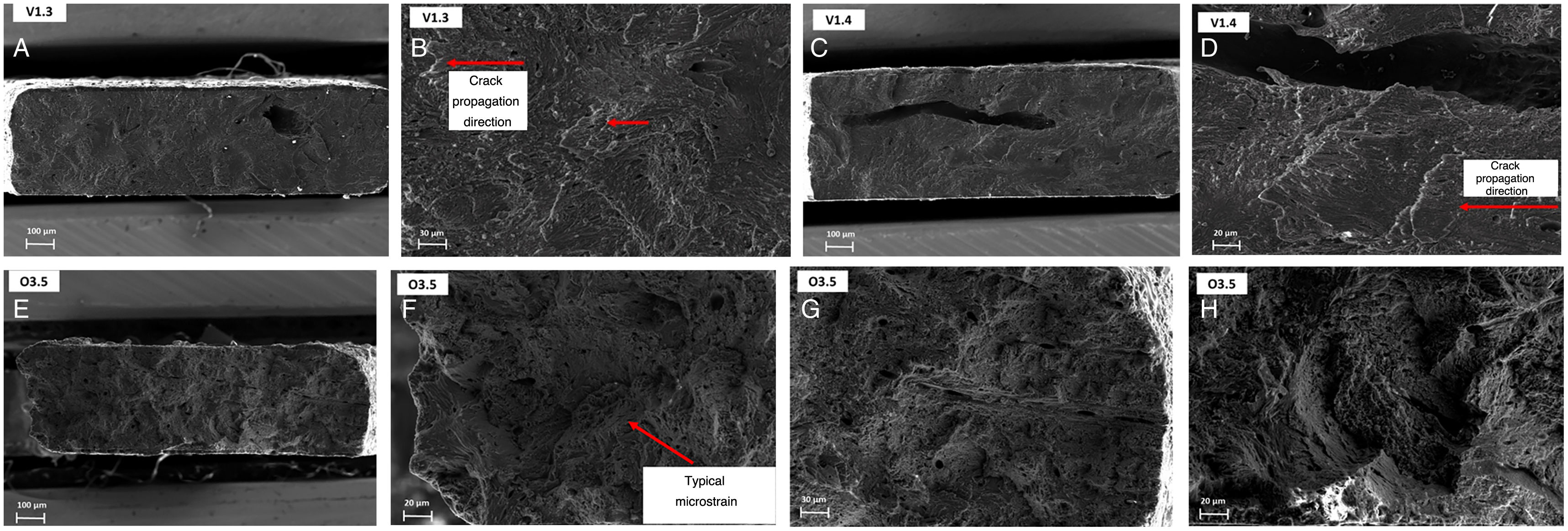
Figure 4 presents the fracture planes for specimens V1.3 and V1.4 (bovine infants). The fracture surface in both cases was very flat, indicative of brittle fracture behaviour. They were fractures that behaved elastically, with no plastic deformation and low energy release, which was confirmed by how clean the surface was and by the stress curves. The fracture planes were oriented toward the left, indicating that the crack started on the right side of the surface, probably due to the presence of pores in the specimens. This type of fracture was evident for all samples made from individuals who were still suckling, as well as those who were transitioning to solid food. In contrast, the fractography of specimen O3.5, which withstood the greatest force before fracture, demonstrated a distinct fracture pattern with signs of microstrain and multiple fracture planes (Fig. 4).
The fractographic analysis showed that the ovine bone was more porous than the bovine bone.
The statistical analysis proved a significant and positive correlation between the modulus of elasticity of the samples and their calcium content. Nevertheless, no significant correlation of the maximum strength with the maximum stress (p=.061) or with the maximum deformation (p=.073) was detected.
DiscussionBone is a complex tissue, exceptional in its properties as a rigid material structure and yet remarkable for its morphological plasticity. These unique mechanical properties derive from the composition of the bone tissue, which combines an inorganic phase, consisting primarily of hydroxyapatite crystals, with an organic phase, in which more than 30 proteins can be found, with collagen type I being the predominant component during growth. The collagen is mineralised.9 The hydroxyapatite crystals are resistant to compression with high strength but low elasticity, while the collagen fibres present low strength and high elasticity. The combination of both gives rise to a material that is harder than each of its individual components.11
The density of a bone is not the same throughout one's lifespan. Studies in different populations have found that the increase in bone density traces a positive and almost linear pattern until the age of 20, after which it increases very slowly, reaching its peak at around the age of 35. Some authors have demonstrated that there is rapid increase in density during puberty, with significant differences in age and sex in both bone mass and density, and the greatest variations found during adolescence.12–14 Following a period of stability, bone loss begins in both sexes, albeit greater in females, who lose 35% of cortical bone and between 50-60% of cancellous bone, while males lose less than 30%.15 In males, the loss of bone density as they age appears to be attributable to decreased bone formation, whereas in females, post-menopause, it is a consequence of increased bone resorption.16-18
We have focused on analysing bone at very early ages with the purpose of detecting the bone mineral/collagen ratio, which has not been examined in depth.19,20 To this end, this study performed quasi-static tensile experiments on young bone specimens divided into three groups based on diet type. Comparing age across different species is complex. For this reason, we resorted to distinguishing between suckling and feed and established an intermediate group in the transition from suckling to solid feed in two different mammalian species, bovine and ovine. What we found in these experiments was that both elastic modulus and bone strength increased with age and these figures were consistent with the results of the compositional analysis. Furthermore, we detected a similar trend in both species analysed.
Other animal studies confirm that elastic modulus and strength maintain a positive correlation with age in juvenile bone tissue. Akkus et al.21 carried out spectroscopic analyses of femurs from female rats aged 3, 8, and 24 months and demonstrated that increased mineralisation and increased crystallinity correlated with decreased elastic deformation with age. Yerramshetty et al.22 looked at the association between mineral crystallinity and the mechanical properties of human cortical bone, revealing that the greater the strength and stiffness of the bone tissue, the greater the crystallinity, while ductility was decreased. They also concluded that crystallinity could be used to predict bone strength. Currey et al.23 studied the effect of mineral content and Young's modulus and concluded that mineral content is positively associated with elastic modulus, but negatively associated with toughness.
Among the limitations of the study, it is worth mentioning that the type of feeding was used as a proxy for the age of the subjects included in this pilot study on bone from young quadrupedal mammals. Despite it being a reasonable approach, it misses a certain amount of control over the age of the bone of the participants and makes it more difficult to identify the variation in the mechanical properties with the age of the subjects more clearly. Furthermore, a thickness of 0.8mm was chosen in the specimen fabrication process to compare our results with other previously published results;24-26 however, the specimens are fragile and at high risk of breakage during processing. Several specimens were lost and only five broke in the area of the shaft. Most of the specimens that broke, did so in the area where it was clamped to the tensile machine. The geometry of this area coincides with an area where stress concentration occurs. This limitation also has an additional implication for the calculation of deformation given that the initial length used for this purpose was the length between the holes that were used to clamp the specimen to the tensile machine (20mm) instead of the 6mm length of the shaft area. This modification makes the means that values of the modulus of elasticity are very small compared to the values in the bibliography. Hence, later work should optimise the design of this type of attachment so as to cause the fracture of the specimen to occur in the area of the shaft. The method of calculating the modulus of elasticity is limited in that it required a subjective assessment by the authors to find a linear model to approximate the slope of the area of elastic behaviour in the specimen. Figure 5 depicts two extreme cases of this approximation: one in which the linear approximation works well, and the stress/deformation curve can be broken down into linear sections, and a second case in which the approximation adopted involves a larger error.
When considering the values of calcium mass percentage detected by EDX, we have found that its content increases as the bone tissue develops in bovine and ovine juveniles. In the group of adult ovine bone, we observed a slightly lower percentage of calcium compared to specimens from animals transitioning to solid food; nevertheless, more specimens would be required to confirm this result.
Fractographic analysis identified differences between species, as the sheep bone samples were more porous than those from bovine specimens, which, in turn, contributes to lower bone mineral density. Variations in calcification and, consequently, in crystallinity appear to be minor in our samples, but having a magnitude such that they contribute to the differences in mechanical properties.
The biomechanical study of bone currently enjoys new technologies that enable us to gain a better understanding of its mechanical behaviour. In our case, we have combined a tensile mechanical study using high-speed cameras with a compositional and fractographic study. Subsequent studies have forced us to modify the design of the specimens in order to avoid sample loss. In our study we have proven that young bone tissue tends to stiffen with age, with increasing weeks of life, and with the change in diet, because as the content of calcium in the bone increases, the elastic modulus of the bone also increases.
Level of evidenceLevel of evidence I.
Conflict of interestsThe authors have no conflict of interests to declare.
Ethical disclosuresThis is a work on fresh bones from slaughterhouses.