Saprochaete capitata (formerly known as Geotrichum capitatum and Blastoschizomyces capitatus) is a ubiquitous fungus found in soil, water, air, plants and dairy products. It colonizes the skin, and bronchial and intestinal tract of healthy people producing serious opportunistic infections in patients with haematological malignancies, especially in those with acute leukaemia. Since 1960s its presence is being increasingly recognized in this group of patients. The clinical spectrum of S. capitata disseminated infections is very similar to that produced by Candida, being easily misinterpreted. The associated high mortality and low susceptibility to fluconazole and echinocandins of S. capitata require the acknowledgement of this emergent infection so that it can be properly treated.
Case reportWe report 5 new cases of S. capitata disseminated infection in patients with advanced haematological malignancies observed in the haematology unit between the years 2004 and 2010, and review the state-of-the-art for diagnosis and treatment of this infection.
ConclusionsBased on our experience, the prophylactic use of or the empirical antifungal treatment with fluconazole and/or echinocandins would not be adequate for oncohaematological patients in those hospitals where S. capitata infection may be highly prevalent.
Antecedentes Saprochaete capitata (previamente conocido como Geotrichum capitatum y Blastoschizomyces capitatus) es un hongo ubicuo que habita el suelo, agua, aire, plantas y productos lácteos. Coloniza la piel, el árbol bronquial y el tubo digestivo de individuos sanos, y provoca infecciones oportunistas graves en pacientes con neoplasias hematológicas, descritas en especial entre los portadores de leucemia aguda. Desde la década de los sesenta se ha incrementado su aislamiento en este tipo de pacientes. El espectro clínico de las infecciones diseminadas por S. capitata es muy similar al producido por Candida, lo que puede dar lugar a errores diagnósticos. Su elevada mortalidad y la baja sensibilidad al fluconazol y a las equinocandinas requieren el reconocimiento de esta infección emergente para instaurar un tratamiento apropiado.
Caso clínicoDescribimos 5 nuevos casos de infección diseminada por S. capitata en pacientes con neoplasias hematológicas avanzadas, observados en una unidad de hematología entre 2004 y 2010, y revisamos los datos más recientes sobre el diagnóstico y tratamiento de esta infección.
ConclusionesDe acuerdo con nuestra experiencia, el uso profiláctico o empírico del fluconazol o las equinocandinas no sería un tratamiento antimicótico adecuado para pacientes con neoplasias hematológicas ingresados en hospitales donde la infección por S. capitata puede ser muy prevalente.
The frequency of invasive fungal infections (IFIs) caused by opportunistic fungi in general hospitals has increased significantly during the last decades. The combination of several predisposing conditions49 makes patients with haematological malignancies or undergoing haematopoietic stem cell transplantation (HSCT) the group at highest risk for development of IFIs.8,43,45 Although Aspergillus and Candida species are the most prevalent,43,44,49 a heterogeneous and growing group of fungi is being identified with increasing frequency. Yeasts of the genera Trichosporon, Saccharomyces, Saprochaete or Rhodotorula, or filamentous fungi as Zygomycetes, or those belonging to genus Pseudallescheria/Scedosporium or Fusarium are some of them.49,53
Saprochaete capitata (teleomorph Magnusiomyces capitatus; formerly known as Geotrichum capitatum and Blastoschizomyces capitatus, teleomorph Dipodascus capitatus) is a ubiquitous microorganism found in soil, water, air, plants and dairy products. Yeasts are urease negative, thermotolerant and can grow in the presence of cycloheximide.52 Phylogenetically it is classified within the ascomycetes, and morphologically it is characterized by its ability to produce anelloconidia and arthroconidia.51
Along with colonizing the skin, bronchial and intestinal tract of healthy people, it can cause disseminated opportunistic infections in patients with cancer, especially with acute leukaemia.6,39,40,55 Like other fungi, S. capitata has been identified in haematology wards as a contaminant of food.5,32 The first cases of IFIs by this organism date back to the 1960s,9 and in 1987 a special virulence was recognized in this group of patients.37
The clinical spectrum of disseminated infections caused by S. capitata is very similar to those produced by other yeasts such as Candida28,39,40 or Trichosporon62 Fever unresponsive to antibiotic treatment during the period of profound neutropenia and the presence of fungemia is common. In addition, like in acute disseminated candidiasis, infections with S. capitata may give rise to skin lesions in the form of a generalized maculopapular eruption.6,39 Nodular lesions in the liver and/or spleen on CT and/or MRI, at the time of the recovery from neutropenia, are indistinguishable from those seen in chronic disseminated candidiasis.6,39
However, in contrast to disseminated candidiasis, the involvement of lung is common in invasive infections caused by S. capitata.39,40 Coughing, expectoration, chest pain, spontaneous pneumothorax and pulmonary infiltrates are frequently observed.6 In this way, the isolation of this microorganism from sputum or bronchoalveolar lavage in neutropenic patients with well documented pneumonia, and in the absence of other pathogens, is indicative of probable pulmonary geotrichosis for some authors.28 Moreover, and contrary to that observed in Candida infections, the role of endovascular catheters as the source of S. capitata IFI has seldom been demonstrated.28 However, like in other IFIs, the catheter removal during episodes of fungemia has been associated with a better evolution of patients.40
Another peculiarity of S. capitata invasive infections is the presence of a soluble antigen that reacts like Aspergillus galactomannan, rendering the Platelia Aspergillus EIA test (Bio-Rad) positive.4,26 In this regard, the existence of clinical data compatible with disseminated candidiasis (such as a maculopapular rash) in a patient with positive Platelia Aspergillus result is highly suggestive of invasive S. capitata infection.4,26 It is essential to correctly interpret these findings since this microorganism is not usually susceptible to caspofungin, one of the most used antifungals for the treatment of candidiasis and aspergillosis.
The mortality attributed to invasive infections by S. capitata is greater than that associated with candidemia in patients with acute leukaemia, fluctuating between 52 and 57% in the former,28,40 and 36% in the latter.44 Since fungemia caused by S. capitata affects deep organs of 60–80% of patients39,40 whereas only 10% of candidemias do so,61 this higher mortality rate could be attributed to greater virulence of the microorganism.
There is no known optimal treatment strategy for S. capitata disseminated infections. Cases of infection are scarce and in vitro antifungal susceptibility findings are sometimes contradictory to those observed in the clinical practice. However, there is a considerable degree of consensus that S. capitata shows adequate susceptibility to polyenes such as amphotericin B6,24,25,29,34,35,39–41,49,54 and azoles such as voriconazole,6,13,25,28,31,34,35,40–42,49,56 posaconazole13,49 and itraconazole.13,18,25,35,40,49,56 Nevertheless, some strains show decreased susceptibility or poor clinical response to itraconazole7,29 or amphotericin B.1,2,7,13,18,20,48 By contrast, S. capitata seems poorly susceptible or even resistant to echinocandins such as caspofungin and micafungin,10,13,15,49 and shows an intermediate dose dependent susceptibility against fluconazole,6,7,11,13,25,27,29,31,34,40,47–49,56 or even clear resistance.16,18 5-Fluorocytosine is not a widely used drug in patients with neutropenia and its usefulness for isolates of S. capitata offers conflicting results; some authors find a suitable susceptibility to this antifungal,6,25,40 while others report lack of susceptibility or even resistance for some isolates.2,13,29,48,49 It has recently been reported that an isolate of S. capitata in a haematology unit was resistant to amphotericin B, 5-fluorocytosine, caspofungin and itraconazole.58 Despite this controversy, voriconazole seems to be a highly active drug against S. capitata, and its association to liposomal amphotericin B is the most frequently recommended treatment,29,40,49 however the recovery from neutropenia and general poor condition of the patients, remain essential for the healing of the infection.
Patients and methodsOur institution is a 950-bed tertiary hospital in northern Spain that attended 282 admissions to the haematology ward, corresponding to 176 adult patients with acute leukaemia or Burkitt's lymphoma between the years 2004 and 2010. Five proven IFIs by S. capitata occurring during this period were retrospectively evaluated in 3 women and 2 men, aged between 41 and 74 years. Four patients were diagnosed acute leukaemia, and one Burkitt's lymphoma. All of them were undergoing intensive chemotherapy in single rooms equipped with HEPA (High Efficiency Particulate Air) filters and positive pressure air flow at the time they developed the IFI. Diagnostic and therapeutic protocols of the institution were followed. The study was approved by the ethics committee of the hospital.
The diagnosis of IFI was established according to the criteria of the consensus group of the EORTC/MSG.19 The clinical and microbiological relevant data of the patients are summarized in Table 1.
Clinical, microbiological and serological characteristics of patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Underlying disease | AML | AML | Burkitt lymphoma | AML | ALL |
| Phase of the disease when S. capitata was isolated | Refractory | Relapsed | Advanced stage | “De novo” | Relapsed |
| Chemotherapy (number of courses)a | Salvage (2) | Salvage (4) | Induction (1) | Induction (1) | Salvage (3) |
| Severe neutropenia accumulated time(<0.1neutrophils×109/L) | 73 days | 84+35 days | 12 days | 13 days | 67 days |
| Prophylaxis | No | Fluconazole | No | No | Fluconazole |
| Clinical manifestations | Maculopapular rash | Pancolitis | Dyspnea | Dyspnea+macular rash | Septic shock |
| Isolation of Saprochaete capitata(day post-chemotherapy) | Blood (+53) | Blood (+19) | Blood (+8) | Lung (post-mortem) | Blood+CVCb(+10, +11, +12, +13) |
| Concomitant microorganisms during neutropenia period (site of isolation)c | None | CNS (blood),Candida albicans (oral smear) | CNS (blood),Candida albicans (oral smear) | Stenotrophomonas maltophilia (sputum),CNS (blood+CVC tip),Candida tropicalis (blood) | Escherichia coli (blood) |
| Galactomannan (number of assays)d | Negative (5) | Negative (1) | Negative (2) | Negative (1) | Negative (1) |
| β-(1–3)-d-Glucane | Non tested | Positive | Non tested | Positive | Non tested |
| Antifungal therapy before//afterS. capitata isolation (days of therapy) | Cas+L-AmB (18), Cas (11)//Vor (34) | Cas (15)//Vor (8), L-AmB (9) | Flu (2), Cas (10)//- | Cas (8)//- | -//L-AmB (5), L-AmB+Vor (2) |
| Recovery from neutropenia | Yes | Yes | No | No | No |
| Outcome | Cure, no relapse | Cure, no relapse | Death | Death | Death |
AML, acute myeloid leukaemia; ALL, acute lymphoblastic leukaemia; CNS, coagulase-negative Staphylococcus; Cas, caspofungin; L-AmB, liposomal amphotericin B; Vor, voriconazole; Flu, fluconazole.
A 41-year-old woman was admitted to the haematology ward by May 2004, for treatment of acute myeloid leukaemia (AML), without maturation according to the WHO classification, and intermediate risk.30,60 She received two cycles of intensive chemotherapy (induction therapy with idarubicin and citarabine, and salvage therapy with high-dose cytarabine plus mitoxantrone) due to refractory leukaemia, entailing a consecutive 73-day period of profound neutropenia (<0.1neutrophils×109/L). On day +44 post-chemotherapy, an erythematous eruption, maculopapular and not pruriginous appeared on the back and forearms. At that time she was afebrile and under broad antibacterial and antifungal coverage: ceftazidime, vancomycin, levofloxacin, and caspofungin. However, in the following days the erythema became widespread, affecting all limbs, and turned to be very pruriginous. On day +53 she developed high fever and S. capitata was isolated in blood cultures obtained from peripheral blood and through the central venous catheter (CVC), while the skin lesions cultures were negative. The substitution of caspofungin for voriconazole, together with the catheter removal on day +55, and the recovery from neutropenia on day +59, achieved the clinical recovery of the patient and the disappearance of the rash and fever. Concurrently, the culture of the tip from the removed CVC was negative. The complete remission of AML was obtained. After long-term voriconazole therapy (34 days) the patient received a new treatment for consolidation and underwent allogeneic HSCT, with no reactivation of the infection by S. capitata.
Patient 2A 60-year-old woman was admitted in March 2009, for salvage therapy of an AML relapse. Seven months earlier, she had been diagnosed with a minimally differentiated AML (WHO classification) of intermediate risk.30,60 Since then, she had undergone three cycles of intensive chemotherapy which entailed over 84 days of profound neutropenia accompanied by fluconazole prophylaxis (200mg/d). Unfortunately 3 months later the disease relapsed. High-dose cytarabine and amsacrine were used as salvage therapy, neutropenia was induced during a 35-day additional period, and fluconazole was administered as prophylaxis. At day +1 post-treatment she started a prolonged febrile syndrome characterized by mucositis, abdominal pain and copious diarrhoea. The abdominal ultrasonography and whole-body CT scan on days +5 and +17 showed pancolitis affecting the terminal ileum and practically the entire colon. Administration of broad spectrum antibiotics did not eliminate the diarrhoea and fever, and on day +8 caspofungin replaced fluconazole. Granulocytic colony stimulating factors (G-CSF) were added. Coagulase-negative Staphylococcus (CNS) was identified in blood culture on days +1 and +7, and on day +19 S. capitata grew in a blood culture obtained through a CVC, and caspofungin was replaced by voriconazole. The CVC was removed but no microbial growth was obtained. The recovery from neutropenia (day +35) was accompanied by the slow recovery of the patient. On day +31, voriconazole had to be replaced by liposomal amphotericin B due to neurological toxicity, and was maintained until day +40. The patient recovered completely, but unfortunately died 5 months later as a consequence of her disease progression. No clinical or microbiological reactivation of S. capitata infection was suspected.
Patient 3A 74-year-old woman with Burkitt's lymphoma in advanced stage (Ann Arbor IVB) and meningeal infiltration was admitted in August 2009. She underwent induction treatment adjusted to patient's age and her poor general condition (ECOG 4) based on: rasburicase, prednisone, cyclophosphamide, methotrexate, ifosfamide, etoposide, dexamethasone, cytarabine, rituximab, and intrathecal treatment with liposomal cytarabine. From admission and throughout the whole period of profound neutropenia, she had fever and CNS was isolated in blood cultures of days −10, −6, −2 and −1 referred to the completion treatment date. Administration of antibiotics and CVC substitution rendered clinical stability. Cultures of the CVC tips removed on days −1 and +5 were negative. At day −3 she set out with cough, expectoration and a condensation area on the chest X-ray. Meropenem replaced piperacillin-tazobactam, and fluconazole administration was started but soon replaced (day 0) by caspofungin due to patient worsening. On the day +2 she had mucositis with oral ulcers and Candida albicans growth, and G-CSF was prescribed. She regained some clinical stability until day +9, when body temperature suddenly rose with the finding of faint pulmonary infiltrates and severe respiratory failure that ended with the death of the patient on day +10. Peripheral blood cultures from day +8 were positive for S. capitata, but results were received post-mortem. She had endured profound neutropenia for 12 days, and necropsy procedure was not performed.
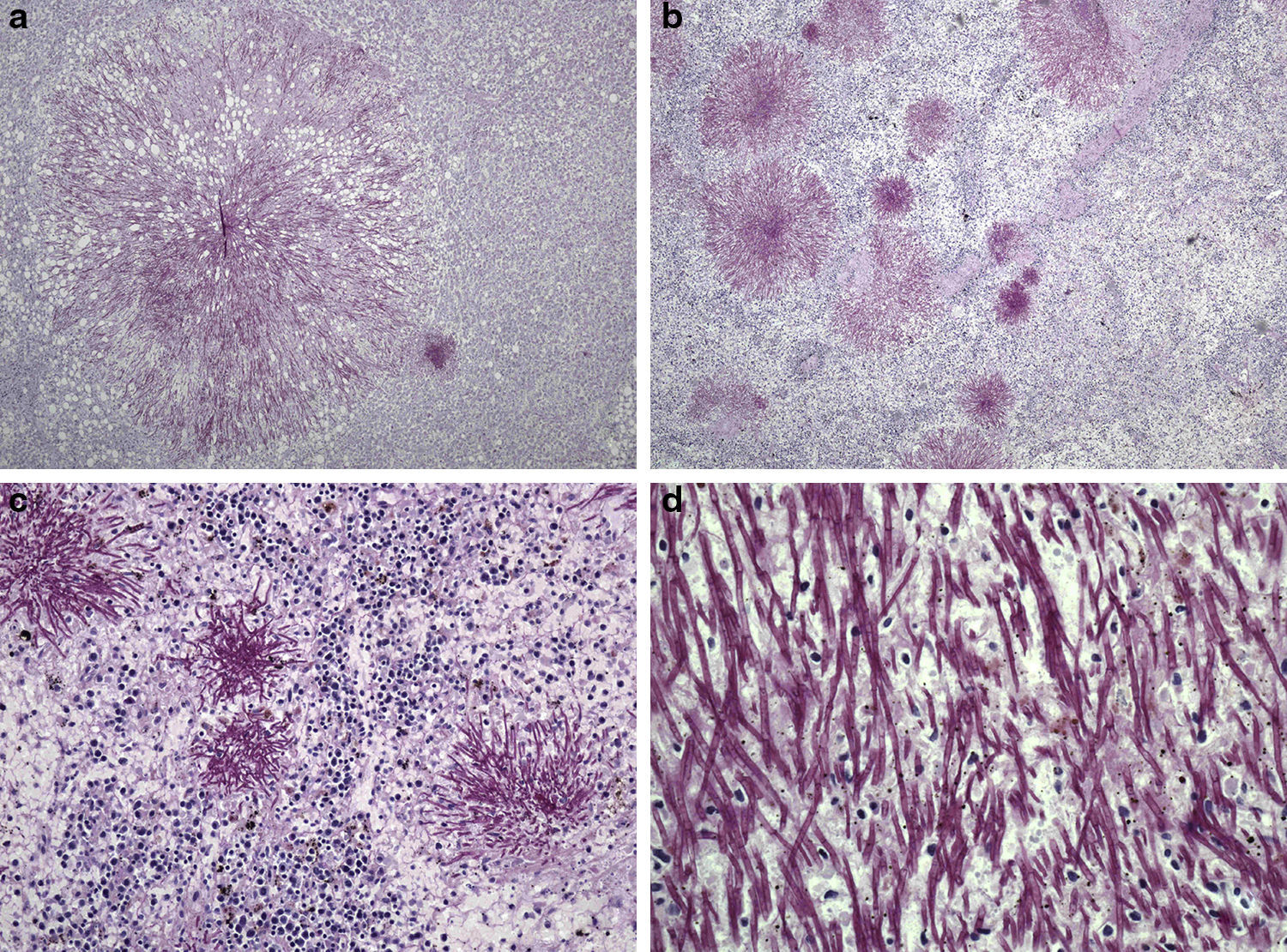
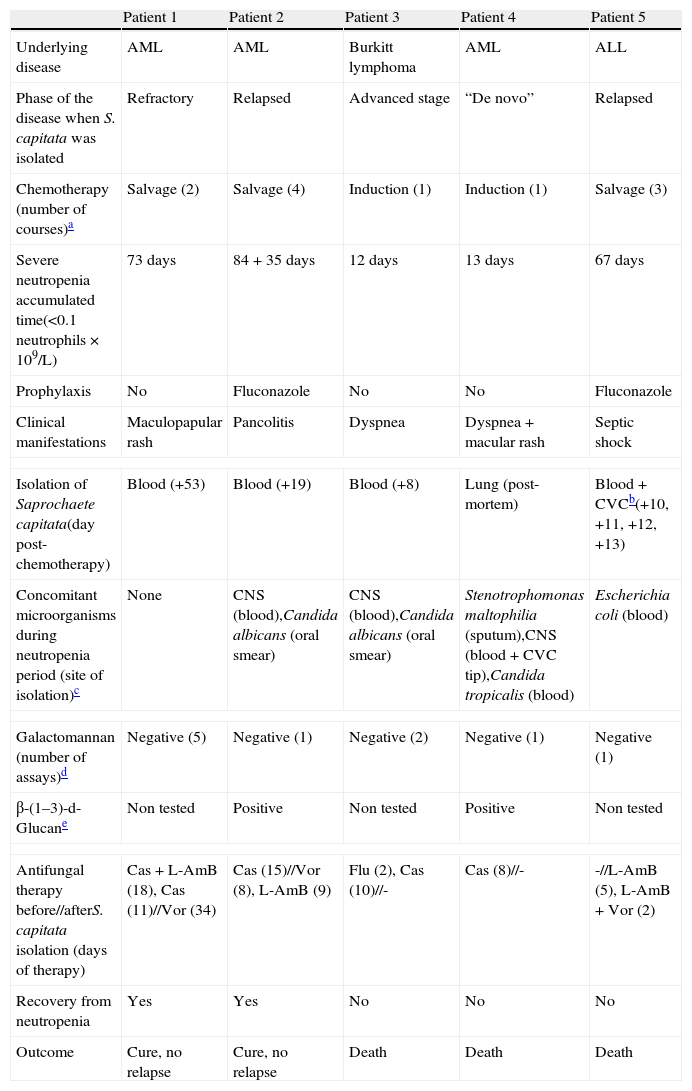
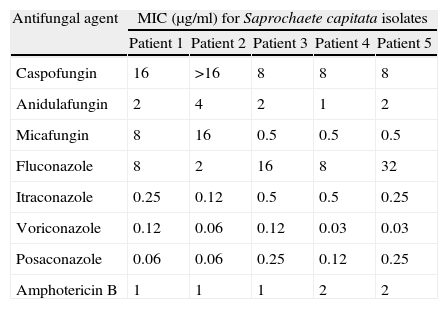
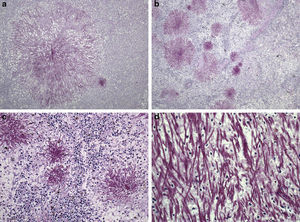
Patient 4A 66-year-old man was admitted to our unit from the intensive care unit (ICU) in November 2009. He had been admitted in the ICU due to severe respiratory failure, pulmonary infiltrates and bilateral pleural effusion that required non-invasive mechanical ventilation (bi-level positive airway pressure). He was diagnosed of acute monocytic leukaemia (WHO classification), and chemotherapeutical induction treatment was initiated with idarubicin, cytarabine and etoposide. The utilization of chemotherapy, supporting measures and broad spectrum antibiotics achieved certain degree of clinical stability that permitted the transfer of the patient to the haematology unit. At that time (day −4) he had fever and distal areas of cyanosis on the toes on both feet with biological data of disseminated intravascular coagulation. Continuous perfusion of sodium heparin was added, together with broad spectrum antibiotic coverage including meropenem, amikacin and teicoplanin. CNS was identified in blood cultures of days −3 and −2 as well as in the tip of the CVC that was removed and replaced on day +2. Since the fever persisted, caspofungin was added on day +2, but despite this, Candida tropicalis was isolated in blood cultures obtained from peripheral blood and through the CVC on days +4 and +7. However, the culture of the removed CVC tip was negative. At day +6 a generalized erythema appeared, and despite all the measures set out the patient died on day +10. The necropsy study revealed filamentous structures in all the studied parenchyma (liver, spleen, kidney, lung, intestine and bone) (Fig. 1), and S. capitata was isolated in the culture of a lung sample. A 13-day neutropenia period was registered.
Periodic-acid-Schiff stained sections of Saprochaete capitata infected organs from Patient 4. (a) Histological section of liver showing a colony of S. capitata (original magnification ×40); (b) histological section of spleen showing lymphoid depletion and abundant colonies of S. capitata mimicking “fireworks” (original magnification ×40); (c) histological section of spleen showing lymphoid depletion and abundant colonies of S. capitata (original magnification ×100); (d) large power field of fungal filaments of S. capitata in the spleen, showing occasional branching (original magnification ×400).
A 55-year-old man, diagnosed in August 2008 with precursor B cells lymphoblastic acute leukaemia (WHO classification), and high risk, was admitted to our unit in April 2010 for salvage treatment after relapse. He received two cycles of chemotherapy with mitoxantrone and cytarabine in high doses, supporting a 56-day period of profound neutropenia, and complete remission was obtained at the end of treatment. Fluconazole (200mg/d) was used as prophylaxis in both cycles. Due to persistence of residual leukaemia in the bone marrow, he underwent a third induction treatment (November 2010) with fludarabine, high dose cytarabine, idarubicin and G-CSF prior to the realization of an unrelated donor HSCT. He was carrying a subcutaneous reservoir (Port-a-Cath® type). During the period of profound neutropenia, he presented two episodes of fever. The first period started on day +5 post-treatment with blood culture isolation of Escherichia coli that responded favourably to piperacillin-tazobactam. At day +8 he experienced a new temperature rise and unproductive cough. The chest X-ray was normal and teicoplanin was added. In the presence of high fever on day +10, meropenem replaced piperacillin-tazobactam and liposomal amphotericin B (4mg/kg/d) was added. However the clinical state worsened quickly to a diminished level of consciousness and hemodynamic instability. Dopamine was started on day +11 and the patient was moved to the ICU. S. capitata was identified in blood cultures obtained through the reservoir on days +10 and +11, from peripheral blood on days +11 and +12, and in the culture of the reservoir membrane (150 CFU, Maki technique) and the tip (200 CFU) of the catheter removed on day +13. Voriconazole was added on day +13 but despite all these measures the patient died of a septic shock on day +15. Necropsy was not accomplished.
Identification and genotypic characterization of yeast isolatesFungal isolates were grown on corn-meal agar and identified morphologically by microscopy, while biochemical characterization was established with ID32C (bioMérieux, Marcy l’Etoile, France). All the isolates grew at 45°C and were unable to hydrolyze urea.
The ribosomal RNA genes internal transcribed spacer region (ITS1-5.8S-ITS2) of the five isolates were amplified with primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS 4 (5′-TCC TCC GCT TAT TGA TAT GC-3′).63 Purified amplicons (NucleoSpin kit, Macherey-Nagel, Duren) were sequenced (Applied Biosystems Genetic Analyzer 3130) and compared in the GenBank database, to confirm their identity as S. capitata.
DNA extracted from each of the five S. capitata isolates were processed by RAPD (Random Amplification of Polymorphic DNA) with four arbitrary sequence decamer primers (B01, B08, B11 and G10, from Operon Technologies Inc., CA, USA). These primers had been selected by Jain et al.36 because of their reproducible profiles among C. albicans isolates, therefore S. capitata DNA was amplified according to their protocol.
Antifungal susceptibilityIn vitro susceptibility testing of S. capitata isolates against the echinocandins caspofungin, anidulafungin and micafungin, the azoles fluconazole, itraconazole, voriconazole and posaconazole, and against amphotericin B (AMB), was conducted with the broth microdilution method, as described in the M27-A3 document of the Clinical and Laboratory Standards Institute (CLSI12). Clinical breakpoints values have recently been reported by Pfaller et al.,50 to define susceptibility to azoles and echinocandins for Candida spp.; however, no clinical breakpoints values are available for S. capitata. In addition, interpretive breakpoints for AMB have not been established12 but strains with MIC>1μg/ml are very likely to be resistant.46
Results and discussionSaprochaete capitata is an emerging fungal pathogen, especially in the Mediterranean area.28 Since early disseminated infections were described in the 1960s, new cases are being reported with increasing frequency.1,3,11,21–23,34,41,57,59 In our haematological unit, between the years 2004 and 2010, 5 cases of proven IFI by S. capitata were registered among 176 adult patients diagnosed of acute leukaemia or related diseases. The differential profiles of randomly amplified DNA bands helped to discard an epidemiologic relationship of the isolated strains. The S. capitata isolate of patient 3 showed the most differentiated bands patterns for all the assayed primers, while the rest of isolates exhibited differences for at least one of the assayed primers within the group. It is odd that the most similar strains belonged to patients 1 and 2, if we consider that their isolation dates were five years apart.
The incidence rate of S. capitata IFI for the whole group of patients was 2.84%, well above the 0.5% rate reported for an Italian multicenter retrospective study conducted on 3000 patients with acute leukaemia, between the years 1992 and 2000.28 As reproduced frequently in the literature, S. capitata infection was registered in patients with a very precarious clinical situation (patient 3) or in those undergoing salvage therapy as a consequence of the advanced state of their illness (patients 1, 2 and 5).1,11,20,34,41 These patients had accumulated long periods of profound neutropenia from previous chemotherapy cycles (at least 73d, 119d and 67d for patients 1, 2 and 5, respectively) that entailed complications such as bacterial infections, antibiotics administration and above all, repetitive handling of CVCs and previous treatment with antifungal agents not active against S. capitata. All these factors may have contributed to the development of the IFIs. In our group of patients, the higher mortality due to S. capitata when compared to that reported for Candida spp.28,40 is difficult to explain because patients with advanced haematological malignancies have inherent higher rates of mortality.
Three out of 5 patients (patients 1, 2 and 5) presented a clinical syndrome of S. capitata IFI indistinguishable from Candida IFI. Coinciding with the isolation of S. capitata in blood cultures, patient 1 presented a generalized very itchy maculopapular rash and patient 2 ileo-pancolitis with identification of C. albicans in the oral mucosa. Few cases such as patient 5 have been described, in which the participation of an endovascular device could be identified as the source of sepsis caused by S. capitata.28 The fungus was isolated from the tip of the catheter and from the membrane of the device, but even though the catheter was removed it was not possible to prevent the death of the patient. The ability of S. capitata and Candida parapsilosis to generate biofilms within the endovascular devices has recently been described.17 In contrast, patient 3 presented another typical manifestation of S. capitata IFI with lung involvement, as it has been frequently reported.39,40 This patient presented severe respiratory failure with faint pulmonary infiltrates which could easily be confused with a trichosporonosis or aspergillosis. Patient 4 had a mixed presentation including erythema without papula, blood culture isolation of C. tropicalis and alveolar infiltrates with respiratory failure. S. capitata was identified in every parenchyma studied, including lung, although the two fungi were likely to have contributed to his death (Fig. 1). All IFIs occurred during the period of profound neutropenia.
Contrarily to other authors,4,26 no positive data for Platelia Aspergillus EIA test were registered during the follow-up of patients, as reported by Schuermans et al.,59 By contrast, the β-glucan determination performed in patients 2 and 4, resulted positive throughout the S. capitata IFI monitoring, which is in agreement with a previous work of our group14 that pointed at β-glucan as a useful biomarker for this type of infections.
Following institutional protocols, patients 2 and 5 were under prophylactic fluconazole treatment, and patients 1, 2, 3 and 4 were receiving caspofungin as empiric treatment, when S. capitata IFI was diagnosed. As shown in Table 2, the MIC values of echinocandins and fluconazole were high for most S. capitata isolates, with the exception of patient 2 isolate, which showed fluconazole MIC 2 mg/ml. In addition, strains from patients 4 and 5 were likely to be resistant to AMB (MIC=2μg/ml46). On the contrary, all the S. capitata isolates showed very low MIC values for voriconazole and posaconazole. These breakthrough fungemia cases, which we found in four of our five patients, have been reported by other authors.39,40,59 In our opinion, the lack of susceptibility of S. capitata to fluconazole and/or echinocandins facilitated its dissemination, given that these antifungal compounds were administered to prevent or treat empirically infections by Candida spp. or Aspergillus spp., which are more prevalent than S. capitata infections in this group of patients.
In vitro antifungal susceptibility of Saprochaete capitata strains isolated from patients. Minimal inhibitory concentration values (MIC) were estimated following the microdilution method of the M27-A3 document of the CLSI.12
| Antifungal agent | MIC (μg/ml) for Saprochaete capitata isolates | ||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Caspofungin | 16 | >16 | 8 | 8 | 8 |
| Anidulafungin | 2 | 4 | 2 | 1 | 2 |
| Micafungin | 8 | 16 | 0.5 | 0.5 | 0.5 |
| Fluconazole | 8 | 2 | 16 | 8 | 32 |
| Itraconazole | 0.25 | 0.12 | 0.5 | 0.5 | 0.25 |
| Voriconazole | 0.12 | 0.06 | 0.12 | 0.03 | 0.03 |
| Posaconazole | 0.06 | 0.06 | 0.25 | 0.12 | 0.25 |
| Amphotericin B | 1 | 1 | 1 | 2 | 2 |
Patients 1, 2, 3 and 4 had received caspofungin treatment for 29, 15, 10 and 8 days, respectively, before the identification of S. capitata in blood cultures or necropsy, and patients 2 and 5 had received prophylactic fluconazole treatment for prolonged periods in the previous chemotherapy courses as well as when S. capitata was isolated. A similar situation has been previously described with other yeasts due to selective pressure exerted by azoles such as fluconazole that is commonly used in patients with haematological malignancies facilitating the emergence of less susceptible species such as Candida glabrata or Candida krusei.33 Lately, it has been suggested that recent exposure to fluconazole or caspofungin, both as prophylaxis and empirical treatment, leads with increasing frequency to the isolation of species less susceptible to these antifungals, such as C. parapsilosis, C. glabrata or C. krusei. These yeasts are capable of causing serious infections in patients at high risk, and therefore they are bringing about a major change in the frequency of the different species of Candida involved in candidemia.38
The emergence of S. capitata strains, which are non susceptible to caspofungin, makes this infection especially dangerous in those haematological units where this echinocandin is routinely used for the empirical treatment of febrile neutropenia, especially in those units where an increased prevalence of this organism has been registered. Moreover, the clinical resemblance with acute invasive candidiasis could make one believe that the infection is been treated adequately when, in fact, it is not. However, the literature reports two cases of S. capitata IFI that had a good clinical response to the combination of caspofungin and voriconazole,22,23 even though the first case showed high echinocandin MIC. In the surviving patients of the present study (patients 1 and 2), in addition to recovering from neutropenia and regaining clinical stability, caspofungin was replaced by voriconazole and liposomal amphotericin B once S. capitata was identified and susceptibility tests were performed. At present the treatment of this rare infection is not clearly defined but, in our experience, to perform of the antifungal susceptibility test of the isolated organism is highly recommended. If there is no microbial isolation and the patient presents with clinical symptoms compatible with disseminated candidiasis, trichosporonosis or aspergillosis that do not respond favourably to antifungal treatment, especially if covered with caspofungin and/or fluconazole, the use of voriconazole and liposomal amphotericin B is advised.
In our group of patients the four cases of fungemia by S. capitata accounted for 20% of total fungemia registered between 2004 and 2010, while other isolates were 7 C. parapsilosis, 4 C. tropicalis, 3 C. krusei, 1 Candida guilliermondii and 1 Fusarium solani. Interestingly, two more isolates of S. capitata were reported in our unit during this period. The first one was isolated in a stool culture of a patient who underwent an autologous HSCT for multiple myeloma one year earlier, and was adequately solved with voriconazole. By contrast, the second one was found in a urine culture of a patient with an advanced chronic lymphoid leukaemia, who died of multiorgan failure with a profound neutropenia. Necropsy was not accomplished. These cases were not analyzed in this study because they were not proven IFIs, however they should be taken into account if we are thinking of S. capitata as an emerging pathogen in our region.
In conclusion, in our institution, S. capitata is an emerging agent responsible for IFIs especially in acute leukaemia or advanced haematological malignancies. The limited extension of this series precludes the categorical conclusion that the IFIs caused by this fungus were facilitated by the previous antifungal treatment with fluconazole or caspofungin, but we found that S. capitata isolates showed low in vitro susceptibility to these antifungals, which in turn did not behave favourably in the clinical set. Therefore, in our opinion, the prophylactic and empirical use of fluconazole and/or caspofungin respectively is not advisable for the treatment of neutropenic fever in high-risk patients, particularly in institutions with high prevalence of this microorganism. From our experience, treatment with voriconazole and liposomal amphotericin B, and early removal of presumably infected CVCs are the most effective measures to control infections by S. capitata, although recovery from neutropenia and clinical improvement of patients are indispensable for success.
Conflict of interestThe authors declare that they have no conflict of interest.
This project has been financed with grants IT-264-07 from Department of Education, Universities and Research, Basque Government (to J.P and M.D.M.), and S-PC10UN05 and S-PC11UN011 from Department of Industry, Innovation, Trade and Tourism, Basque Government (to M.D.M.).
The present work is dedicated to Dr. Jose Pontón, a great friend and better researcher, who passed away on July 2010.