The differentiation and classification of pathogenic Cryptococcus species provides useful data for epidemiological studies and for the clinical diagnosis and treatment of patients.
AimsThe aim of this study was to characterise 40 clinical Cryptococcus isolates obtained from patients at the Tropical Medicine Foundation of Amazonas (FMTAM) from 2006 to 2008.
MethodsIt was used phenotypic (i.e., enzyme production and antifungal resistance) and molecular biological (URA5-RFLP) experiments.
ResultsPatients with HIV/AIDS were most affected with cryptococcosis. Thirty-one (75.5%) of the clinical isolates were classified as Cryptococcus neoformans and 9 (22.5%) as Cryptococcus gattii. High amounts of protease and phospholipase enzymes were produced by most of the isolates. Using the disk diffusion test (CLSI M44-A), 81, 35 and 100% of the C. neoformans isolates were characterized as susceptible to fluconazole, itraconazole and amphotericin B, respectively, whereas 78, 56 and 100% of the C. gattii isolates were susceptible to these antimicrobial agents. The average of Minimal Inhibitory Concentration (MIC) for C. neoformans and C. gattii isolates was 0.26 and 0.58μg/mL, respectively. The 9 isolates of C. gattii had a fingerprint pattern comparable with the VGII molecular type, while all 31 isolates of C. neoformans presented with a pattern consistent with the VNI type.
ConclusionsThis study confirms the importance of HIV/AIDS for the cryptococcosis epidemiology, the susceptibility of the isolates to amphotericin B and the high prevalence of the molecular genotypes VNI and VGII in the north of Brazil.
La diferenciación y clasificación de las especies patógenas del género Cryptococcus aporta datos importantes para la asistencia clínica y para estudios epidemiológicos.
ObjetivosEl objetivo de este trabajo fue caracterizar 40 aislamientos clínicos del complejo Cryptococcus neoformans de pacientes que fueron atendidos en la Fundación de Medicina Tropical de Amazonas desde 2006 hasta 2008.
MétodosSe utilizaron métodos fenotípicos (producción de enzimas y pruebas de sensibilidad a los antifúngicos) y moleculares (URA5-RFLP).
ResultadosLos pacientes con VIH/sida fueron los más afectados de criptococosis. Se observó que 31 (75,5%) y 9 (22,5%) de los aislamientos fueron Cryptococcus neoformans y Cryptococcus gattii, respectivamente. La producción de proteasa y fosfolipasa fue alta en la mayoría de las cepas. Utilizando la prueba de difusión en disco (CLSI M44-A) se observó que el 81, 35 y 100% de los aislamientos de C. neoformans fueron sensibles al fluconazol, itraconazol y amphotericin B, respectivamente, mientras que 78, 56 y 100% de los aislamientos de C. gattii fueron sensibles a estas sustancias. El valor promedio de la concentración mínima inhibitoria (CMI) para C. neoformans y C. gattii fue de 0,26 y 0,58mg/ml, respectivamente. Todos los aislamientos (9) de C. gattii presentaron un patrón de electroforesis compatible con el genotipo VGII, y todos los aislamientos (31) de C. neoformans presentaron el genotipo VNI.
ConclusionesEste estudio confirma la importancia del HIV/sida para la epidemiología de la criptococosis, la sensibilidad de los aislamientos a la anfotericina B y la alta prevalencia de los genotipos moleculares VNI y VGII en el norte de Brasil.
Cryptococcosis is an opportunistic fungal disease caused by the encapsulated yeast species Cryptococcus neoformans and Cryptococcus gattii.17 The infection is acquired by the inhalation of desiccated yeasts or spores and can develop to the brain or other systemic organs.18 Infection with Cryptococcus spp. is the major cause of fungal meningitis in immunocompromised patients, resulting in elevated morbidity and mortality rates.21 Furthermore, immunocompromised status is the most common risk factor for infection with C. neoformans. Additionally, there are increasing numbers of immunocompetent patients with fungal meningitis who are infected with C. gattii.21,32 The preferred treatment for cryptococcal meningitis is amphotericin B, but a side effect of the drug manifested by nephrotoxicity limits its clinical use and increases the importance of antifungal susceptibility testing.10,12
Based on serological studies, using antibodies against the fungal capsule, seven serotypes of Cryptococcus have been identified: A, B, C, D, AB, AD and BD.3,5,20C. neoformans serotypes A, D and AD are found in cities, whereas the C. gattii serotypes B and C are predominately localised in tropical and subtropical regions.1,13,19 In 1999 an outbreak of C. gattii occurred in Canada, raising the possibility that this species could be localised in both tropical and temperate climates.11
In addition to the serotypes of C. neoformans and C. gatti, molecular analyses, including M13 fingerprinting, URA5-RFLP (Restriction Fragment Length Polymorphism) and AFLP (Amplified Fragment Length Polymorphism), have identified 8 molecular genotypes: VNI and VNII (Serotype A); VNIII (Hybrid AD); VNIV (Serotype D); and VGI, VGII, VGIII and VGIV (Serotypes B and C).17 Molecular genotyping contributes to better and more accurate clinical diagnoses and characterizes genetic diversity for global epidemiological studies.24
The aim of this work was to characterize forty Cryptococcus isolates from patients at the Tropical Medicine Foundation of Amazonas (FMTAM) during 2006–2008 by using phenotypic and molecular biological experiments.
1Materials and methods1.1Isolates and strainsForty isolates were obtained from patients with cryptococcal infection who were hospitalized in FMTAM between March 2006 and February 2008. A single sample was collected from each patient and stored at 4°C in the FMTAM fungal collection. They were grown on Sabouraud agar at 30°C for 48h. For molecular genotyping, the following standard strains were used: WM 148 (serotype A,VNI), WM 626 (serotype A, VNII), WM 628 (serotype AD, VNIII), WM 629 (serotype D, VNIV), WM 179 (serotype B, VGI), WM 178 (serotype B, VGII), WM 161 (serotype B, VGIII) and WM 779 (serotype C, VGIV). These reference strains were kindly provided by the fungal collection at FIOCRUZ-Rio de Janeiro-Brazil. Also, the reference strains WM 148 and WM 178 were used as reference strains for l-canavanine glycine bromothymol blue (CGB) species determination, enzyme production and susceptibility assays using antifungal agents.
1.2Patient informationThe epidemiological profile of the patients (age, sex, immunological status and residence) was obtained by analysis of the examination requisition.
1.3Identification of the speciesCGB agar was utilized to differentiate the species C. neoformans and C. gattii as previously described.13C. gattii strains use the glycine in the media as a source of carbon and nitrogen, are resistant to canavanine and produce a blue cobalt colour during incubation, whereas C. neoformans do not exhibit any colour change in the media.
1.4Enzyme quantificationThe production of proteinases and phospholipases were measured as previously described.23,25,33 The fungal isolates were inoculated at equidistant points on plates of proteinase agar and phospholipase agar, and the enzyme activity (Pz) was calculated using the relation between the diameter of the colony (dc) and the diameter of the colony and precipitation zone (dcp). The results were classified as negative (Pz=1), positive (0.64≥Pz<1) or strongly positive (Pz<0.64).
1.5Susceptibility to antifungal agentsSusceptibility tests were performed according to the Clinical and Laboratory Standards Institute (CLSI) document M44-A.4 Disks impregnated with fluconazole (25μg), itraconazole (10μg) or amphotericin B (100μg) (Cecon-Sensifungidisc, São Paulo, Brazil) were used in the assays. The diameter of the inhibition haloes was used to determine the susceptibility of the yeast to the antifungal compounds. Cut-off values were as follows: Amphotericin B>10mm – susceptible (S), <10mm –resistant (R); itraconazol >20mm – S, 19–12mm – intermediate (I), <1mm – R and fluconazole >19mm – S; 19–14mm – I; <14mm – R. The determination of the MIC for amphotericin B was performed using the E-test-AB BIODISK method as described previously.14
1.6Characterization of molecular genotypesThe genotypic characterization was carried out as described previously.17 Fungal DNA was extracted with the QIAamp tissue kit (Qiagen, Germany) according to the manufacturer's protocol for DNA extraction from yeast, including digestion with lyticase and RNAase. PCR of the URA5 gene was conducted in a final volume of 50μL, and each reaction contained 50ng of DNA, 1× PCR buffer (10mM Tris–HCl, pH 8.3, 50mM KCl and 1.5mM MgCl2); 0.2mM each of dATP, dCTP, dGTP and dTTP; 3mM magnesium acetate; 1.5U AmpliTaq DNA polymerase (Invitrogen, Carlsbad, California) and 50ng of each primer URA5 (5′ATGTCCTCCCAAGCCCTCGACTCCG3′) and SJ01 (5′TTAAGACCTCTGAACACCGTACTC3′). The PCR was performed in a PerkinElmer thermal cycler (model 480) for 35 cycles of a 2min initial denaturation at 94°C, 45s denaturation at 94°C, 1min annealing at 61°C and 2min extension at 72°C, followed by a final extension cycle for 10min at 72°C. The PCR products were double digested with Sau96I (10U/μL) and HhaI (20U/μL) for 3h or overnight and separated by 3% agarose gel electrophoresis at 100V for 5h.
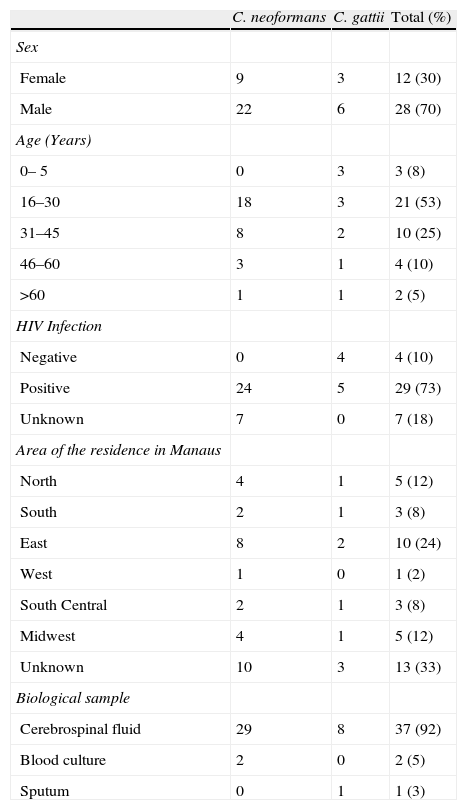
2ResultsThe epidemiological characteristics of the patients with cryptococcosis were evaluated. Males were more commonly infected (70%) than females, and the age group most affected was between 16 and 30 years (53%; median=28.5 years) (Table 1). The majority of patients with cryptococcosis were also infected with HIV (72%) and resided in the east zone of the city of Manaus (25%) (Table 1). Cerebrospinal fluid (CSF) was collected for the isolation of the fungal agents from most patients (93%). Isolates were inoculated on a CGB medium; 9 were positive for the presence of C. gattii, and 31 were positive for C. neoformans.
Epidemiological characteristics of patients with cryptococcosis.
| C. neoformans | C. gattii | Total (%) | |
| Sex | |||
| Female | 9 | 3 | 12 (30) |
| Male | 22 | 6 | 28 (70) |
| Age (Years) | |||
| 0– 5 | 0 | 3 | 3 (8) |
| 16–30 | 18 | 3 | 21 (53) |
| 31–45 | 8 | 2 | 10 (25) |
| 46–60 | 3 | 1 | 4 (10) |
| >60 | 1 | 1 | 2 (5) |
| HIV Infection | |||
| Negative | 0 | 4 | 4 (10) |
| Positive | 24 | 5 | 29 (73) |
| Unknown | 7 | 0 | 7 (18) |
| Area of the residence in Manaus | |||
| North | 4 | 1 | 5 (12) |
| South | 2 | 1 | 3 (8) |
| East | 8 | 2 | 10 (24) |
| West | 1 | 0 | 1 (2) |
| South Central | 2 | 1 | 3 (8) |
| Midwest | 4 | 1 | 5 (12) |
| Unknown | 10 | 3 | 13 (33) |
| Biological sample | |||
| Cerebrospinal fluid | 29 | 8 | 37 (92) |
| Blood culture | 2 | 0 | 2 (5) |
| Sputum | 0 | 1 | 1 (3) |
All of the isolates had high protease production (Pz<0.64) and an average Pz=0.3 for both Cryptococcus species. All but 1 isolate was positive for the production of phospholipase (average Pz=0.53). Twenty-six C. neoformans isolates (83.8%) presented strong positive results (Pz<0.64), and 4 isolates (12.9%) presented positive results (Pz≥0.64 and < 1.0), whereas all 9 isolates of C. gattii were classified as strong-positive producers of phospholipase.
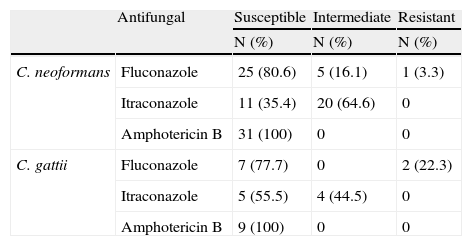
Using the disk diffusion test (CLSI M44-A), 81, 35 and 100% of the isolates of C. neoformans were identified as susceptible to fluconazole, itraconazole and amphotericin B, respectively, and 78%, 56% and 100% of the isolates of C. gattii were susceptible to these antifungal compounds (Table 2). The average MIC for amphotericin B, evaluated by E-test methodology, was 0.26±0.22μg/mL for C. neoformans and 0.58±0.53μg/mL for C. gattii.
- Susceptibility of C. neoformans and C. gattii to antifungal compounds, as measured by disk diffusion.
| Antifungal | Susceptible | Intermediate | Resistant | |
| N (%) | N (%) | N (%) | ||
| C. neoformans | Fluconazole | 25 (80.6) | 5 (16.1) | 1 (3.3) |
| Itraconazole | 11 (35.4) | 20 (64.6) | 0 | |
| Amphotericin B | 31 (100) | 0 | 0 | |
| C. gattii | Fluconazole | 7 (77.7) | 0 | 2 (22.3) |
| Itraconazole | 5 (55.5) | 4 (44.5) | 0 | |
| Amphotericin B | 9 (100) | 0 | 0 |
DNA was extracted from all clinical isolates and was used to identify the molecular genotypes by URA5-RFLP. All 9 isolates of C. gattii possessed a fingerprint pattern consistent with the VGII molecular genotype, and all 31 isolates of C. neoformans had the profile of the VNI molecular type.
3DiscussionThe total number of the inhabitants in the state of Amazonas is approximately 3.5×106 and according to the Mycology Service of the Foundation of Tropical Medicine of Manaus, 20–25 new cases of cryptococcosis occur annually in the region. Manaus, the capital of Amazonas State, is located near the centre of the Amazon Basin on the left bank of the Rio Negro, consisting of Tertiary sediments on sandy-clay land. The region has an average rainfall of 2300mm/year and is thus associated with a hot and humid climate and lush vegetation.31 In this study, the incidence of cryptococcosis was not related to these weather patterns, as the number of isolates was similar throughout the year. The identification of potential environmental sources of C. neoformans and C. gatii that are introduced into the human population requires further investigation. It is hypothesise that the sources of contamination are similar to those described in other countries: human infection with the Cryptoccocus type VNI primarily occurs from contact with bird faeces, and infection with the VGII type usually occurs following contact with decomposing vegetal biomass.
This is one of the first studies to characterise cryptococcosis in the Amazonas State in Brazil. This study determined that the patients most affected by cryptococcosis were male, young (16–30 years old), HIV-positive and inhabitants of the east zone of the city of Manaus.
The number of cases of HIV/AIDS reported annually in the Amazonas State is approximately 700, half of them receive HAART treatment.30 AIDS is the most important risk factor for cryptococcosis, due to the suppression of immune responses by the HIV virus.9,13,26C. neoformans was the species most frequently isolated (n=31; 77.5%), which is consistent with the 82.3% reported in the literature.17,19C. neoformans almost exclusively causes disease in immunocompromised patients, and the majority of the isolates that we obtained came from patients with AIDS.17,19 Among the nine C. gattii isolates, three were obtained from immunocompetent patients, four from AIDS patients and two from patients without documented immune status.
In this study, we obtained three isolates from children aged 0–15 years. C. gattii was the primary causative agent of meningitis in these patients. In a previous study in the Amazonas State from 1988 to 1998, Martins15 reported that the frequency of cryptococcosis in children represented 33% of the total cases. In the Pará state, neighbouring the Amazonas State, 19–24% of cryptococcosis cases were reported in children over the last 10 years.28,29 These studies demonstrate that in the Amazon Rainforest, the prevalence of cryptococcosis in children is high and is also associated with the molecular type VGII. Further epidemiological studies and environmental sampling are necessary to define the importance of the environmental conditions in the incidence of cryptococcosis cases in children.
The production of lipases and proteases is related to the virulence of Cryptococcus pathogenic species.7,8 These enzymes are involved in the destruction of cellular structures to both obtain nutrients for the fungal pathogens and to facilitate spread throughout tissues. Both C. neoformans and C. gattii produced proteases and lipases in similar quantities, and no difference was observed between molecular types.
Antifungal cryptococcosis testing showed 80% and 77% susceptibility to fluconazole in C. neoformans and C. gattii, respectively. This maintenance drug is commonly used in patients with AIDS to prevent opportunistic fungal diseases, and the emergence of resistant strains has been described.6,9C. neoformans and C. gattii had 35% and 55% susceptibility to itraconozole, respectively, and all of the clinical isolates were susceptible to amphotericin B. These results are consistent with previous studies.14,22,27 Amphotericin B is considered to be the gold standard for cryptococcosis treatment.2 In one study, amphotericin B resistance was reported in 10% of clinical isolates,5 but other studies demonstrate low in vitro resistance to this compound.14,27 Although all isolates were considered to be susceptible to amphotericin B (MIC<2μg/mL), the average MIC of the C. gattii isolates (0.56μg/mL) was greater than that of C. neoformans isolates (0.26μg/mL). This result has been previously reported14 and highlights the potential for differential efficacy of therapeutics in the treatment of C. neoformans and C. gattii.
The identification of pathogenic Cryptococcus species and sub-species are important for both clinical guiding and as well as for epidemiology. Recently, false-positive C. gattii results have been reported using the conventional CGB differentiation method due to the existence of C. neoformans isolates that are resistant to canavanine.13 Meanwhile, PCR fingerprinting and PCR-RFLP are being utilized more frequently.9,20,24 In the current study, PCR-RFLP was used to characterize the Cryptococcus isolates as the molecular types VNI and VGII. Furthermore, these results are consistent with previous studies that identified C. neoformans VNI as the primary agent of cryptococcosis.13,16,19 A study that characterized 63 Brazilian isolates described the prevalence of the molecular types VNI (82.3%), VGII (13.6%) and VNII (3.0%),17 and more recent studies in the Pará state reinforce the prevalence of VNI and VGII as being the principal molecular types of C. neoformans and C. gattii, respectively, in the northern region of Brazil.17
Data from this study can assist with current and future management of cryptococcosis in Northern Brazil by identifying the frequency of C. neoformans and C. gattii in patients as well as their susceptibility to commonly used antifungal drugs. These data also contribute to epidemiological studies of the distribution of different molecular types of Cryptococcus species in the Brazilian Amazon.
Conflict of interestThe authors have no conflict of interest to declare.
The authors are grateful to Fundação de Amparo a Pesquisa do Estado do Amazonas (FAPEAM) for the financial support.