The use of bone cement is widespread in orthopedic surgery. Most of the mechanical tests are performed in dry medium, making it difficult to extrapolate the results. The objective of this study is to assess if the mechanical properties of polymethylmethacrylate (PMMA), obtained in previous reports, are still present in a liquid medium.
Materials and methodsAn experimental study was designed with antibiotic (vancomycin) loaded PMMA. Four groups were defined according to the medium (dry or liquid) and the pre-conditioning in liquid medium (one week or one month). Wear and flexural strength tests were performed according to ASTM and ISO standards. Volumetric wear, friction coefficient, tensile strength, and Young's modulus were analyzed. All samples were examined by scanning electron microscopy.
ResultsThe samples tested in liquid medium showed lower wear and flexural strength values (P<.05). The kind of wear was modified from abrasive to adhesive in those samples studied in liquid medium. The samples with a pre-conditioning time showed lower values of wear (P<.05).
ConclusionsCaution is recommended when extrapolating the results of previous PMMA results. The different mechanical strength of the cement in a liquid medium, observed in saline medium, is much closer to the clinical situation.
El uso del cemento óseo esta muy extendido en COT, existiendo multitud de estudios experimentales que lo avalan. La mayoría de los ensayos mecánicos están realizados en seco, lo que cuestiona la extrapolación de los resultados a la clínica. El objetivo de este estudio es evaluar si las propiedades mecánicas del polimetilmetacrilato (PMMA) obtenidas en series previas en seco, se mantienen en un medio fisiológico.
Material y métodoSe ha diseñado un estudio experimental para evaluar este aspecto, utilizando PMMA con antibiótico (vancomicina). Cuatro grupos fueron definidos en función del medio estudiado (seco o líquido) y de la realización de un acondicionamiento previo en suero fisiológico (una semana o un mes). Se hicieron estudios de desgaste y resistencia a flexión según las normativas ISO y ASTM, valorando el desgaste, el coeficiente de fricción, la resistencia a la rotura y el modulo de Young. Las muestras fueron analizadas mediante microscopía electrónica.
ResultadosLas muestras ensayadas en medio líquido presentaron menores valores de desgaste, así como menor resistencia a flexión, obteniéndose significación en el desgaste. El tipo de desgaste se modificó de un desgaste abrasivo a uno adhesivo en aquellas muestras estudiadas en medio líquido. El tiempo de acondicionamiento proporcionó menores valores de desgaste (P<0,05).
ConclusionesSe recomienda precaución a la hora de extrapolar los resultados de los estudios sobre PMMA en seco dado el diferente comportamiento mecánico del cemento en un medio líquido mucho más cercano a la situación clínica real, como es el suero fisiológico.
Since the use of polymethylmethacrylate (PMMA) by Dr. Charnley in the 1960s as a method of fixation for hip arthroplasties,1,2 PMMA has been shown to be one of the best methods of implant fixation in orthopedic surgery, with survival rates of more than 90% at 15 years in knee4 and hip3 arthroplasties. In addition to its usefulness in fixation, PMMA has been shown to be the best vehicle to date for using high amounts of antibiotics in the treatment of osteoarticular infection (and especially in prosthetic infection), achieving high local concentrations with very few systemic effects. This is particularly important, given that an increase of nearly 50% in the rate of prosthesis review caused by infection is projected by 2030.5
The usefulness of PMMA as a method of fixation, as well as growing interest in its use in the treatment and prophylaxis of prosthetic infection, has made it into one of the most frequently studied materials in our environment; this in turn has led to the appearance of multiple commercial preparations and the publication of minimum requirements by international agencies such as the ISO and the ASTM.6–8 Given that the great majority of studies performed to date have been under experimental conditions of dry medium, it is difficult to extrapolate these results correctly to clinical practice. This difficulty undoubtedly is of special interest when studying PMMA used with antibiotics, whose elution in liquid medium inevitably affects the mechanical characteristics of PMMA.
The objective of this study was to assess the influence of a liquid medium such as saline solution on the resistance and wear of bone cement mixed with an antibiotic, and whether such influence varied over time.
Materials and methodsWe performed an experimental study to assess the effect on liquid medium on bone cement. The PMMA used was bone cement that was quick setting, of high viscosity and preloaded with 0.5g of gentamicin Palacos® R+G (Heraeus Medical GmbH, Wehrheim, Germany), because of its wide use and its good survival data in various series.9,10 To each commercial preparation of cement, we added a vial of 1g of powdered vancomycin (Normon EFG, Tres Cantos, Madrid, Spain) manually to reproduce the treatment conditions of an infected arthroplasty.
Two types of samples were prepared: circular and rectangular. Before sample preparation the cement had been stored at controlled temperature and relative humidity. The components were not cooled beforehand; they were mixed in a tray manually. We added vancomycin independently, following the method of Frommelt and Kühn11 for manual addition of antibiotics, previously adding the same amount of powdered antibiotic as of polymer in a tray for a minute until we achieved a homogeneous mixture of the powder. Room temperature at the time of mixing was 23°C, with a relative humidity of 55–60%. The mixing process was carried out following the recommendation of the manufacturer and ASTM F451-996 and ISO 5833:20028 regulations. Each 20-ml vial of monomer was mixed with a 40-g sachet of polymer, added in that order in a reusable plastic tray. The mixture was prepared by stirring in a clock-wise direction in homogeneous frequency for a timed period of 30s. Once the paste was achieved, it was left to set until it was shown that the mixture would not stick (2min at work temperature), then beginning the preparation of the samples.
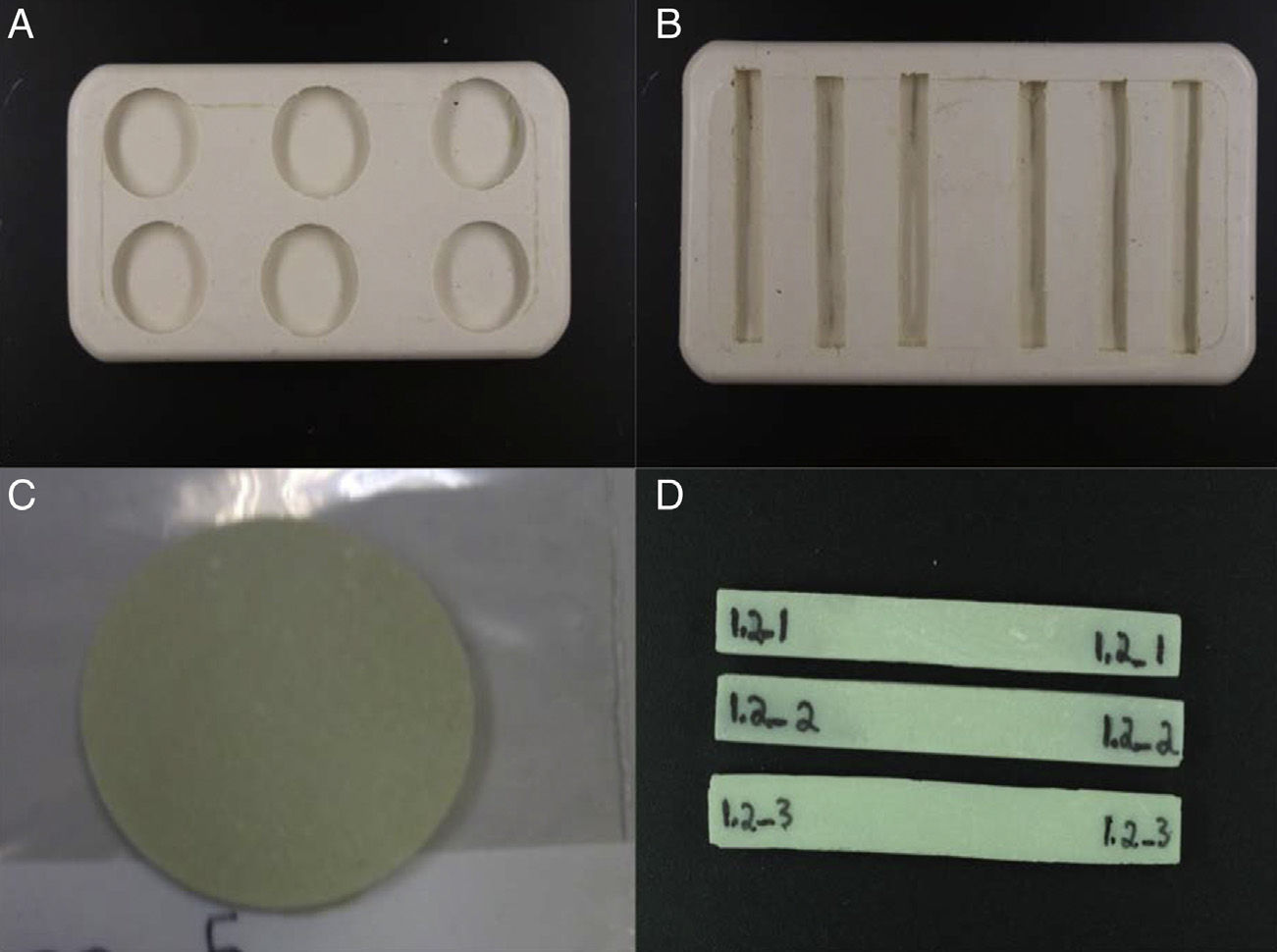
To prepare the samples we used a predesigned mold made of silicone (Fig. 1A and B), with circular samples 30mm in diameter and 4mm thick (Fig. 1C) and rectangular samples 79mm long, 10mm wide and 4.5mm high (Fig. 1D). The process of preparing these was similar, using cement in ductile phase to fill the molds, leaving them to set under a continuous weight of 10kg to become compact and to obtain homogeneous samples. Following a hardening period (10–12min) we removed the samples from the molds. Each bag of cement yielded enough for the preparation of 6 samples in 1 of the molds. The circular samples were used to study wear, while the rectangular ones were used to study resistance to fracture.
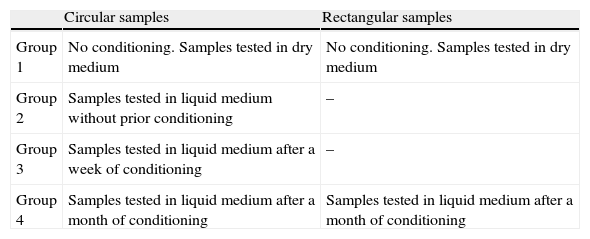
We defined various groups based on the conditioning process to which they were submitted, yielding 4 groups for the circular samples and 2 groups for the rectangular samples (Table 1). The conditioning process consisted of immersing the samples in isotonic saline solution (B. Braun, Melsungen, Germany) during the appropriate time for each group, maintaining the samples at a constant temperature of 37°C by incubation with a heater. These conditions attempted to replicate a medium similar to that which exists in a joint. Three homogeneous samples were prepared for each study group.
Definition of the 4 groups based on conditioning process.
| Circular samples | Rectangular samples | |
| Group 1 | No conditioning. Samples tested in dry medium | No conditioning. Samples tested in dry medium |
| Group 2 | Samples tested in liquid medium without prior conditioning | – |
| Group 3 | Samples tested in liquid medium after a week of conditioning | – |
| Group 4 | Samples tested in liquid medium after a month of conditioning | Samples tested in liquid medium after a month of conditioning |
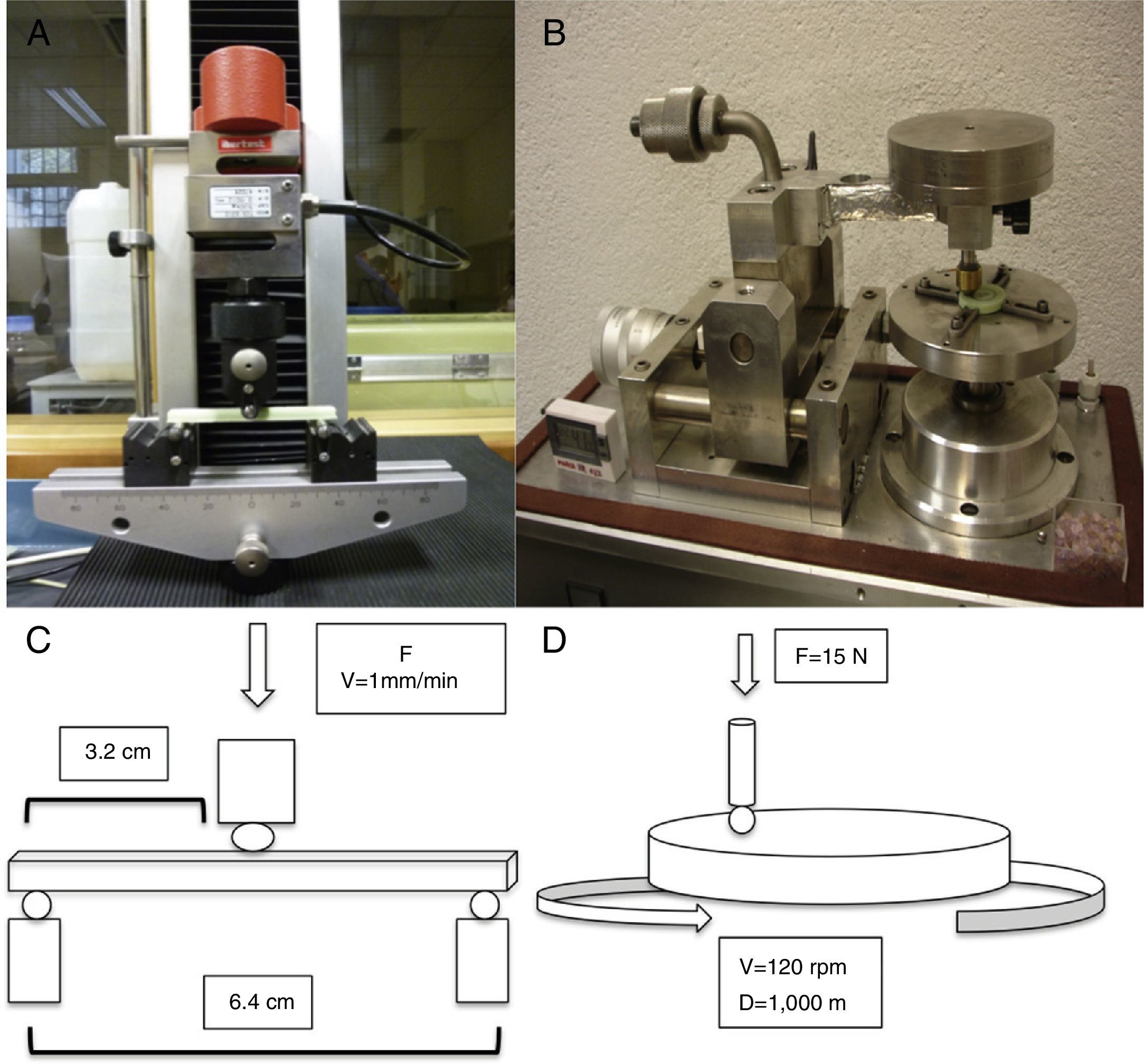
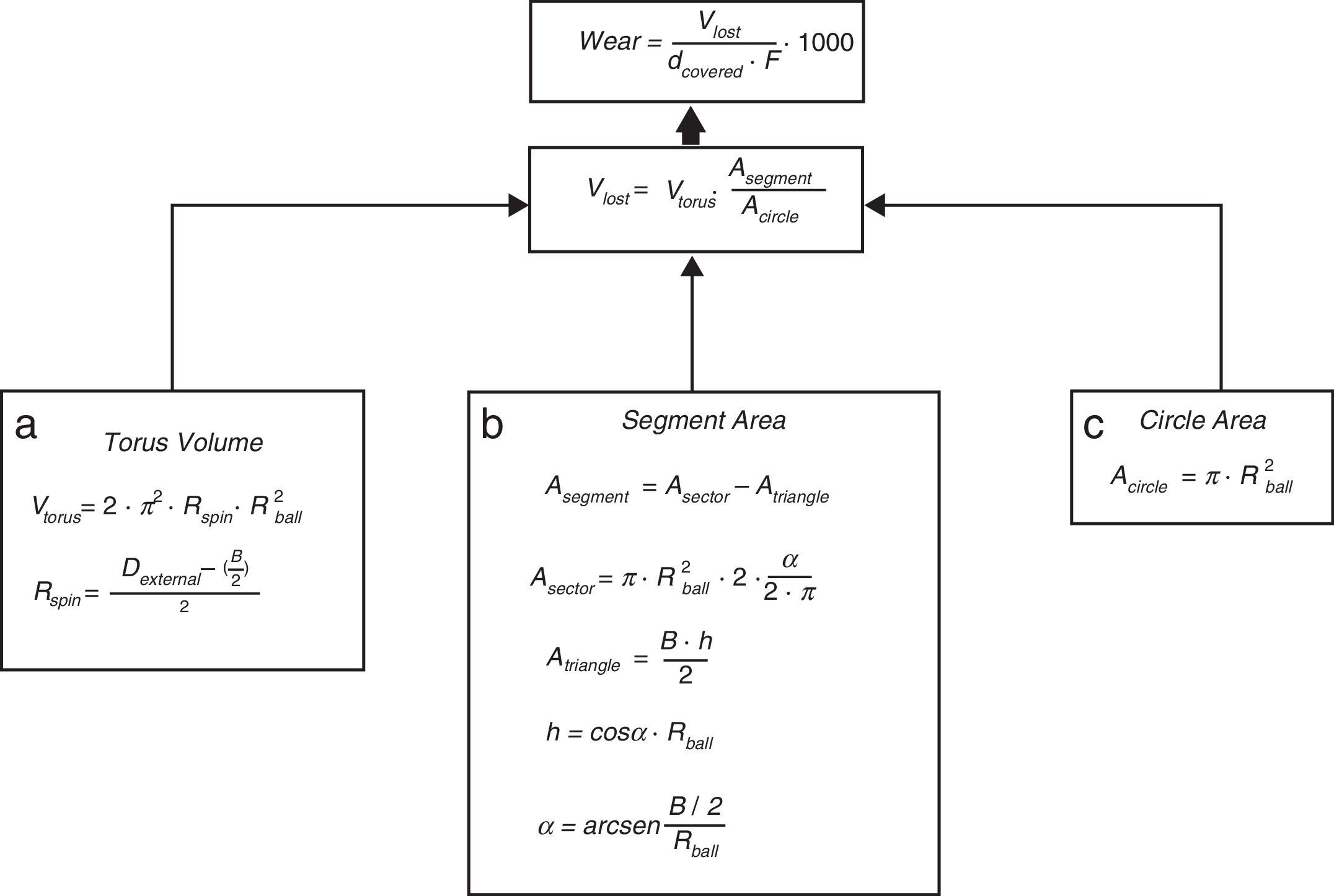
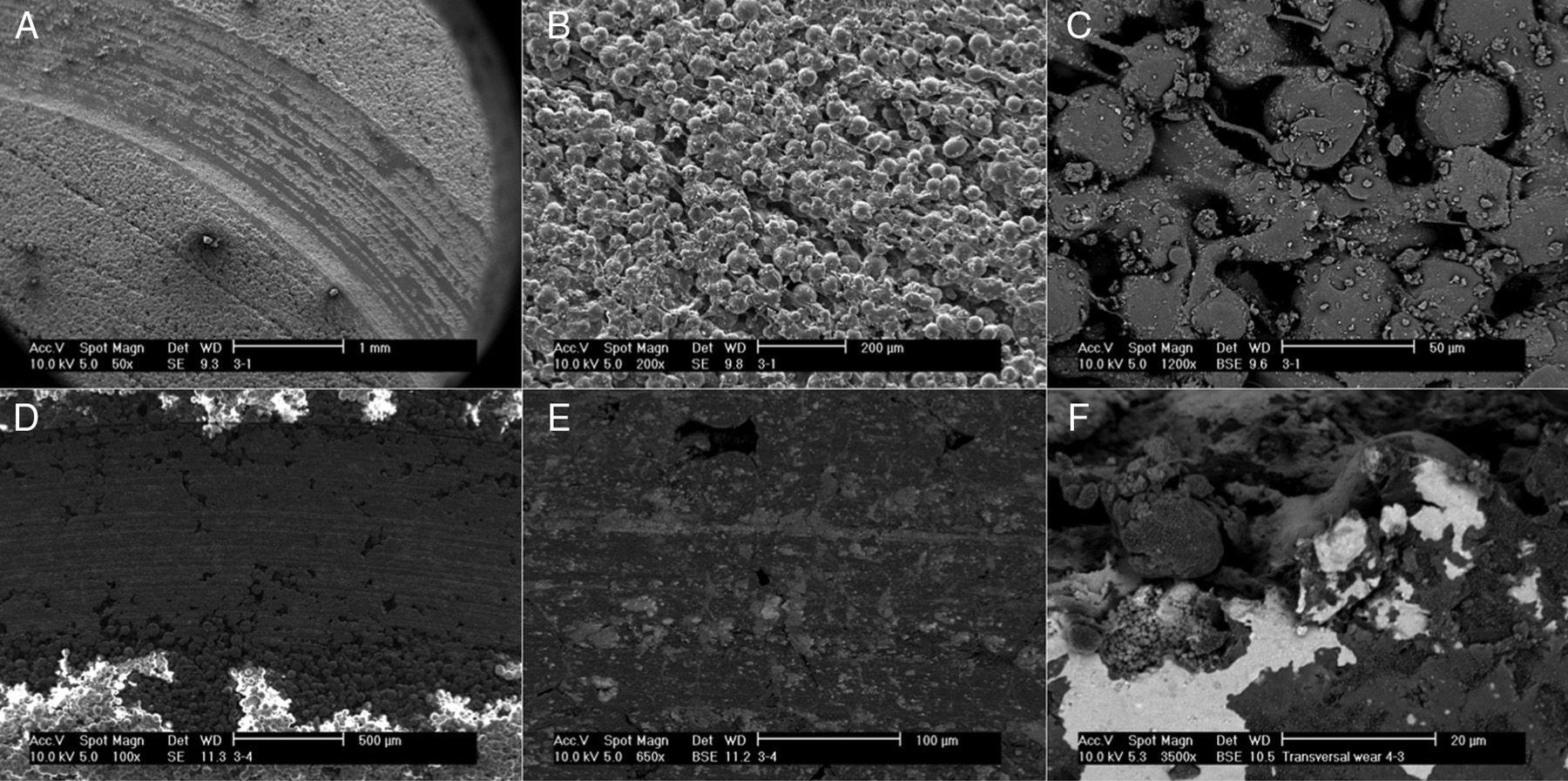
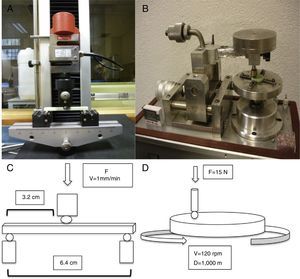
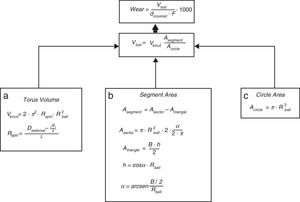
For the determination of wear and of the coefficient of friction, we performed mechanical testing following Standard ASTM G99-05.7 For the production of a wear track, we used a PIN-ON-DISK tribometer (Microtest, Madrid, Spain), with a 304 stainless steel ball 6mm in diameter. The test conditions used were with a normal load applied of 15N, a described path of 1000m, and a frequency of 120rpm (Fig. 2B and D). For the tests performed with the samples submerged in saline solution, a recipient was attached to the tribometer plate, where the samples covered in saline solution were introduced. The measurement of the wear track and its width was done with a V-20A Nikon Profile Projector (Nikon instrument INT, NY, USA). The use of a trigonometric model let us measure the wear obtained, defining the volume lost in mm3/Nm (Fig. 3). All of the samples tested were observed using a scanning electron microscope, with a previous requisite gold dust coated by sputtering.
Trigonometric model used to calculate wear; d (distance covered=1000m), F (force applied=15N), Vlost (volume lost due to wear), Vtorus (volume of the rotation torus), Rspin (radius of the wear track), Rball (radius of the wear ball=6mm), Dexternal (external diameter of the wear track), B (width of the track), A (area), and h (height).
To assess the resistance of the samples at a moment of flexion, we carried out testing using the international standard ISO 178:1993 and its Spanish modification UNE-EN-ISO 178. We used a universal testing machine (IB-MU4, IBERTEST, Ibertest group, Madrid, Spain) with 3-point flexion testing jaws, placing each sample over the lower points and applying a central force over the upper face of the sample. The distance between both lower supports was 64mm, applying the force in the central point at a speed of 1mm/min (Fig. 2A and C).
For the statistical analysis, we used the SPSS® version 15.0 computer package for MS-Windows (SPSS Inc., Chicago, USA). The ANOVA test was used for the comparison of the variables studied among the various groups for the wear tests, and Student's t-test for independent samples in the mechanical resistance studies. Statistically significant differences were defined as those having P values lower than 5% (P<.05).
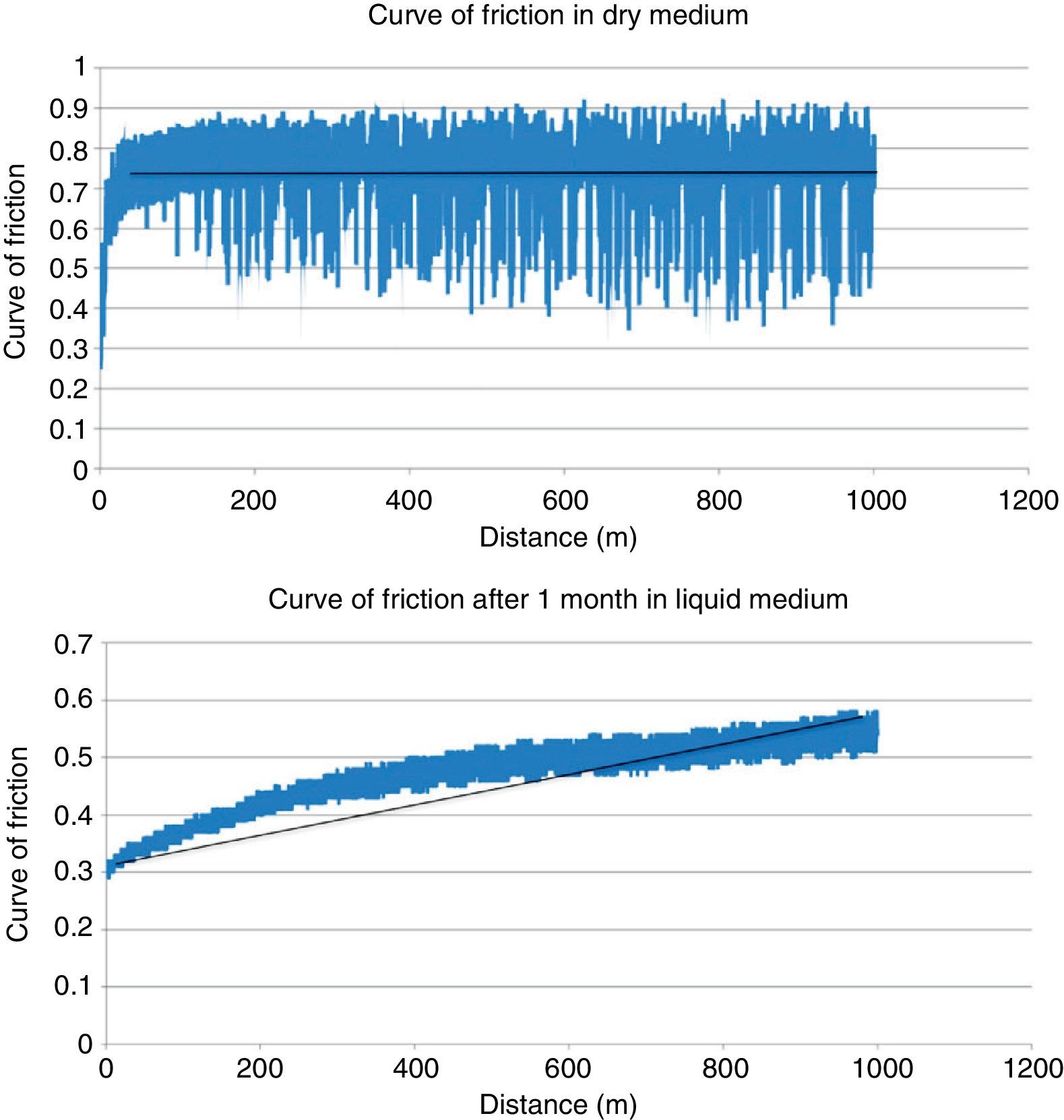
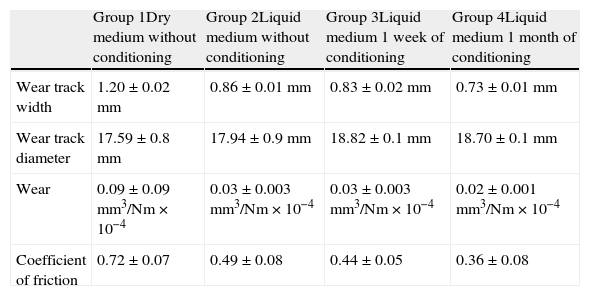
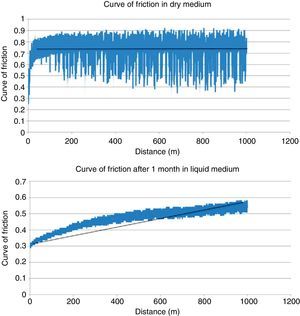
ResultsTable 2 shows the results obtained in the wear testing. In the samples tested in dry medium there were wear and coefficient of friction values significantly greater than those tested in liquid medium, whatever the process of conditioning (P<.05). The medium of study also conditioned how wear was produced, in that continuous wear was observed in the samples in dry medium in comparison to exponential wear in samples studied in liquid medium (Fig. 4). The decrease in wear observed was more than 60% (Table 2). The performance of a previous process of conditioning (Groups 3–4) diminished both wear and the coefficient of friction obtained in comparison to the samples that presented a lack of conditioning (Group 2), although there was no statistically significant difference. Increasing the time of conditioning from 1 week (Group 3) to 1 month (Group 4) produced a significantly greater decrease in the values of wear and coefficient of friction (P=.04).
Mean results and standard deviation obtained in wear testing.
| Group 1Dry medium without conditioning | Group 2Liquid medium without conditioning | Group 3Liquid medium 1 week of conditioning | Group 4Liquid medium 1 month of conditioning | |
| Wear track width | 1.20±0.02mm | 0.86±0.01mm | 0.83±0.02mm | 0.73±0.01mm |
| Wear track diameter | 17.59±0.8mm | 17.94±0.9mm | 18.82±0.1mm | 18.70±0.1mm |
| Wear | 0.09±0.09mm3/Nm×10−4 | 0.03±0.003mm3/Nm×10−4 | 0.03±0.003mm3/Nm×10−4 | 0.02±0.001mm3/Nm×10−4 |
| Coefficient of friction | 0.72±0.07 | 0.49±0.08 | 0.44±0.05 | 0.36±0.08 |
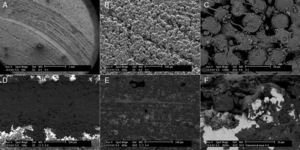
Scanning electron microscopy (SEM) showed a smaller size of the wear tracks tested in dry medium. The wear was of the abrasive type, changing to mainly adhesive when testing the samples in liquid medium (Fig. 5).
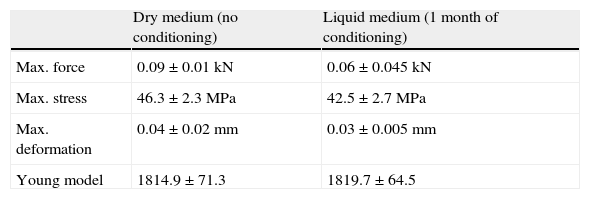
The results obtained in the flexion testing are summarized in Table 3. Samples tested in liquid medium presented lower resistance to breaking, bearing forces of lesser magnitude and suffering deformation to a lesser degree, than the samples studied in dry medium. The stress registered in the samples studied in liquid medium was also lower than that obtained in dry medium. No variations in the model of elasticity were observed.
DiscussionThe results of our study were influenced by the choices of bone cement, environmental conditions, and antibiotic. For this study we used Palacos® R+G (Heraeus Medical GmbH, Wehrheim, Germany) as it is one of the most widely used commercial cements that had the best long-term results.12,13 The environmental conditions selected were defined attempting to imitate the normal conditions existing in operating rooms where bone cement is used.14,15 The industrial addition of a small amount of an antibiotic like gentamicin has not been shown to affect the mechanical properties in previous studies.16 The choice of vancomycin as the antibiotic was based on its wide distribution, its use as treatment of prosthesis infection associated to PMMA, and its reduced price.9,17 All samples were mixed manually. This method increases porosity in PMMA, which leads to reducing the mechanical resistance and increasing the elution of PMMA.3,18–20 This has been called into question by McLaren et al.21
The effect of immersing PMMA in a liquid medium has been studied previously. Schmitt et al. described an increase in cement mass when it was submerged in saline solution.22 Other authors observed a decrease in the model of elasticity as water was absorbed,23 with PMMA even becoming saturated at 3 months without any observable changes in its properties although the water acted as an initiator of fractures.24 The plasticizing effect of the water and its influence on the mechanical properties of the bone cement have been described in detail,22,24,25 with it being due to the absorption of water by the PMMA, a process described in other polymers,26 being cyclic.
In our study we did not find statistical significance in the changes observed in the mechanical properties of PMMA studied in dry medium as compared to that studied in liquid medium. However, there was a clear tendency to a decrease in these properties when we introduced the PMMA in a medium with liquid. These results are the same as those found in the literature. Nottrott27 studied the behavior of Palacos® R+G and CMW 3 Genta® stored for 5 years in a dry medium against for 3 weeks in a medium with water; they found an increase in resistance to stress and in the model of elasticity in the samples that had been stored dry.
The wear observed in our study was low compared to the coefficients of friction obtained, with significantly lower wear in the samples studied in liquid medium. This could be due to the lubricating effect of water, the modification of the wear pattern from an abrasive behavior to an adhesive one, and to the deposit itself of substances in the wear track of the samples tested in liquid (Fig. 5A–F), although the authors have no knowledge of any prior descriptions of this phenomenon.
Bone cement is not a static component; its mechanical properties vary over time. Cizmecioglu et al.28 described the influence of PMMA “aging” on its elasticity, determined by the amount of residual monomer, which has a damaging effect on PMMA resistance.29 In our study, we obtained less wear in the samples that had undergone a process of conditioning, observing an influence of period of adaptation in this decrease; the samples submitted to a month of conditioning presented significantly less wear than the samples submitted to a week. To the best of our knowledge, there is no previous study that evaluates the time of aging in a liquid medium on PMMA resistance to wear.
In contrast to previous studies in the literature, in our study we used PMMA wear as another variable to measure the influence of the liquid medium on mechanical properties. An increase in wear could be prejudicial to implant fixation; Saleh et al.30 and Goodman31 described how the particles of bone cement wear could be involved in periprosthetic osteolysis, although excessive wear could be useful in other situations because of the increase in elution of antibiotic present on the surface of articulating spacers. This study is an initial step in attempting to understand what happens on the surface of these spacers.
In our study we wanted to imitate the environmental conditions in a joint. To this end, we used a medium of study with saline solution, but we must accept certain limitations. A healthy joint contains, in addition to synovial liquid, various substances such as proteins, cells, glucose and so on that give it different rheological properties from those of saline solution. Using a watertight medium for producing the conditioning and wear also differs from the normal behavior of a joint, where there is a flux between absorption/production, in addition to movement, which can have an effect on the particles of wear being deposited on the cement itself and can consequently change the wear values obtained. We feel that it is important to keep these considerations in mind when interpreting the results. We do not believe that the limited number of samples represents a limitation for the results of this study, although it could indeed have limited the obtaining of statistical significance in specific groups. Other publication of special relevance in biomaterials have used similar sample sizes; examples are Unemori et al.25 who utilized 3 samples to observe the absorption of water in modified PMMA, and Bridgens et al.32 who used 2 samples to study the elution and mechanical resistance of various cements mixed with PMMA.
In conclusion, the results obtained in this study show the damaging effect of the liquid medium on PMMA, which brings into question the use of the data obtained in previous studies carried out in dry medium extrapolated to clinical practice. Nevertheless, further studies that back this up are required, as well as additional studies to evaluate if the decrease in PMMA resistance and wear with vancomycin observed in this study continues in other PMMA isolated or in combination with other antibiotics.
Level of evidenceLevel of evidence 1.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments on human beings or animals were performed for this research.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sanz-Ruiz P, Paz E, Abenojar J, del Real JC, Forriol F, Vaquero J. Influencia del medio fisiológico sobre las propiedades mecánicas del cemento óseo. ¿Son los estudios actuales extrapolables?. Rev Esp Cir Ortop Traumatol. 2014;58:3–10.
This study received the 2013 SECOT Award for Basic Research in Orthopedic Surgery and Traumatology.