The Mitogen-Activated Protein Kinase (MAPK) signaling pathways constitute one of the most important and evolutionarily conserved mechanisms for the perception of extracellular information in all the eukaryotic organisms. The MAPK pathways are involved in the transfer to the cell of the information perceived from extracellular stimuli, with the final outcome of activation of different transcription factors that regulate gene expression in response to them. In all species of fungi, the MAPK pathways have important roles in their physiology and development; e.g. cell cycle control, mating, morphogenesis, response to different stresses, resistance to UV radiation and to temperature changes, cell wall assembly and integrity, degradation of cellular organelles, virulence, cell–cell signaling, fungus–plant interaction, and response to damage-associated molecular patterns (DAMPs).
Considering the importance of the phylogenetically conserved MAPK pathways in fungi, an updated review of the knowledge on them is discussed in this article. This information reveals their importance, their distribution in fungal species evolutionarily distant and with different lifestyles, their organization and function, and the interactions occurring between different MAPK pathways, and with other signaling pathways, for the regulation of the most complex cellular processes.
Las vías de señalización de la proteína-cinasa activada por mitógenos (abreviadas como MAPK por sus siglas en inglés) son uno de los mecanismos más importantes y evolutivamente conservados para la percepción de información extracelular en organismos eucarióticos. Las vías MAPK están involucradas en la transferencia a la célula de la información recibida de estímulos extracelulares, que ofrecen como resultado final la activación de diferentes factores de transcripción que regulan la expresión de genes en respuesta a aquellos. En todas las especies de hongos, las vías MAPK tienen importantes funciones en su fisiología y desarrollo como, por ejemplo, el control del ciclo celular, el apareamiento, la morfogénesis, la respuesta a diferentes tipos de estrés, la resistencia a la luz UV y a los cambios de temperatura, la formación e integridad de la pared celular, la degradación de los orgánulos, la virulencia, la señalización célula-célula, la interacción hongo-planta y la respuesta a patrones moleculares asociados con el daño (abreviado como DAMP, por sus siglas en inglés).
Dada la importancia de las vías MAPK en hongos, en esta revisión se discute el conocimiento adquirido más recientemente sobre ellas. Esta información revela su importancia, su distribución en especies de hongos evolutivamente distantes y con estilos de vida diferentes, su organización y función, y las interacciones que ocurren entre diferentes vías MAPK, y entre estas y otras vías de señalización que regulan los procesos celulares más complejos.
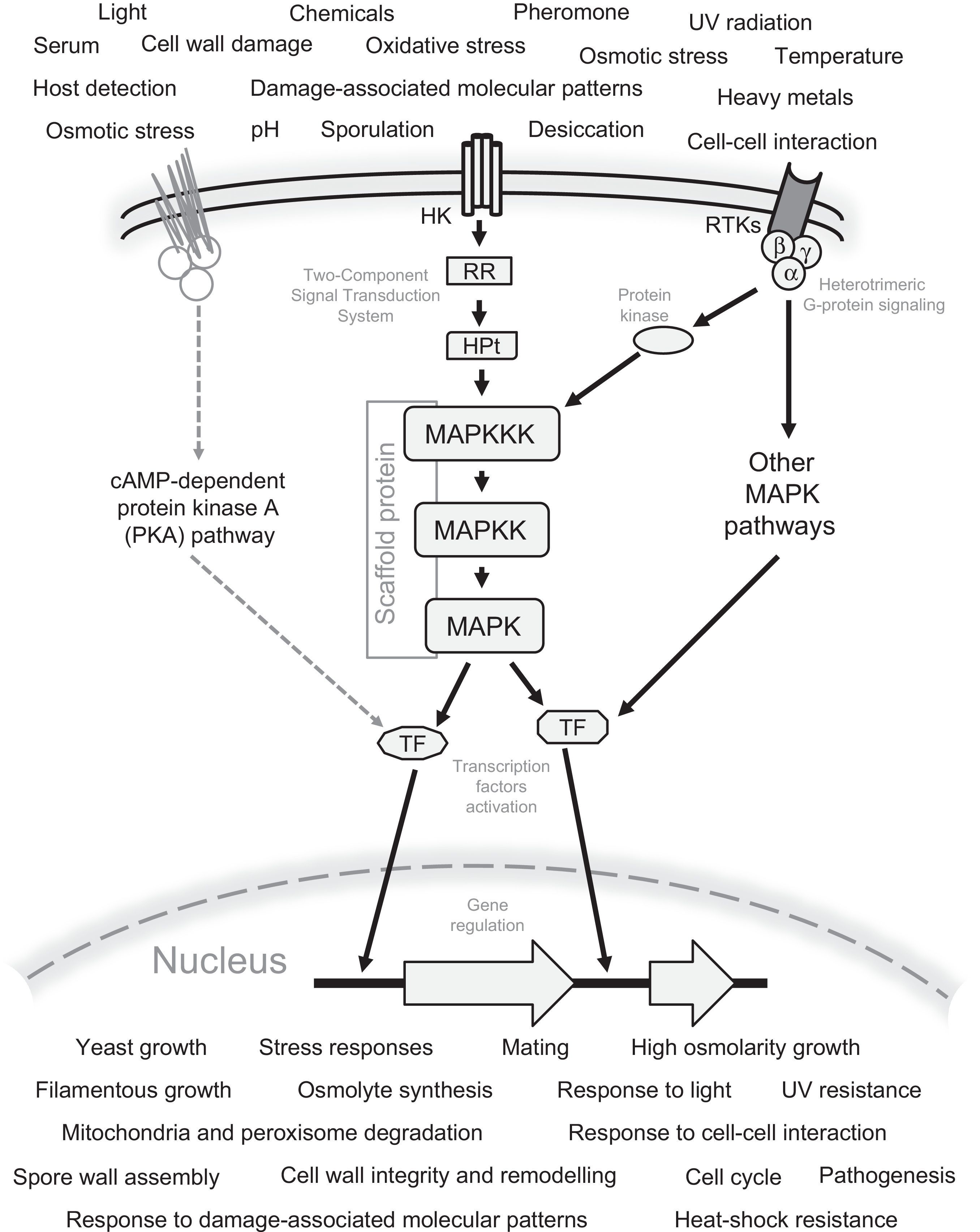
Mitogen-activated protein kinase (MAPK) pathways are one of the most important and evolutionarily conserved mechanisms of cellular signaling existing in eukaryotic organisms including animals, plants and fungi.23,79 The signal transduction processes in which MAP kinases are involved start with the sensing of environmental stimuli by receptors and proteins anchored to the cell membrane, such as the two-component signal transduction systems (TCS), receptor tyrosine kinases (RTKs) or multiprotein structures such as eisosomes, which in turn are attached to heterotrimeric or monomeric G proteins-coupled to receptors.3,23,39,50,99 These can interact with adaptor proteins, or directly activate a MAPKKK (MAP kinase kinase kinase) which in turn activates a MAPKK (MAP kinase kinase) by the phosphorylation of serine/threonine residues. This latter protein phosphorylates one or several MAPKs (MAP kinases) in serine/threonine/tyrosine residues, that finally give rise to the activation of transcription factors that induce or repress genes involved in the cellular adaptation or response to the sensed stimuli.6,32 In some of these pathways a scaffold (anchor) protein keeps associated with the different MAPKs.41,53,78
Fungi are eukaryotic organisms with different lifestyles that possess protein kinases, including those organized in the form of MAPK systems, with high homology to kinases from animals, such as flies, worms, and humans.23,51 In fungi, the MAPK pathways are involved in different physiological and developmental processes, including cell cycle, mating, morphogenesis, sporulation, cell wall assembly and integrity, autophagy, pathogenesis, UV and heat-shock resistance, cell–cell signaling, fungus–fungus interactions, fungus–plant interactions (e.g. mycorrhiza), response to different forms of stress, response to damage-associated molecular patterns (DAMPs), etc.3,21,23,25,29,32,41,49,52,64,79
Taking into consideration that some of these processes occur in higher eukaryotic organisms, involving also the action of MAPK modules, it may be concluded that fungi constitute excellent model organisms for the study and understanding of the mechanisms that operate in the signaling systems occurring in eukaryotic organisms in general. On these bases, in this review we analyze the functions of the MAPK pathways, the interactions between different MAPK pathways, and their interaction with other signaling pathways occurring in fungal species evolutionarily distant, and with different lifestyles.
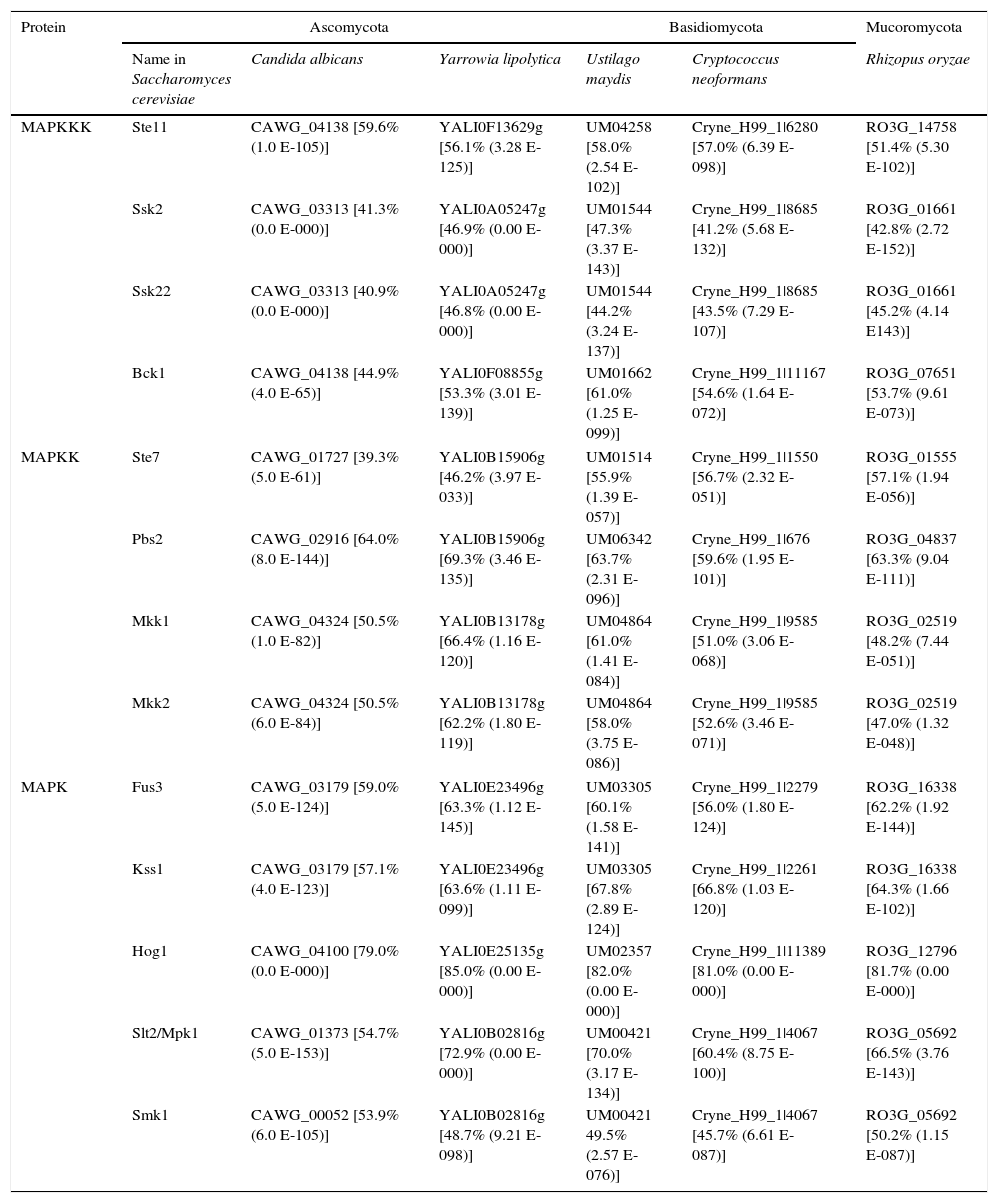
An overview of the MAPK pathways in fungiAs described above, MAPK pathways are very important fungal systems involved in many physiological and developmental processes, stress response, virulence, interaction with other organisms, etc. They are generally conserved in all the species studied thus far and they have a very similar organization and functions3,21,23,25,29,32,41,49,52,64,79,93 (Fig. 1). The saprophytic yeast Saccharomyces cerevisiae and the human pathogen yeast Candida albicans gather the most information available about the fungal MAPK pathways. Indeed, S. cerevisiae was the first organism where genes related to sensing signals of mating were cloned.3 These genes were named STE2 and STE3 because their mutation caused sterility.69 The MAPKs characterized in S. cerevisiae have homologues in many fungi belonging to different divisions, and with different lifestyles (e.g. see Table 1). In some of these fungi, MAPKs, and even the complete MAPK pathways, have been also characterized by molecular or biochemical studies, or predicted by bioinformatic analysis.
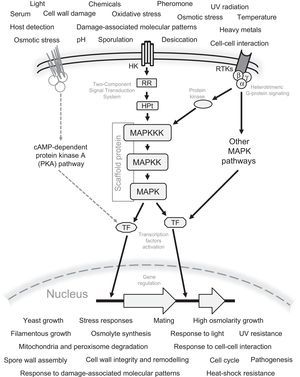
General schematic representation of the signaling mechanism by the MAPK pathways in fungi. All possible sensed signals and cellular responses to these signals are included. Also the Two-Component Signal Transduction (TCS) system, G protein-coupled receptors, receptor tyrosine kinases (RTKs), and the interaction of MAPK with other signaling pathways, e.g. the cAMP-dependent protein kinase A (PKA) pathway are represented. The Scaffold protein is generally present in the MAPK pathway involved in mating. The following abbreviations are used: MAPKKK, MAP kinase kinase kinase; MAPKK, MAP kinase kinase; MAPK, MAP kinase; TF, transcription factors; HK, histidine kinase; RR, response regulator; HPt, histidine-containing phospho-transmitter.
MAPK components described in Saccharomyces cerevisiae, and their possible homologues in representative fungi of different phyla.
| Protein | Ascomycota | Basidiomycota | Mucoromycota | |||
|---|---|---|---|---|---|---|
| Name in Saccharomyces cerevisiae | Candida albicans | Yarrowia lipolytica | Ustilago maydis | Cryptococcus neoformans | Rhizopus oryzae | |
| MAPKKK | Ste11 | CAWG_04138 [59.6% (1.0 E-105)] | YALI0F13629g [56.1% (3.28 E-125)] | UM04258 [58.0% (2.54 E-102)] | Cryne_H99_1|6280 [57.0% (6.39 E-098)] | RO3G_14758 [51.4% (5.30 E-102)] |
| Ssk2 | CAWG_03313 [41.3% (0.0 E-000)] | YALI0A05247g [46.9% (0.00 E-000)] | UM01544 [47.3% (3.37 E-143)] | Cryne_H99_1|8685 [41.2% (5.68 E-132)] | RO3G_01661 [42.8% (2.72 E-152)] | |
| Ssk22 | CAWG_03313 [40.9% (0.0 E-000)] | YALI0A05247g [46.8% (0.00 E-000)] | UM01544 [44.2% (3.24 E-137)] | Cryne_H99_1|8685 [43.5% (7.29 E-107)] | RO3G_01661 [45.2% (4.14 E143)] | |
| Bck1 | CAWG_04138 [44.9% (4.0 E-65)] | YALI0F08855g [53.3% (3.01 E-139)] | UM01662 [61.0% (1.25 E-099)] | Cryne_H99_1|11167 [54.6% (1.64 E-072)] | RO3G_07651 [53.7% (9.61 E-073)] | |
| MAPKK | Ste7 | CAWG_01727 [39.3% (5.0 E-61)] | YALI0B15906g [46.2% (3.97 E-033)] | UM01514 [55.9% (1.39 E-057)] | Cryne_H99_1|1550 [56.7% (2.32 E-051)] | RO3G_01555 [57.1% (1.94 E-056)] |
| Pbs2 | CAWG_02916 [64.0% (8.0 E-144)] | YALI0B15906g [69.3% (3.46 E-135)] | UM06342 [63.7% (2.31 E-096)] | Cryne_H99_1|676 [59.6% (1.95 E-101)] | RO3G_04837 [63.3% (9.04 E-111)] | |
| Mkk1 | CAWG_04324 [50.5% (1.0 E-82)] | YALI0B13178g [66.4% (1.16 E-120)] | UM04864 [61.0% (1.41 E-084)] | Cryne_H99_1|9585 [51.0% (3.06 E-068)] | RO3G_02519 [48.2% (7.44 E-051)] | |
| Mkk2 | CAWG_04324 [50.5% (6.0 E-84)] | YALI0B13178g [62.2% (1.80 E-119)] | UM04864 [58.0% (3.75 E-086)] | Cryne_H99_1|9585 [52.6% (3.46 E-071)] | RO3G_02519 [47.0% (1.32 E-048)] | |
| MAPK | Fus3 | CAWG_03179 [59.0% (5.0 E-124)] | YALI0E23496g [63.3% (1.12 E-145)] | UM03305 [60.1% (1.58 E-141)] | Cryne_H99_1|2279 [56.0% (1.80 E-124)] | RO3G_16338 [62.2% (1.92 E-144)] |
| Kss1 | CAWG_03179 [57.1% (4.0 E-123)] | YALI0E23496g [63.6% (1.11 E-099)] | UM03305 [67.8% (2.89 E-124)] | Cryne_H99_1|2261 [66.8% (1.03 E-120)] | RO3G_16338 [64.3% (1.66 E-102)] | |
| Hog1 | CAWG_04100 [79.0% (0.0 E-000)] | YALI0E25135g [85.0% (0.00 E-000)] | UM02357 [82.0% (0.00 E-000)] | Cryne_H99_1|11389 [81.0% (0.00 E-000)] | RO3G_12796 [81.7% (0.00 E-000)] | |
| Slt2/Mpk1 | CAWG_01373 [54.7% (5.0 E-153)] | YALI0B02816g [72.9% (0.00 E-000)] | UM00421 [70.0% (3.17 E-134)] | Cryne_H99_1|4067 [60.4% (8.75 E-100)] | RO3G_05692 [66.5% (3.76 E-143)] | |
| Smk1 | CAWG_00052 [53.9% (6.0 E-105)] | YALI0B02816g [48.7% (9.21 E-098)] | UM00421 49.5% (2.57 E-076)] | Cryne_H99_1|4067 [45.7% (6.61 E-087)] | RO3G_05692 [50.2% (1.15 E-087)] | |
The existence of sensing proteins, such as Sln1, Sho1, Msb2, Opy2, Snf1, and even phytochrome (FphA), auxiliary proteins that interact with the external sensors Wsc, Gpr1, Ypd1, Ssk1, Cdc42, Bem4, and that subsequently activate the corresponding MAPK pathways, have been described in different fungal species, such as S. cerevisiae, C. albicans, Cryptococcus neoformans, Aspergillus nidulans, Fusarium graminearum, Fusarium oxysporum, Verticillium dahliae, Parastagonospora nodoorum, Beuveria bassiana, Kluyveromyces lactis, Botritis cinerea, Magnaporthe oryzae, Ustilago maydis, etc.9,17,30,31,36,38,42,43,45,48,60,66,74,75,83,86,88–90,93
These sensory proteins and auxiliary proteins form the two-component signal transduction (TCS) system, which together with G protein-coupled receptors (GPCRs), have been described in fungi to be involved in the signal transfer from the extracellular medium to the MAPK core, mainly under conditions of stress and virulence (see reviews in Rispail et al.79; Velázquez-Zavala et al.89; Ma and Li50; Hagiwara et al.32; Kou et al.41; Alvaro and Thorner3). These signal transduction mechanisms upstream of the MAPK core are discussed below.
The MAPK pathways not always regulate the same processes or induce the same cellular responses in the different fungal species. For example, in C. albicans the cell wall integrity (CWI) pathway is an important virulence factor,2 but in contrast its homologue CWI pathway in the Basidiomycota phytopathogenic fungus U. maydis is only involved in sensing damage in the cell wall, forcing the cell to escape from the G2 phase of the cell cycle.13 This phenomenon occurs similarly in S. cerevisiae, with the difference that in the latter the homologue MAPK pathway induces a cell cycle arrest at the G2 phase when its cell wall is damaged.13
Mechanisms of transfer of the environmental signals through the MAPK pathway, and their connection with the downstream componentsSignal transfer to MAPK pathways by the two-component signal transduction systems, and G-proteins coupled to receptorsThe two-component signal transduction (TCS) system (originally described in bacteria, but now known to be present also in fungi and plants,32,50,73,79,86,90 and not in animals.50), and G-proteins coupled receptors (GPCRs), are known to be the main mechanisms involved in receiving extracellular signals and in the further activation of MAPK or other pathways. In fungi, this system is involved in several processes including development, for example: osmotic and oxidative stress, cell and sexual cycle regulation, virulence, etc.32,50,74,90 In these organisms the TCS system is made by three components or signal transducers present in one or more copies: a histidine kinase (HK), a response regulator (RR), and a histidine-containing phospho-transmitter (HPt), that in turn phosphorylates the MAPKKK of the corresponding MAPK core.32
Heterotrimeric G protein signaling occurs by its activation through a membrane G protein-coupled receptor (GPCRs). This occurs during the perception of an extracellular stimulus, in which the GPCR undergoes changes in its conformation, giving rise to the dissociation of the G proteins into a dimer, Gβ-Gγ, and a monomer, GTP-Gα. These components act downstream interacting with protein kinases which subsequently phosphorylate the MAPKKK of the MAPK core. After MAPK protein activation occurs, GTP bound to Gα is hydrolyzed, and re-association with the Gβ-Gγ heterodimer takes place.8,22,41,47,73,76
Transfer of the signal received through the MAPK coreThe extracellular signal perceived by receptors, and transmitted by the mechanisms described above is finally received by the MAPKKK protein. The activation of MAPK protein occurs by phosphorylation of specific amino acid residues. MAPKKK actives the MAPKK protein, and this protein in turn activates the MAPK protein, which finally activates transcription factors involved in the transcriptional regulation of cellular response.6,32 MAPK proteins have the characteristic domain S_TKc (serine/threonine protein kinase), as well as ATP binding sites, and a phosphotransferase domain. It should be noticed that MAPKKKs also have SAM domains (sterile alpha motif domains involved in protein interaction and signal transduction), and Ras_bdg_2 domains (domains involved in its interaction with the Ras G proteins that allow the transfer of the perceived signals by trans-membrane receptors). These catalytic domains are generally conserved in the MAPKs of different fungal species, and their high homology is an evidence that they are involved in the same physiological phenomena and, accordingly, receive a similar name (Table 2). For example: Kss1 is involved in filamentous growth; Fus3, in pheromone response and mating; Hog1, in osmotic and oxidative stress response; Slt2/Mpk1, in cell wall integrity; etc.2,3,9,32,41,50,73 Under this idea, Hog1, the principal component of the high-osmolarity glycerol (HOG) pathway is probably the most conserved MAPK protein in fungal species, and has high similarity mainly in the regions coding for their functional domains: STKc_Sty1_Hog1, catalytic domain; S_TKc, serine/threonine protein kinase; phosphotransferase; and ATP binding site (Fig. 2). Recently, in addition to the indispensable role of the HOG pathway in response to stress and osmosensing, an additional function was described in A. nidulans.91 Accordingly, it was described that this pathway was involved in the response to light sensed by phytochrome FphA, and its interaction with protein phosphotransferase YdpA.
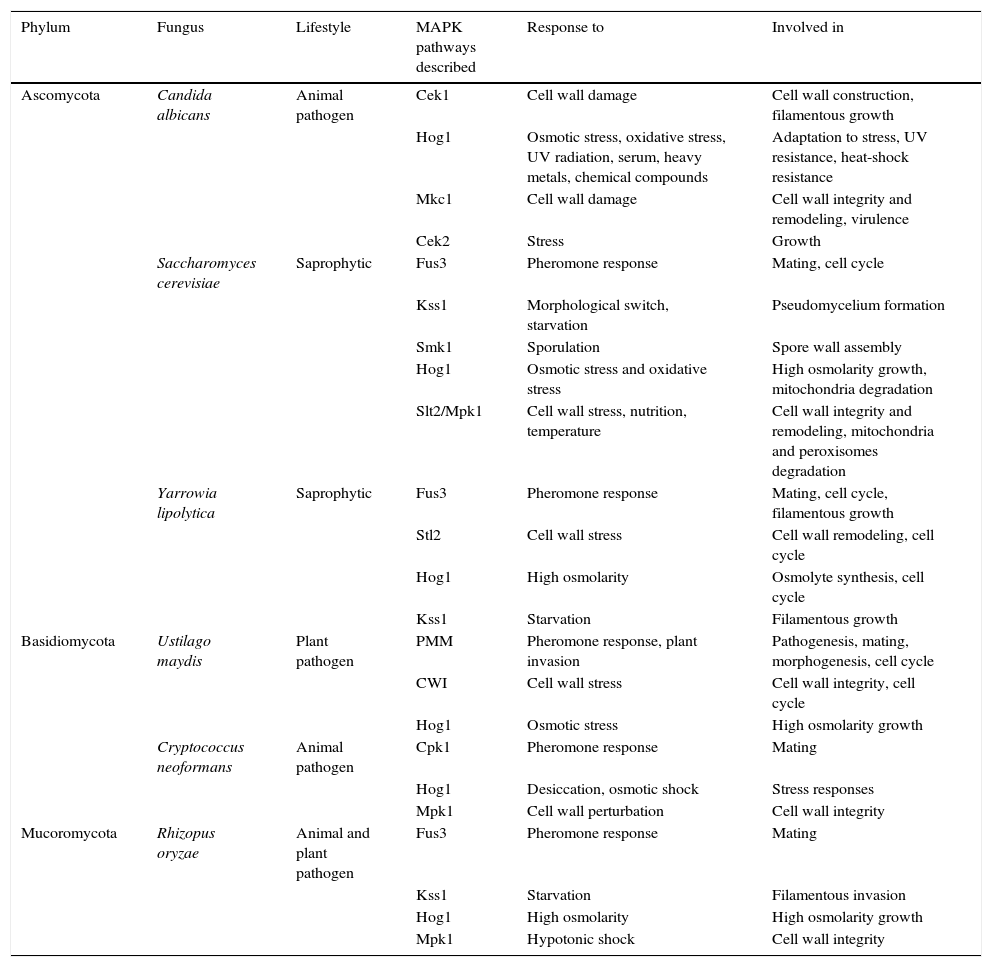
MAPK pathways described in phylogenetically distant fungi and with different lifestyles.
| Phylum | Fungus | Lifestyle | MAPK pathways described | Response to | Involved in |
|---|---|---|---|---|---|
| Ascomycota | Candida albicans | Animal pathogen | Cek1 | Cell wall damage | Cell wall construction, filamentous growth |
| Hog1 | Osmotic stress, oxidative stress, UV radiation, serum, heavy metals, chemical compounds | Adaptation to stress, UV resistance, heat-shock resistance | |||
| Mkc1 | Cell wall damage | Cell wall integrity and remodeling, virulence | |||
| Cek2 | Stress | Growth | |||
| Saccharomyces cerevisiae | Saprophytic | Fus3 | Pheromone response | Mating, cell cycle | |
| Kss1 | Morphological switch, starvation | Pseudomycelium formation | |||
| Smk1 | Sporulation | Spore wall assembly | |||
| Hog1 | Osmotic stress and oxidative stress | High osmolarity growth, mitochondria degradation | |||
| Slt2/Mpk1 | Cell wall stress, nutrition, temperature | Cell wall integrity and remodeling, mitochondria and peroxisomes degradation | |||
| Yarrowia lipolytica | Saprophytic | Fus3 | Pheromone response | Mating, cell cycle, filamentous growth | |
| Stl2 | Cell wall stress | Cell wall remodeling, cell cycle | |||
| Hog1 | High osmolarity | Osmolyte synthesis, cell cycle | |||
| Kss1 | Starvation | Filamentous growth | |||
| Basidiomycota | Ustilago maydis | Plant pathogen | PMM | Pheromone response, plant invasion | Pathogenesis, mating, morphogenesis, cell cycle |
| CWI | Cell wall stress | Cell wall integrity, cell cycle | |||
| Hog1 | Osmotic stress | High osmolarity growth | |||
| Cryptococcus neoformans | Animal pathogen | Cpk1 | Pheromone response | Mating | |
| Hog1 | Desiccation, osmotic shock | Stress responses | |||
| Mpk1 | Cell wall perturbation | Cell wall integrity | |||
| Mucoromycota | Rhizopus oryzae | Animal and plant pathogen | Fus3 | Pheromone response | Mating |
| Kss1 | Starvation | Filamentous invasion | |||
| Hog1 | High osmolarity | High osmolarity growth | |||
| Mpk1 | Hypotonic shock | Cell wall integrity |
The corresponding citation appears in the text.
Conservation of Hog1 MAPKs in some phylogenetically distant fungi, and their functional domains. Functional domains are indicated: remarked in gray, STKc_Sty1_Hog1; bold letters, Kinase and Phosphotransferase; and underlined, ATP binding site. Ca, Candida albicans; Sc, Saccharomyces cerevisiae; Yl, Yarrowia lipolytica; Ro, Rhizopus oryzae; Um, Ustilago maydis; Cn, Cryptococcus neoformans.
The last part in the transduction of extracellular signals by the MAPK pathways is the activation by phosphorylation of downstream master transcription factors, which in turn regulate the transcription of other transcription factors and other genes involved in the response to the extracellular signal sensed. Interestingly, the transcription factors are one of the most important points of interaction and interconnection between different MAPK pathways, as well as in other signaling pathways. An excellent example of all the aspects described above is the U. maydis transcription factor Prf1. This transcription factor regulates two fundamental processes in this fungus, mating and the pathogenic process, and it can be activated by both the cAMP-dependent protein kinase A (PKA) pathway (during mating) and MAPK PMM pathway (during the pathogenic process) (revised by Brefort et al.8).
Throughout the study of MAPK and other pathways in fungi, different transcription factors involved in transcriptional regulation of cellular responses have been described. For example, as previously mentioned, Yap2 is the point of interconnection between S. cerevisiae CWI and HOG pathways63; in A. nidulans, AtfA is involved in the response to stress37; in U. maydis, the role of PacC/Rim101 as a crossover point between CWI, HOG, PMM and Pal/Rim pathways during response to pH and dimorphism have been suggested.24,58,59 Also in C. albicans, the transcription factors Cph1, Efg1 and Tec1 activated by the Cek1 MAPK pathway, regulate filamentous growth and virulence.9,96
Likewise, the downstream activation by MAPK pathways of the transcription factors Sst2, Bni1, Far1, Ste12, Sko1, Rck1, Rck2, Msn2, Msn4, Hot1, Smp1, Rlm1, Mcm1, Msg5, Sdp1, Pir3, Mbp1, Swi6, Swi4, Fks2, Ppz1, Ppz2, Smp1, Far1, Flo8, Sfl1, Sbf1, NapA, Pap1, etc., has been described as important or indispensable for many important physiological processes in several and different fungi.32,79,96
Finally, it is important to mention that also an epigenetic regulation has been suggested for some of the transcription factors described here, and therefore the cellular processes that they regulate.55,73
Interaction between MAPK pathways, or with other signaling pathwaysSeveral MAPK pathways may be involved in more than one cellular processIn C. albicans the already mentioned HOG pathway, described to be involved in the adaptation to high osmolarity stress, interacts with CWI pathway, and they together are involved in morphogenesis, integrity of the cell wall, growth, response to stress, and virulence35,77,80; it was revised by Brown et al.9 These interactions were confirmed by the mutation of the upstream components (SHO1, SLN1, YDP1, SSK1, MSB2, OPY2) of these two pathways (HOG or CWI). The mutant strains were avirulent in mice and Galleria mellonella (the greater wax moth or honeycomb moth35); a different behavior and susceptible phenotype to different types of stress was also shown.88 The same behavior was also found in the Ascomycota fungus B. bassiana, a fungal pathogen of insects.90 An important evidence of interaction between MAPK pathways is the TCS system, since in general the sensors and proteins belonging to this system can interact with more than one MAPK pathway, as demonstrated in different fungi, such as S. cerevisiae, C. albicans, B. bassiana, V. dahlie, F. oxysporum, B. cinerea, K. lactis, Aspergillus fumigatus, etc.,17,33,45,74,75,86,88–90 revised in Ma and Li,50 and Higiwara et al.33
In fungi, the interaction among proteins belonging to different MAPK pathways, apart from the interaction of different whole MAPK pathways, may take place. Thus, the S. cerevisiae MAPK Ste7 interacts generally with Fus3 in the mating reaction of the yeast, but also with Kss1 for pseudomycelium formation. Similarly, the MAPK Ste11 interacts with Ste7 for mating, but also with Pbs2 during osmotic stress.96 The same cross interactions take place in other fungi, e.g. C. albicans, U. maydis, and Rhizopus oryzae.79 Also in S. cerevisiae the interaction between CWI and HOG pathways has been suggested by the existence of the same transcription factor in both pathways, Yap2.63 In A. nidulans, the MAPK SakA and the transcription factor AtfA are components of multiple signaling pathways involved in response to stress and in development (e.g. mating, DNA damage response, mRNA stability, protein synthesis, cell cycle regulation, and mitochondrial function).37 Besides, in Fusarium verticillioides the MAPK pathway FvBCK1 involved in growth and development is also involved in cell wall biogenesis and in the response to osmotic and oxidative stresses,94 suggesting the interconnection of this MAPK pathway (growth and development) with other MAPK pathways present in this fungus (CWI and HOG).
More than one MAPK pathway is involved in a single phenomenonThe HOG pathway (Hog1) is associated with the CWI pathway (Mkc1) (described to be involved in the regulation of glucan and chitin synthesis71,72) and the filamentous growth pathway (Cek1) (involved in the regulation of genes encoding protein-O-mannosyltransferases12), to maintain the integrity of the cell wall of C. albicans.9 Under the same concept, in S. cerevisiae it has been described the requirement of two MAPK pathways for the process of autophagy: CWI (Stl2) and HOG (Hog1) pathways, involved in the degradation of mitochondria, although the STL2 gene is also independently involved in the degradation of peroxisomes.52 Again in S. cerevisiae, the CWI, HOG and filamentous growth pathways are together involved in the response to stress.27
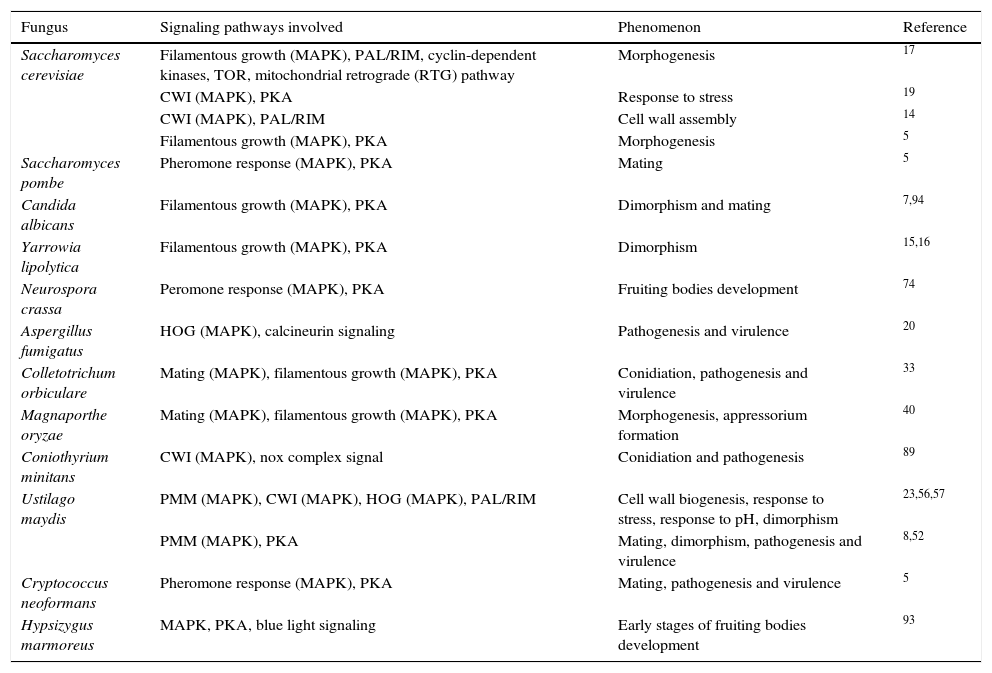
Interaction of MAPK pathways with other signaling pathwaysIn addition to these interactions among the MAPK pathways, the interaction of MAPK pathways with other signal transduction pathways has been also described in fungi. In U. maydis, it has been demonstrated that the PMM (pathogenesis, mating and morphogenesis) pathway, the CWI, and also possibly the HOG MAPK pathways, interact with the Pal/Rim pathway involved in sensing and response to pH24 and dimorphism.59 This association is evidenced by the up-regulation of the transcription factor PACC/RIM101 by the PMM pathway in this fungus58; most likely this transcription factor is the crossover point between both pathways. Similarly, the interconnectedness between CWI MAPK and Pal/Rim pathways during assembly of the cell wall has been described in yeasts.14 The participation of CWI (MAPK), and the cAMP-dependent protein kinase A (PKA) pathway in response to stress has been also described in S. cerevisiae.19 These and other interactions between MAPK pathways and other signaling pathways are presented in Table 3. These interactions have been described as essential for different physiological processes of S. cerevisiae, C. albicans, U. maydis, A. fumigatus, M. oryzae, Schizosaccharomyces pombe, Yarrowia lipolytica, Neurospora crassa, Colletotrichum orbiculare, Coniothyrium minitans, C. neoformans, Hypsizygus marmoreus, etc.,5,8,14–17,19,20,24,34,41,54,58–60,76,91,94,96 including morphogenesis and pathogenesis (see following subtopics).
Interaction of the MAPK pathways with other signaling pathways in different fungi.
| Fungus | Signaling pathways involved | Phenomenon | Reference |
|---|---|---|---|
| Saccharomyces cerevisiae | Filamentous growth (MAPK), PAL/RIM, cyclin-dependent kinases, TOR, mitochondrial retrograde (RTG) pathway | Morphogenesis | 17 |
| CWI (MAPK), PKA | Response to stress | 19 | |
| CWI (MAPK), PAL/RIM | Cell wall assembly | 14 | |
| Filamentous growth (MAPK), PKA | Morphogenesis | 5 | |
| Saccharomyces pombe | Pheromone response (MAPK), PKA | Mating | 5 |
| Candida albicans | Filamentous growth (MAPK), PKA | Dimorphism and mating | 7,94 |
| Yarrowia lipolytica | Filamentous growth (MAPK), PKA | Dimorphism | 15,16 |
| Neurospora crassa | Peromone response (MAPK), PKA | Fruiting bodies development | 74 |
| Aspergillus fumigatus | HOG (MAPK), calcineurin signaling | Pathogenesis and virulence | 20 |
| Colletotrichum orbiculare | Mating (MAPK), filamentous growth (MAPK), PKA | Conidiation, pathogenesis and virulence | 33 |
| Magnaporthe oryzae | Mating (MAPK), filamentous growth (MAPK), PKA | Morphogenesis, appressorium formation | 40 |
| Coniothyrium minitans | CWI (MAPK), nox complex signal | Conidiation and pathogenesis | 89 |
| Ustilago maydis | PMM (MAPK), CWI (MAPK), HOG (MAPK), PAL/RIM | Cell wall biogenesis, response to stress, response to pH, dimorphism | 23,56,57 |
| PMM (MAPK), PKA | Mating, dimorphism, pathogenesis and virulence | 8,52 | |
| Cryptococcus neoformans | Pheromone response (MAPK), PKA | Mating, pathogenesis and virulence | 5 |
| Hypsizygus marmoreus | MAPK, PKA, blue light signaling | Early stages of fruiting bodies development | 93 |
MAPK pathways are involved or are required to carry out different morphogenetic processes in fungi, like dimorphism. Dimorphism is a morphogenetic and differentiation phenomenon defined as the property of fungi to grow as budding yeasts or mycelium, depending on the environmental conditions.81 This phenomenon is considered a model process of cell differentiation in eukaryotes. Its importance relies on the fact that many pathogenic fungi undergo a dimorphic transition during the colonization of their hosts,81 and a role of the MAPK pathways in the yeast-to-mycelium transition in different fungal species has been revealed. In C. albicans this phenomenon is controlled by the Cek1 pathway,9 in U. maydis by the PMM pathway both in vitro54 and in vivo conditions,4,61 and in Y. lipolytica by the Kss1 pathway.15 Similarly, the transition from yeast to the pseudomycelial morphologies in S. cerevisiae (that properly does not grow in a filamentous form), involves the Kss1 MAPK pathway.26 Recently, in addition, the participation of a number of different signaling pathways – the filamentous growth MAPK, PKA, PAL/RIM, TOR (targets of rapamycin) and RTG (mitochondrial retrograde), as well as the cyclin-dependent kinases – were found by means of a genetic screen to be involved in the pseudomycelium formation of S. cerevisiae.17
As mentioned above, the Cek1 MAPK pathway in C. albicans, a homologue of the S. cerevisiae Kss1 MAPK pathway that is involved in pseudomycelium formation, is necessary for the filamentous growth. Mutations in the components of the core Cek1 pathway (Ste11, Hst7, Cek1), adaptor proteins (St20, Ras1, Cdc42), or transcription factors (Cph1, Efg1, Tec1) controlled by this pathway, affect or suppress the mycelial growth of C. albicans, and significantly attenuate or suppress its virulence.9,96 A similarly phenomenon occurs when the gene HOG1 is deleted in this fungus,35 demonstrating the requirement of the filamentous growth and HOG pathways during the distinctive and pathogenic processes of C. albicans. In addition, mutation of the TCS system (upstream interactive proteins) in this pathway affects the C. albicans polarized growth under nutrient limitation conditions.75
As already pointed out, fungal MAPK pathways can interact with other signal transduction pathways during the morphogenetic processes (Table 3). For example, in C. albicans the MAPK (Cek1) and PKA pathways operate in synergy during the yeast-to-mycelium transition (dimorphism),96 whereas in Y. lipolytica15,16 and U. maydis54 MAPK is required for the mycelial growth, and PKA for growing in the yeast form. That is to say, in these latter fungi these pathways are functionally antagonistic.
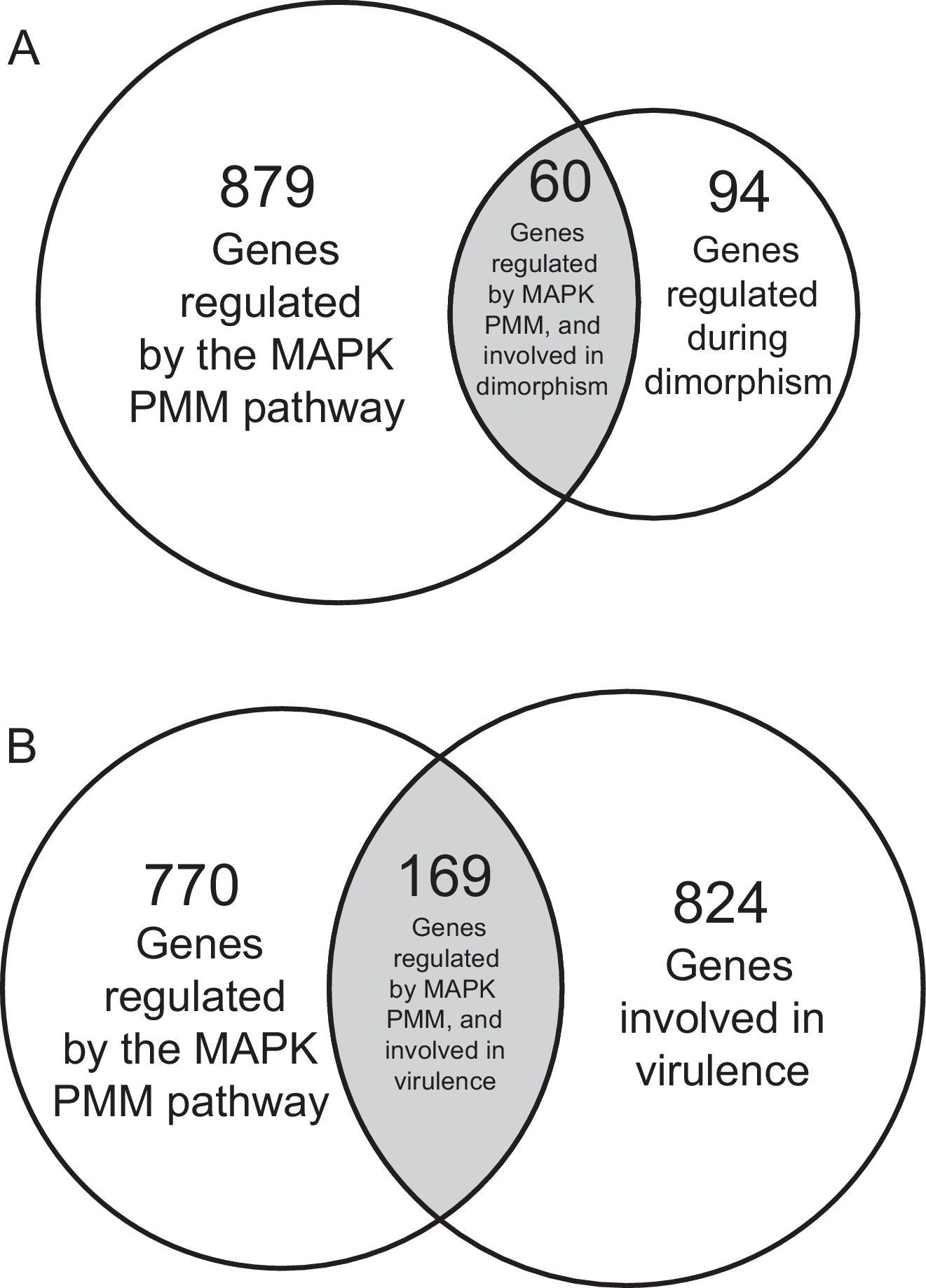
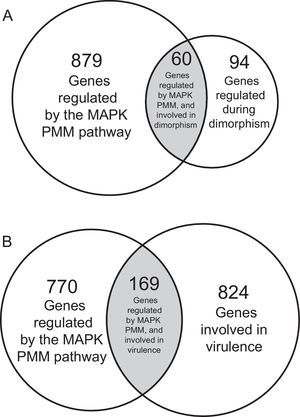
As an example of the regulation of dimorphism by the MAPK pathway in fungi, Fig. 3A shows an important number of genes regulated by the MAPK PMM pathway during the dimorphic transition from yeast to mycelium in U. maydis.58,59 This study represents the first analysis where gene regulation of the whole eukaryotic genome by a MAPK pathway was done.58 In U. maydis, approximately 14% (equivalent to 939 genes) of its genome was found to be regulated by the MAPK pathway. Among the genes differentially regulated, there were genes encoding proteins involved in cell cycle regulation [cyclin-dependent kinases (cdk), cell-division cycle (CDC), Hos4-subunit of the Set3 complex, DIP1, etc.]; transcription factors [PacC (response to pH changes), AtfA (response to different types of stress), white collar 1 (response to light), etc.]; cellular transport and secretion (transport of substances, metals, effectors, etc.); signal transduction mechanism [Sok1-protein kinase; Pbs2-tyrosine protein kinase, GTPases, Crk1 (MAPK protein), etc.]; synthesis of cell wall [chitin deacetylase, glucan synthase (Kre6), chitinases, chitin synthases, proteins involved in N-glycosylation and O-mannosylation (Rot1)]; and finally in differentiation processes [proteins involved in organization of the actin cytoskeleton, cell polarity (Kin7a-motor protein), vesicle trafficking; actin polymerization (Ysc84-protein), etc.]. Also genes involved in pathogenesis and virulence were also differentially expressed (see below).
Venn diagram showing the number of genes regulated by the MAPK (PMM) in Ustilago madis dimorphism and virulence. (A) Venn diagram showing 60 genes regulated by the MAPK pathway during the U. maydis dimorphism. (B) Venn diagram showing 169 genes regulated by the MAPK pathway involved the U. maydis pathogenic process.
In other fungi, such as C. albicans70 and Y. lipolytica,67 genes involved in similar processes have been also identified by transcriptomic analysis during their dimorphic transition, corroborating the importance of the MAPK pathway in the differentiation processes of these fungi.
MAPK pathways in the development of fruiting bodiesAnother morphogenetic phenomenon that occurs in some fungi is the formation of fruiting bodies, complex structures involved in sexual reproduction. Interestingly, the role of a MAPK pathway in developing ascocarps of N. crassa has been described, as well as its possible interaction with the PKA pathway under the same mechanisms described above.76 It is known that during the formation of these structures, the corresponding extracellular stimuli are transferred through heterotrimeric G or Ras proteins, and then to the MAPK and PKA pathways, which regulate gene expression leading to the formation of ascocarps.76 Accordingly, a recent study described the importance of MAPK, PKA and blue light signaling pathways in the early stages of the formation of fruiting bodies (mycelial knot, mycelial pigmentation, and primordium) in the edible Basidiomycota species H. marmoreus.95 Similarly, in the Shiitake fungus Lentinula edodes, the importance of the MAPK pathway, also in early stages of the fruiting bodies development, has been described.84 In the Ascomycota fungi Cordyceps militaris,97Sordaria macrospora,85 and Phaeosphaeria nodorum38 the role of MAPK pathway in fruiting bodies formation has been suggested, particularly the HOG pathway in the latter fungus.38 Moreover, in the Basidiomycota fungus Pleurotus eryngii, differentially expressed genes involved in MAPK signaling, particularly in the cell wall biosynthesis (MAPK CWI), were identified during fruiting body formation.25 In U. maydis, despite its taxonomic classification, the ability to form basidiocarps under controlled conditions has been described.11 Interestingly, mutant strains in genes encoding MAPK core proteins were unable to form basidiocarps (León-Ramírez et al., in preparation). This observation confirms the fundamental role of MAPK pathways during basidiocarps formation in fungal species. Nevertheless, despite these studies, there is still scant information about how the MAPK pathways regulate the phenomenon of fruiting body formation in fungi.
MAPK pathways involved in fungal virulenceIn all plant or animal pathogenic fungi thus far studied, MAPK pathways have been identified to be involved in their respective virulent processes. For example, the role of MAPK pathways during pathogenesis has been described for the plant pathogens U. maydis,8Alternaria alternata,18B. cinerea,45,82F. graminearum,30,31F. oxysporum,74C. orbiculare,34Magnaporthe grisea,10,41V. dahliae,86Cochliobolus heterostrophus,46P. nodorum,38Fusarium verticillioides,94C. minitans,91 inter alia, and for the human pathogens C. albicans,9,35,77,80C. neoformans,36 and A. fumigatus.20,87,92 Also a similar role in the pathogenesis was disclosed in the MAPK pathway of the insect pathogen B. bassiana.90 In other types of interactions between fungi and plants, the signaling by the MAPK pathways has been described as necessary; e.g. in the Ascomycota fungus Epichloë festucae, a MAPK plays a crucial role during its symbiotic interaction with the plant Lolium perenne.7 A similar requirement occurs in the mycorrhizal relationship between Rhizophagus irregularis and its soy bean host.49
Regarding the molecular mechanism of the MAPK signaling pathways in virulence, deeper analyses have been performed with C. albicans, probably the most important fungal pathogen in humans. This fungus has the ability to colonize different parts of the human body changing its morphology from yeast to an invasive mycelium.40 When C. albicans infects and colonizes a human being, the fungus is faced with a hostile environment caused by the defense system of the host. Under these conditions the HOG, the filamentous growth, and CWI MAPK pathways play a key role, firstly by sensing the hostile environmental conditions, and secondly orchestrating the alterations in the genetic machinery of the fungus that leads to a physiological alteration associated to virulence such as change in cell morphology, changes in cell wall composition and structure, ability to respond to several stresses, secretion of different proteins including hydrolytic enzymes, etc.9,35,80,98 Of the four known C. albicans MAPK pathways, CWI (Mkc1), HOG (Hog1), filamentous growth (Cek1), and growth (Cek2) (see Table 2), CWI and HOG are involved in the response to the stress imposed by the immune system of the host during the infection process, including the activity of the phagocytes.2,35,77,80 It is therefore not surprising that inactivation of the Hog1 and Mkc1 pathways reduces the virulence of C. albicans in mice,1,2,9 and that mutant strains in these genes cannot bind to, or infect, the intestinal mucosa, also being susceptible to bile salts.77 It has been described the role of the sensor protein Msb2, a glycoprotein of the Cek1 pathway involved in cell wall integrity and filamentous growth. Msb2 is secreted to the medium and prevents the inflammatory response caused by the antimicrobial peptides (AMPs) produced by the host.83 Mbs2 is part of the TCS system, and the C. albicans mutant strains in the genes of the TCS system are susceptible to different stresses.88 Similarly, genes encoding proteins of this system are essential at the early stages of the pathogenesis processes developed by other fungi: U. maydis,43V. dahlie,86B. bassiana,90B. cinerea,45F. oxysporum,74M. oryzae,41,60 and several species of Aspergillus.32
Interactions of MAPK pathways with other signaling pathways during the pathogenic processes of fungi have been described. For example, in A. fumigatus, the HOG and calcineurin signaling pathways are required for its virulence.20 Similarly, in C. minitans, a fungal species used as a biocontrol agent, the Nox complex signal along with CWI (Stl2) pathway regulate its pathogenicity and conidiation,91 and in U. maydis two important phenomena, mating and virulence, are regulated by the MAPK PMM and PKA pathways (revised by Brefort et al.8). U. maydis, is the causal agent of Zea mays smut disease, and under controlled conditions can infect different plants.44,56,57,65 Apparently in this fungus, and in contrast to C. albicans, only the MAPK PMM pathway is involved in virulence8 through the MAPKs Kpp268 and Crk1.28 Therefore when the genes encoding proteins of the PMM pathway are deleted, the virulence of the fungus over the maize is reduced or eliminated.61,62 In Fig. 3B we show the number of genes putatively regulated by the MAPK PMM pathway and required for pathogenic processes or the acting of some molecules in U. maydis: degradative proteins of plant cell wall, effectors, virulence factors, or regulating transcription factors are some of them. Other genes implied in virulence and identified by its deletion or by bioinformatic analysis are also shown. The regulation of these genes by the MAPK PAM pathway confirms the key role in the signal transduction processes occurring during the pre-penetration and pathogenic process of this fungus, similarly to what occurs in many other pathogenic fungi.
Conclusions and perspectivesIt is evident that MAPK pathways are signal transduction and cellular communication mechanisms highly conserved in all fungi, similarly to what occurs in higher eukaryotic organisms. Accordingly, MAPK pathways are involved in the most essential physiological and development processes occurring in fungal species, and their mutation leads to aberrant phenotypes, severe damages under different growth conditions, alterations in development and differentiation, and decrease or loss of virulence in the pathogenic species. The data accumulated in the study of fungal species analyzed thus far evidence that, practically, with only subtle variations, the same MAPK pathways exist in all the analyzed species, although their number may be variable. It may be concluded also that, although they act very similarly and work by the same mechanism, Receptor – Two-Component Signal Transduction system (TCS) – MAPK core – Transcription factors – Gene regulation (Fig. 1), the regulated processes are not always the same. It is also important to recall that during practically all important physiological processes in fungi, there is an active interconnection between MAPK pathways and also with other signaling pathways, especially with the PKA. In many cases, this interaction of the MAPK pathways with other pathways occurs upstream of the pathways involved, for instance in the sensory and auxiliary proteins.
It seems necessary to point out that, although large advances on the study of MAPK pathways in fungi have been recently uncovered, more information on some phenomena or processes that occur in these organisms is necessary. For example, the nature of different receptors, as well as the interactions occurring between the different signaling pathways present in fungi are still unknown. Similarly, the role of the MAPK pathways in the formation of fruiting bodies in fungi is still a poorly studied matter, and no information is available on the signaling pathways that are regulating this process in coordination with the MAPK pathways. Also necessary in this aspect is some information on the nature of the genes regulated in several fungal processes.
Regarding the pathogenic processes, it is important to have more information on whether the MAPK pathways regulate the synthesis of virulence factors, effector proteins, etc., involved in the pathogenic processes per se, and of the systems used by fungi to avoid the host defense mechanisms, especially in fungi with biotrophic or hemibiotrophic life styles. It would be important also to increase the knowledge on how MAPK pathways regulate fungus symbiotic relationships with other organisms: fungus–plant (e.g. mycorrhizae, orchids), fungus–algae (e.g. lichens), and fungus–insects (e.g. Ambrosia beetles, ant gardens), etc. Finally, it is necessary to study in depth the understanding of the epigenetic regulation of the physiological and developmental processes where MAPK pathways are involved.
Despite these unfilled aspects it is obvious that our knowledge on the roles played by MAPK pathways in fungal cells has widened very rapidly in the most recent years, and that the knowledge gathered on them makes clear their importance in all the living processes of the members of the Phylum Fungi.
Conflict of interestThe authors declare that they have no conflicts of interest.
Some of the experimental work of the authors discussed in this review were partially supported by Consejo Nacional de Ciencia y Tecnología (CONACYT), México.