Onychomycosis is the most common nail disease and represents around 50% of nail disorders. Accurate diagnosis with adequate evidence is ideal before starting any treatment. Current diagnostic methods offer low specificity and sensitivity.
AimsTo create a new method for the diagnosis of onychomycosis, and to compare its sensitivity and specificity with the existing methods.
MethodsOne hundred and ninety-two samples with clinical suspicion of onychomycosis were included and underwent modified PAS stain (M-PAS), KOH/chlorazol black (KOH/CB) and culture testing. Sensitivity, specificity, positive and negative predictive values were calculated.
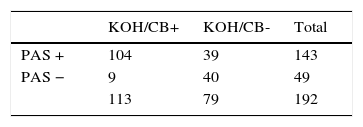
ResultsIn 152 out of 192 samples (79.2%) fungi structures were found in at least one of the three tests performed, and the patients were diagnosed with onychomycosis; 40 samples out of 192 (20.8%) were negative. Using M-PAS, filaments and/or spores were seen in 143 samples from the 152 positive (94%); 39 of them were negative to KOH/CB and positive to M-PAS (25.6%). With KOH/CB, filaments and/or spores were seen in 113 cases from the 152 positive samples (73.8% of the onychomycosis cases). Thirty-five cultures were positive, of which 77% were identified as Trichophyton rubrum; 117 onychomycosis cases were diagnosed despite the negative culture (76.9%). M-PAS showed 92.5% sensitivity and 55.55% specificity, a 67.5% positive predictive value and a 81.6% negative productive value.
ConclusionsThis procedure, a combination of the existing methods to diagnose onychomycosis, KOH/CB together with a nail clipping biopsy, proved to have high sensitivity, as well as being rapid, easy, inexpensive and readily available in most hospital settings. M-PAS allowed us to diagnose 39 cases (25.6% of the cases of onychomycosis) that were false negative using only KOH/CB and culture.
La onicomicosis es la enfermedad más común de las uñas y representa un 50% del total de las enfermedades que afectan a esta parte del cuerpo. Antes de iniciar un tratamiento, es muy recomendable contar con un diagnóstico preciso y pruebas suficientes. En la actualidad, los métodos diagnósticos ofrecen una sensibilidad y especificidad bajas.
ObjetivosCrear un nuevo método de diagnóstico de la onicomicosis y comparar su sensibilidad y especificidad con los métodos diagnósticos existentes.
MétodosSe recogieron ciento noventa y dos muestras con sospecha clínica de onicomicosis en las que se aplicaron las pruebas de examen directo con KOH/Negro de clorazol (KOH/CB), cultivo y examen directo teñido con PAS (M-PAS). Se calcularon la sensibilidad, la especificidad, y los valores predictivos positivo y negativo.
ResultadosEn 152 de las 192 muestras (79,2%) se hallaron estructuras micóticas en una de las tres pruebas realizadas como mínimo, y se diagnosticó onicomicosis en dichos pacientes; 40 de las 192 muestras (20,8%) dieron resultados negativos. Mediante M-PAS, se observaron filamentos o esporas en 143 de las 152 muestras (94%); 39 de ellas resultaron negativas con KOH/CB y positivas con M-PAS (25,6%). En el caso de KOH/CB, se observaron filamentos o esporas en 113 de las 152 muestras, (73,8% de los casos de onicomicosis). Treinta y cinco cultivos dieron resultados positivos, conel 77% de los aislamientos obtenidos identificados como Trichophyton rubrum; se diagnosticaron 117 casos de onicomicosis a pesar de los resultados negativos en el cultivo (76,9%). La sensibilidad de M-PAS fue del 92,5%, la especificidad del 55,55%, y los valores predictivos positivo y negativo de 67,5% y 81,6%, respectivamente.
ConclusionesEste procedimiento, una fusión de métodos ya existentes para el diagnóstico de la onicomicosis, que aplica KOH/CB junto con una biopsia de fragmentos de uña, mostró una gran sensibilidad. Es además un método rápido, fácil, económico y disponible en la mayoría de los ámbitos hospitalarios. M-PAS permitió diagnosticar 39 casos (25,6% de los pacientes con onicomicosis) con resultados falsos negativos al utilizar únicamente KOH/CB y cultivo.
Onychomycosis accounts for 50% of all nail diseases.1,14 It affects 2–13% of the general population, and this percentage increases with age, reaching up to 40% in the elderly.4,7 The main differential diagnosis is with psoriasis, lichen planus and traumatic onychodystrophy.1,2,9,11
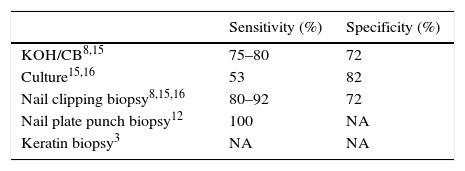
KOH/CB is the most used diagnostic tool because it is fast, non-expensive, and non-invasive. The sample is obtained by scraping the subungueal hyperkeratotic area of the affected nail, placed on a slide with KOH/CB and then observed with light microscopy (10× and 40×).6,14
The rest of the scales are placed in a Sabouraud with cycloheximide medium for culture. This procedure is considered the gold standard for species identification.5,15,16 The most widely used diagnostic methods are summarized in Table 1.
Other available diagnostic methods are biopsies in different variants such as nail clipping, nail plate punch or keratin biopsy, where the collected samples are processed in the same way as skin samples, and stained with PAS. These are excellent methods but not easy to perform.3
Diagnostic accuracy is important because oral antimycotic treatment is lengthy, expensive and significant drug interactions can occur. The currently employed methods do not offer enough specificity and sensitivity for the diagnosis.7,15 The objective of this study was to create a new method for the diagnosis of onychomycosis, and compare its sensitivity and specificity with those of the current methods.
Materials and methodsWe designed a new diagnostic procedure that consists of taking a sample obtained by scraping the subungueal hyperkeratotic area of the affected nail, fixating it on a slide with nitrocellulose–toluene–formaldehyde (transparent nail polish), and then staining the sample with PAS (M-PAS) in the histology department. PAS stain was performed in the usual manner: the slides were oxidized in 0.5% periodic acid solution for 5min, rinsed in distilled water, placed in Schiff reagent for 15min, washed in lukewarm tap water for 5min, counterstained in hematoxylin for 1min, washed in tap water for 5min, and finally coverslipped using a synthetic mounting medium.
These slides were observed in light microscopy at 10×, 20× and 100× by an experienced mycologist that was blinded to the results obtained in the routine tests, KOH/CB and culture.
We considered as inclusion criteria samples of patients that had the enough material for the three studies (M-PAS, KOH/CB, and culture), with clinical suspicion of onychomycosis that were referred to the mycology section of our hospital for a routine workup for onychomycosis diagnosis.
The exclusion criteria included those patients that had received topical or systemic treatment 1 or 3 months before taking the sample, respectively, and those with other ungueal diagnoses such as lichen planus, psoriasis, or traumatic dystrophy.
The elimination criteria were the loss of a sample during any of the proceedings involved.
Every culture was performed on BBL Mycosel Agar (Becton Dickinson and Company, Sparks, MD, USA), and incubated up to 4 weeks. If a colony developed, the macroscopic and microscopic characteristics were analyzed to determine the fungal species. We considered a positive result when fungal structures were identified.
The KOH/CB test was accomplished in the usual manner. This test was considered positive when fungal structures such as hyphae or spores were visualized.
The M-PAS stain exam was performed as follows: (i) the slide was properly marked with an identification number; (ii) nitrocellulose–toluene–formaldehyde was applied to the slide, in a enough amount for the scale to adhere; (iii) part of the subungueal hyperkeratotic sample was placed on top of the nitrocellulose–toluene–formaldehyde; (iv) after the sample had dried (about 5min) it was sent to the histopathology department for PAS staining; (v) the sample was considered positive if hyphae or spores were observed.
For the evaluation of the sensitivity and specificity, we considered culture together with KOH/CB as the gold standard for comparison, based on the fact that these two methods are sensitive and specific when performed in combination and evaluated by trained personnel. Moreover, these are the two methods we use as routine diagnostic tests for onychomycosis.
The diagnosis of onychomycosis was made when one or more of the three diagnostic tests were positive.
ResultsWe included 192 samples of patients with clinical suspicion of onychomycosis. All of them underwent the three diagnostic methods. One hundred and fifty-two (79.2%) were positive in at least one of the tests.
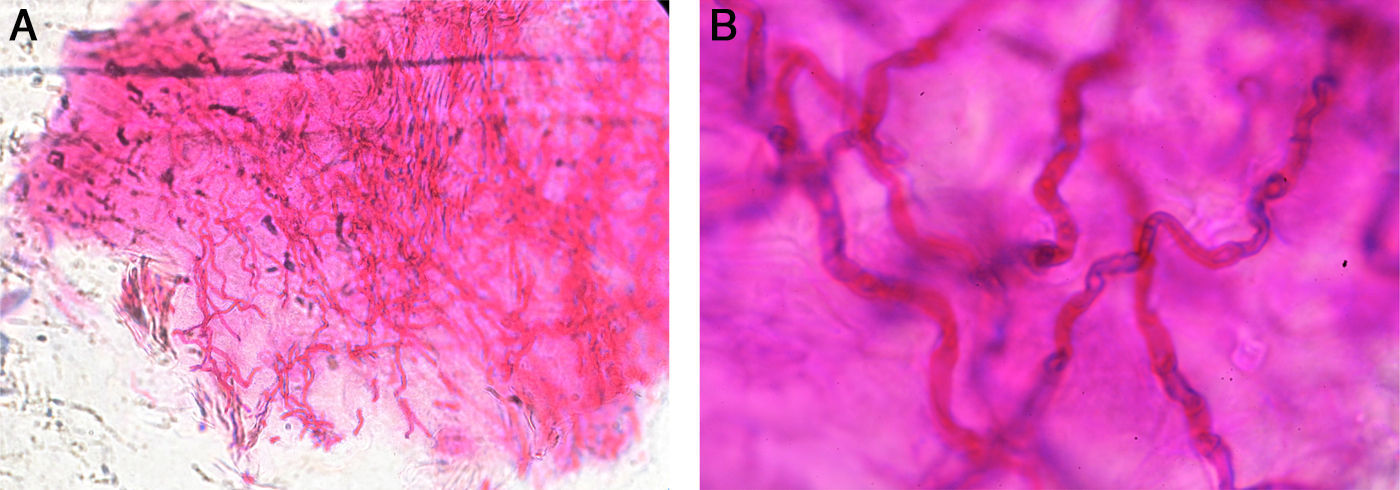
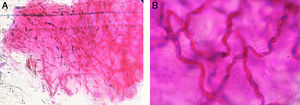
In the slides with M-PAS, fungal structures were shown in 143 samples out of the 152 that were positive (94%) (Fig. 1). Nine samples were false negative with the PAS stain (5.9%), but resulted positive with KOH/CB. In the slides studied with KOH/CB we found 113 positive samples out of 152 (73.8%). Thirty-nine false negative samples with KOH/CB (25.6%) were exclusively positive with the PAS stain (Table 2). Sensitivity and specificity were estimated in 92.5% and 55.55%, respectively. We obtained 35 positive cultures from the 152 positive cases (23%). Trichophyton rubrum was the etiologic agent in 77% of the isolates (Table 3).
In the pursuit of simplifying the diagnosis we created a new procedure that is a combination of the existing techniques for the observation of fungal structures. This procedure proved to have a high sensitivity and feasibility, as well as low cost. The good cost–benefit of this diagnostic test is confirmed with the fact that the approximate cost of this diagnostic method is less than 1 US dollar.
We found that M-PAS has a sensitivity of 92.5% and a specificity of 55.55%, whereas KOH/CB has 75% sensitivity and 72% specificity.15 The low specificity of our procedure is due to the false negative results in the KOH/CB group.
This technique allowed us to make the diagnosis in 39 cases (25.6%) that were false negative with the routine diagnostic tests. Therefore, we consider that these findings are of great benefit for those patients and represent a new feasible option for routine diagnosis.
Onychomycosis is a common condition in the medical practice1 and although the clinical picture can be very suggestive of the diagnosis, this should be confirmed by any of the routine methods before starting any treatment, particularly if it will be systemic, since antimycotic drugs can have adverse effects, possible drug interactions and are expensive.12 The routine methods used in our hospital for the diagnosis of onychomycosis are KOH/CB, that has a reported sensitivity between 75 and 80%,8,15 and culture, that has a low sensitivity for the identification of dermatophytes, and usually have false negative results in up to 60% of the cases.13,14 The high frequency of false negative results made us conceive a new diagnostic procedure that would be easy, fast, low-cost and with high sensitivity. Multiple studies report that when the routine methods are negative but the clinical picture is suggestive, a histological analysis either by a punch biopsy or nail-clipping specimens stained with PAS are indicated because this is a reliable method with a high reported sensitivity 80.8–92%8,15 However, it is technically demanding, invasive and can have morbidity if performed by inexperienced personnel, is time consuming and more expensive than the routine tests.10
The keratin biopsy described by Chang et al. is a technique in which the subungueal hyperkeratosis is analyzed in the same way a skin biopsy is, and better results are reported (97% of positivity) when compared with a PAS stained nail clipping biopsy.3,9,10,13
The limitations for our diagnostic procedure are that this method requires a histopathology department, a qualified histology technician, and a trained observer. The quality of the sample will also determine the sensitivity and specificity of the test.
In conclusion, our recommendation is to perform this test only when the routine methods are negative and the clinical picture is highly suggestive.
Financial disclosureThis work was funded by the first place award of the Foundation La Roche Posay Latin-America for researcher dermatologists.
Conflict of interestAll authors agree that no conflict of interest exists for the publication of this paper.
The authors would like to thank La Roche Posay Latin-America for their support.