Vulvovaginal candidiasis is a common infection among women worldwide, being Candida albicans the most commonly isolated species. Therefore, controlling this opportunistic yeast is one of the key factors for reducing nosocomial infection.
AimsWe investigated several virulence properties of 28 vaginal strains of Candida isolated from Tunisian women suffering from vulvovaginitis. We also analyzed the virulence properties of a clinical Candida krusei strain and five Candida reference strains.
MethodsCandida strains were subjected to microscopic analysis and culture in Candida ID2 chromogenic medium. The adhesive properties of these strains were estimated by the microtiter plate – the safranin-staining – and the Congo red agar (CRA) methods, for determining yeast ability to form biofilms on biomaterials used in urinary catheter manufacturing. Their potency to produce hydrolytic enzymes was also studied.
ResultsOur results showed that nine out of the total studied strains produced phospholipase. In addition, very high protease activity was detected in 23 Candida strains. All Candida strains were beta-hemolytic and adhered to polystyrene microtiter plates in varying degrees. Two vaginal C. albicans strains were strongly adhesive to polystyrene and glass slides. Also, our results showed that vaginal Candida strains were more adhesive to the three tested materials than the reference strains.
ConclusionsThis study shows the presence of a range of virulence and adhesion factors in clinical isolates of vaginal Candida. Consequently, control and treatment of vaginal candidiasis as a means to prevent biofilm formation on urinary catheters is of crucial importance.
La candidiasis vulvovaginal es una infección habitual entre las mujeres de todo el mundo. Candida albicans es la especie aislada más común, por lo que su control es un factor fundamental para reducir la infección nosocomial.
ObjetivosEl objetivo del presente estudio fue investigar algunas propiedades concernientes a la virulencia de 28 cepas vaginales clínicas del género Candida, procedentes de mujeres tunecinas afectadas de vulvovaginitis. También se estudiaron las propiedades de virulencia de una cepa clínica de Candida krusei y cinco cepas de referencia de Candida.
MétodosLas distintas cepas de Candida se analizaron mediante microscopía y cultivo en el medio cromogénico Candida ID2. Se estudiaron las propiedades de edhesión de las distintas cepas empleando placas de microtitulación. La capacidad de formar biopelículas sobre el material empleado en los catéteres urinarios se examinó mediante los métodos de tinción con safranina y en agar con rojo Congo. Las actividades proteasa y fosfolipasa también fueron determinadas.
ResultadosDe las cepas analizadas, 9 presentaron actividad fosfolipasa y en 23 cepas se detectó una alta actividad proteasa. Todas las cepas ensayadas presentaron actividad β-hemolítica y diferentes grados de adherencia a las placas de microtitulación. De las cepas de C. albicans, dos de los aislamientos vaginales mostraron una elevada adherencia al poliestireno y al vidrio. En general, los aislados vaginales mostraron una mayor adherencia que las cepas de control empleadas.
ConclusionesEn este estudio se demuestra la presencia de factores de virulencia y adherencia en aislamientos clínicos vaginales de Candida. El control y el tratamiento de las candidiasis vaginales como medio para prevenir la formación de biopelículas de origen candidiásico en catéteres urinarios es de gran importancia.
Vulvovaginal candidiasis (VVC) is a yeast infection that affects a large proportion of women. Almost 75% of women experience at least one episode of VVC at some point during their lifetime and 5% experience recurrent VVC.44 Recurrent vulvovaginal candidiasis remains a common problem affecting women's well-being. Previous epidemiological studies have shown Candida albicans to be the most commonly isolated species in women with vulvovaginal candidiasis followed by Candida glabrata, Candida krusei, and Candida parapsilosis.2,44
The development of Candida vaginitis involves a balance breakdown between aggressive fungal traits and host active immune surveillance that allows Candida growth and mucosal colonization.2
Several virulence factors contribute to the pathogenicity of Candida spp., including the ability to adhere to epithelial cells and medical biomaterial, production of hydrolytic enzymes such as extracellular proteinases and phospholipases, and production of haemolytic factor.17 In fact, the secretion of specific hydrolytic enzymes by Candida has been suggested as a possible virulence factor.30 Phospholipases are hydrolytic enzymes that attack phospholipids, common to all cell membranes. In addition, some authors have also demonstrated that lipases could aid microorganism growth in environments with lipids as the sole carbon source.45 In turn, secretory aspartyl proteinases (Sap) constitute a family of enzymes that are able to degrade several physiologically important substrates such as albumin, immunoglobulins and skin proteins.7 Also, haemolysins are known to be putative virulence factors facilitating hyphal invasion in disseminated candidiasis.
The major complication associated with the use of medical implants such as intravascular catheters, urinary catheters or joint prostheses is infection. Microorganisms can colonize these devices and form biofilms consisting of layers of cells embedded within a matrix material.18
An essential and necessary first step in successful colonization, pathogenesis development and infection is the adherence of C. albicans to host cells or biomaterials such as polyvinyl chloride or polyethylene.6,19 Therefore, determining biofilm production in Candida spp. may be important for the management of invasive infections.47 In fact, various methods are used in routine laboratories for the detection of biofilm production, such as the tissue culture plate, the tube method or the Congo-red agar assay.35
The purpose of the current study was to investigate a range of virulence properties of 28 Candida strains isolated from Tunisian women suffering from vulvovaginitis, such as their ability to form biofilms on biomaterials used in urinary catheter manufacturing, as well as their potency to produce hydrolytic enzymes.
Materials and methodsPatientsThis study was conducted following ethical approval. The subjects of the study were twenty six patients (women) from the region of Sousse (Centre of Tunisia) suffering from different vaginal infections and admitted to a range of services (Pediatric, Gynecology, Family Planning, Neonatal, Medical Intensive Care and Maternity) of the Farhat Hached University Hospital Complex (Sousse) (Table 1).
Clinical characteristics of patients.
| Patient | Strain | Service | Body site |
|---|---|---|---|
| 1 | 1 | Pediatric | Urine culture |
| 2 | 2 | Gynecology | Vagina |
| 3 | 3+3′a | Family Planning | Vagina |
| 4 | 4 | Family Planning | Urine culture |
| 5 | 5 | Gynecology | Vagina |
| 6 | 6 | Gynecology | Vagina |
| 7 | 7 | Gynecology | Vagina |
| 8 | 8 | Maternity | Vagina |
| 9 | 9 | Family Planning | Vagina |
| 10 | 10 | Maternity | Vagina |
| 11 | 11 | Gynecology | Vagina |
| 12 | 12 | Gynecology | Vagina |
| 13 | 13 | Maternity | Vagina |
| 14 | 14+14′a | Maternity | Vagina |
| 15 | 15+15′a | Gynecology | Vagina |
| 16 | 16 | Maternity | Vagina |
| 17 | 17 | Family Planning | Vagina |
| 18 | 18 | Neonatal | Urine culture |
| 19 | 19 | Medical Intensive Care | Vagina |
| 20 | 20 | Gynecology | Vagina |
| 21 | 21 | Family Planning | Vagina |
| 22 | 22 | Gynecology | Vagina |
| 23 | 23 | Maternity | Vagina |
| 24 | 24 | Gynecology | Vagina |
| 25 | 25 | Family Planning | Vagina |
A total of 28 vaginal Candida isolates were used in the present study. Samples were collected from the vaginal cavity with a sterile cotton swab (Nippon Menbo, Tokyo, Japan), which was immediately cultured on Sabouraud Chloramphenicol agar and on Candida ID2 chromogenic medium for 24–48h at 35°C.
A C. krusei strain (code Roma) from Italy, and five type strains, C. albicans SC 5314, C. albicans ATCC 90028, C. glabrata ATCC 90030, C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as positive and negative controls for the tests in this study (Table 2).
Distribution of hydrolytic enzymes in Candida strains.
| Strain | Species | Phospholipase | Proteinase | Hemolytic factor | |||
|---|---|---|---|---|---|---|---|
| A | PZ±DS | A | PZ±DS | A | HI | ||
| C. tropicalis | |||||||
| 1 | C. tropicalis | – | 1±0 | – | 1±0 | β | 2.38±0.57 |
| C. krusei | |||||||
| 3′ | C. krusei | – | 1±0 | +++ | 0.26±1 | β | 2.11±0.57 |
| ATCC 6258 | C. krusei | – | 1±0 | +++ | 0.41±1 | β | 2.64±1.04 |
| Roma | C. krusei | – | 1±0 | +++ | 0.2±0 | β | 1.83±0 |
| C. albicans | |||||||
| 3 | C. albicans | – | 1±0 | +++ | 0.23±1 | β | 1.55±0.57 |
| 4 | C. albicans | ++ | 0.36±0.57 | +++ | 0.28±0.57 | γ | 1±0 |
| 6 | C. albicans | ++ | 0.6±0 | +++ | 0.26±1.15 | β | 1.83±0 |
| 7 | C. albicans | ++ | 0.4±0 | +++ | 0.22±1 | γ | 1±0 |
| 10 | C. albicans | – | 1±0 | – | 1±0 | α | 2.38±0.57 |
| 14 | C. albicans | – | 1±0 | +++ | 0.26±0 | γ | 1±0 |
| 15 | C. albicans | ++ | 0.36±1.15 | – | 1±0 | γ | 1±0 |
| 16 | C. albicans | – | 1±0 | +++ | 0.18±0 | γ | 1±0 |
| 18 | C. albicans | ++ | 0.48±0.57 | – | 1±0 | γ | 1±0 |
| 19 | C. albicans | – | 1±0 | +++ | 0.21±0.57 | β | 2.33±1 |
| 20 | C. albicans | – | 1±0 | – | 1±0 | γ | 1.055±0.57 |
| 22 | C. albicans | – | 1±0 | – | 1±0 | γ | 1±0 |
| 23 | C. albicans | – | 1±0 | – | 1±0 | γ | 1±0 |
| 24 | C. albicans | ++ | 0.54±1 | +++ | 0.12±0 | β | 3.055±0.57 |
| SC5314 | C. albicans | – | 1±0 | +++ | 0.18±0.15 | β | 1.55±0.57 |
| ATCC 90028 | C. albicans | – | 1±0 | +++ | 0.26±0.57 | β | 1.88±0.57 |
| C. glabrata | |||||||
| 2 | C. glabrata | – | 1±0 | – | 1±0 | β | 2.38±0.57 |
| 5 | C. glabrata | – | 1±0 | – | 1±0 | α | 2.22±1.15 |
| 8 | C. glabrata | ++ | 0.41±0.57 | – | 1±0 | β | 1.33±0 |
| 9 | C. glabrata | – | 1±0 | +++ | 0.17±0.57 | β | 1.66±0 |
| 11 | C. glabrata | – | 1±0 | +++ | 0.22±1 | β | 4.88±0.57 |
| 12 | C. glabrata | – | 1±0 | – | 1±0 | β | 2.055±0.57 |
| 13 | C. glabrata | – | 1±0 | +++ | 0.21±1.15 | β | 2.88±1.15 |
| 14′ | C. glabrata | – | 1±0 | +++ | 0.24±0 | β | 2.72±0.57 |
| 15′ | C. glabrata | – | 1±0 | +++ | 0.4±0 | β | 2.11±1.15 |
| 17 | C. glabrata | ++ | 0.53±0.57 | +++ | 0.2±0.57 | β | 1.5±0 |
| 21 | C. glabrata | ++ | 0.41±0.57 | +++ | 0.18±1.52 | γ | 1±0 |
| 25 | C. glabrata | – | 1±0 | +++ | 0.28±1.52 | β | 1.88±0.57 |
| ATCC 90030 | C. glabrata | – | 1±0 | +++ | 0.28±1.15 | α | 2.77±1.15 |
| C. parapsilosis | |||||||
| ATCC 22019 | C. parapsilosis | – | 1±0 | +++ | 0.2±1.15 | β | 1.5±0 |
A: Activity; Pz=Diameter of the colony/(colony diameter+light area); SD: Standard Deviation; IH: Hemolytic Index; –: no activity; ++ and +++: very high activity.
The genomic confirmation at species level of all C. albicans and C. glabrata strains tested was done according to the protocol described by Noumi et al.33 by amplification of a RPS0 gene fragment. The sequences of synthetic oligonucleotides used as primers were INT1 (5′-AAGTATTTGGGAGAAGGGAAAGGG-3′) and INT2 (5′-AAAATGGGCATTAAGGAAAAGAGC-3′) for C. albicans,5 and CG1 (5′-ACATATGTTTGCTGAAAAGGC-3′) and CG2 (5′-ACTTTTTCTTAGTGTTCAGGACTTC-3′) for C. glabrata. The amplification was performed in an automated thermocycler in a final volume of 25μl containing 2.5μl of 10× buffer, 1μl of 50mM of MgCl2, 2.5U of Eco Taq polymerase (Ecogen), 2.5μl of dNTPs (2.5mM each; Sigma, St. Louis, MO, USA), and optimum concentrations of each primer (4μM). One μl of DNA suspension (30–50ng) was amplified in a PCR thermal cycler (PTC-150 MinicyclerTM) by using 1 cycle at 95°C for 5min and then 35 cycles as follows: 30s of denaturation at 95°C, 30s of annealing at 56°C, and 90s of primer extension at 72°C. At the final cycle, 10min of incubation at 72°C were added to complete polymerization. The resultant fragments of amplified DNA were analyzed by electrophoresis through 1% agarose gels, run in Tris–acetate–EDTA buffer (TAE) for 1h at 90V. A 100-bp ladder (Fermentas) was used as a size marker. Gels were stained in a solution of ethidium bromide (10μg/ml) and photographed with a Gel printer plus and a Scion Image analysis software (TDI. S.A. Madrid, Spain).
Cell surface hydrophobicity (CSH)The hydrophobicity of Candida strains was measured according to the protocol described by Rosenberg et al.40 which consists in measuring the adherence of yeasts to hydrocarbons, such as cyclohexane.
The tested strains were grown overnight in 5ml of yeast peptone dextrose (YPD, 10g yeast extract, 10g peptone, 10g of glucose in 1000ml of distilled water) at 28°C. Cells were washed with phosphate buffered saline (PBS) and concentrated to obtain a solution corresponding to OD600=1. For adhesion assays, 3ml of the cell suspension were mixed with 150μl of cyclohexane in an acid-washed glass tube. The sample was vigorously mixed using a vortex for 1min. After 20min at room temperature, aqueous phase absorbance at 600nm (A1) was measured and compared with that obtained prior to the mixing procedure (A0). The percentage of cells in the cyclohexane layer (adhered cells) was used to estimate the hydrophobicity using the following formula: Percent cell adhesion=(A1/A0)×100.
Myceliation in Lee mediumThe test consisted in comparing the ability of various strains of C.albicans to form mycelia. For this purpose, cells were grown in a small volume (15ml) of Lee medium, until they reached the end of the exponential phase. Cells were harvested by centrifugation at 2000×g for 5min and washed twice with sterile water for medium removal. The cell pellet was then resuspended in sterile water to an OD600=0.6 and incubated for 2h at 28°C to stop cell cycle. Cells were then kept at 4°C for 48–72h in order to induce a metabolic starvation. Cells were next recovered by centrifugation at 2000×g for 5min and resuspended in pre-warmed Lee medium to an OD600nm=0.3. Cells were then incubated at 28°C, and the morphology transition was followed hourly by phase contrast microscopy.32
Qualitative detection of exopolysaccharide productionSafranin methodSlime production was determined using the safranin method as described by Christensen et al.9 for coagulase negative staphylococci and modified by Davenport et al.13 Briefly, a loopful of microorganisms from the surface of a Sabouraud dextrose agar plate was inoculated into a tube containing 10ml of Sabouraud broth supplemented with glucose (8% final concentration) The tubes were incubated at 35°C for 24h and examined for the presence of a viscid slime layer. Slime production by each isolate was scored as negative, weak (+), moderate (++), or strong (+++). Each isolate was tested at least three times and read independently by two different observers.
Detection of slime production by the Congo Red Agar (CRA) methodThe slime-producing ability of Candida strains was tested according to the protocol described by Dag et al.12 All yeasts were cultured on Congo red agar plates prepared by adding 0.8g of Congo red (Sigma) and 36g of glucose to 1L of brain heart infusion agar. The Congo red stain was prepared as a concentrated aqueous solution and autoclaved separately at 115°C for 10min, and was added when the agar had been cooled down to 55°C. Plates were incubated at 37°C for 24h under aerobic conditions and subsequently overnight at room temperature. After incubation, pigmented colonies (generally black in color) were considered as slime positive, whereas unpigmented colonies (white, pinkish-red, smooth colonies with a darkening at the center) were interpreted as slime-negative strains.43
Enzymatic characterizationProduction of phospholipase, proteinase and haemolysins by Candida strains was determined as described by Price,36 Aoki et al.3 and Manns,26 respectively.
Phospholipase assayCultures from maintenance media were transferred onto Sabouraud Chloramphenicol agar plates and incubated at 37°C for 48h for enzymatic tests. Strains were transferred to flasks containing 5ml of YPD medium. The flasks were incubated at 37°C for 18h. Following incubation, 1.5ml of the yeast culture was transferred to an Eppendorf tube and centrifuged at 2000×g, for 5min. The pellets obtained were washed twice by resuspension in PBS, and centrifuged under the same conditions to remove residual culture medium. The suspensions were adjusted at 5 McFarland with a densitometer (bioMérieux, France), and 1μl was then plated in duplicate, at equidistant points, in phospholipase agar medium (10g of peptone, 30g of glucose, 57.3g of NaCl, 0.55g of CaCl2, 100ml of egg yolk emulsion and 900ml of distilled water). The inoculated plates were incubated at 37°C for 4 days.
The presence of phospholipase was determined by the formation of an opaque zone around the yeast colonies. Enzyme activity (Pz) was measured by dividing the diameter of the colony by the diameter of the colony plus the precipitation zone. We distinguished three types of phospholipase activity: Pz=1 meant that the isolate was phospholipase negative, Pz>0.63 meant that the strain was phospholipase positive and when Pz<0.63, the phospholipase activity was very strong.36
Proteinase assayDetermination of proteinase production was performed according to the method described by Aoki et al.3 The test medium consisted of agar plates containing BSA (Sigma Chem Co., St. Louis, Mo., USA). Briefly, a solution containing 0.04g MgSO4·7H2O, 0.5g K2HPO4, 1g NaCl, 0.2g yeast extract, 4g glucose and 0.5g BSA was prepared in a volume of 60ml. The pH was adjusted to 3.5. The solution was sterilized by filtration and then mixed with 15g of agar. The medium (20ml) was poured into Petri dishes. Each strain was inoculated in triplicate, and the plates were incubated at 37°C for 7 days. The presence of proteinase was determined by the formation of a transparent halo around the yeast colonies.
Proteinase activity was measured according to the method described by Price et al.36 calculating the ratio between the diameter of the colony and that of the colony plus the precipitation zone. The Pz coefficients of the Candida strains analyzed were grouped into 4 classes: Pz between 0.9 and 1, with very low proteinase activity; 0.89–0.80,low proteinase activity; 0.79–0.70, high protinase activity, and Pz<0.69, very high proteinase activity.3
Production of haemolytic factorHaemolysin production was evaluated using the method described by Manns et al.26 with modifications. A loopful of the stock culture was streaked onto Sabouraud Chloramphenicol agar and incubated at 37°C for 24h. Cells were harvested and washed with sterile PBS. Ten microlitres of yeast suspension (108cells/ml) was spotted onto a sugar enriched sheep blood agar medium prepared by adding 7ml of fresh sheep blood to 100ml of Sabouraud Chloramphenicol agar supplemented with 3% glucose (w/v).26 The final pH of the medium was adjusted at 5.6±0.2. The plates were incubated at 37°C in 5% CO2 for 48h. The presence of a distinct translucent halo around the inoculum site, viewed with transmitted light, indicated positive haemolytic activity. The diameter of the lysis zone and that of the colony were measured, and the ratio resulting from dividing the first by the second (equal to or larger than 1) was used as a haemolytic index to represent the intensity of the haemolysin production by different Candida species. The assay was conducted in triplicate on two separate occasions for each isolate.
Adhesion assay on polystyreneGrowth conditionsAll Candida strains were cultured at 37°C for 18h in Sabouraud Chloramphenicol agar. A loopful of yeasts was then inoculated in Yeast Nitrogen base medium (Sigma–Aldrich, St. Louis, Mo., USA) supplemented with 100mM glucose. After overnight culture, yeasts were harvested in exponential growth phase, washed twice with 5ml of PBS, the optical density of the suspension was adjusted to 0.38 at 520nm (107cells/ml), and the cell suspension was used immediately. Previous works have demonstrated that optimal biofilm formation occurs at this particular density.25
Biofilm formationBiofilms were produced on commercially available presterilized polystyrene flat-bottom 96-well microtiter plates (Iwaki, Tokyo, Japan).25 One hundred microlitres of a standardized cell suspension (107cells/ml) was transferred to each well of a microtiter plate, and the plate was incubated for 1.5h at 37°C in a rotary shaker (SI-600, Lab. Companion) at 75rpm to allow yeasts to adhere to the well surfaces.25 As negative controls, three wells of each plate were handled in an identical way, except that no Candida suspensions were added.
Following the adhesion phase, cells suspensions were aspirated, and each well was washed twice with 150μl of PBS to remove loosely adherent cells. A total of 100μl of yeast nitrogen base medium was transferred into each washed well with a pipette, and the plates were incubated at 37°C in a shaker at 75rpm. Biofilms were allowed to develop for up to 66h, and then yeasts were quantified by the XTT (Sigma–Aldrich, Inc., Germany) reduction assay.27 The medium was replenished daily by the aspiration of the spent medium and the addition of fresh medium. All assays were carried out on three different occasions.
XTT reduction assayThe XTT reduction assay was carried out according to the methods described by Kuhn et al.25 Briefly, the XTT solution (1mg/ml) was prepared in PBS, sterilized through a 0.22μm pore size filter (Millipore, Sartorius Minisart CE 0297, Germany) and stored at −70°C. Menadione (Sigma–Aldrich, Switzerland) solution (0.4mM) was prepared in acetone and sterilized immediately before each assay. The biofilms were first washed five times with PBS, and subsequently, 200μl of PBS and 12μl of the XTT-menadione solution (5 volumes of XTT solution were mixed with 1 volume of menadione solution) were added to each of the prewashed wells and the control wells. The microtiter plate was then incubated for 2h in the dark at 37°C.
Following incubation, 100μl of the solution was transferred to new wells, and the color change in the solution was measured with a microtiter plate reader (SpectraMAX 340 Tunable Microplate Reader, Calif.) at 492nm. The absorbance control values were then subtracted from the tested-well values to eliminate spurious results due to background interference.
Adherence of Candida spp. on the polyvinyl chloride, polyethylene and glassTo test the ability of vaginal Candida strains to adhere to the surface of three commonly used materials in the manufacture of urinary catheters, we chose materials like polyvinyl chloride (PVC), polyethylene (PE) and glass. PVC and polyethylene were cut with a laser. The PVC and glass strips were round-shaped, with diameter of 8mm, whereas the polyethylene strips were square-shaped and measured 8mm×8mm. Once cut, the strips were washed in an alcohol–acetone bath and dried at 50°C to be used later in the process of biofilm formation.23
Adhesion and biofilm formationThe ability of Candida strains to form a biofilm on different surfaces (PVC, PE, glass) was tested according to the previously described procedure by Baillie and Douglas.4 Briefly, Candida cells were grown in liquid YNB medium plus 50mM glucose and incubated at 37°C for 24h with shaking. Cells were then washed twice with PBS and adjusted to a cell density of 107cells/ml. Afterwards, 80μl of Candida cell suspension were inoculated onto the surface of the biomaterial slices contained in 24-well tissue plates and allowed to adhere for 90min at 37°C (adhesion phase). After this, slices were gently washed with PBS in order to remove non-adhered cells and then placed in 3ml of YNB medium with 50mM glucose. The plates were incubated at 37°C for 48h (biofilm formation phase). Afterwards, the YNB medium was removed and the biomaterial slices were washed with PBS. Biomaterial slices without Candida cells were used as a negative control.
Biofilm quantificationQuantification of biofilm was performed by a colorimetric assay based on the determination of the color reduction of tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium (MTT), a water-soluble formazan salt. For this purpose, the PVC, PE and glass slices were transferred into new cell culture plates (24 wells) containing 3ml of PBS/well and 50μl of MTT (5mg/ml in PBS) in each well. Plates were then incubated at 37°C for 5h. After incubation, the optical density of the purplish blue formazan salt present in the supernatant was measured at 570nm by using an ELISA reader (BIO-TEK, Model Synergy HT).29
The tested yeasts were classified according to Stepanovic et al.46 as: Non-adhesive (0): OD≤ODc; weakly adhesive (+): ODc<OD≤2×ODc; moderately adhesive (++): 2×ODc<OD≤4×ODc; very adhesive (+++): 4×ODc<OD. This classification was based on the comparison of the measured optical densities (OD) with the optical density of the control (ODc), which is defined as the average of three values obtained for the negative control.
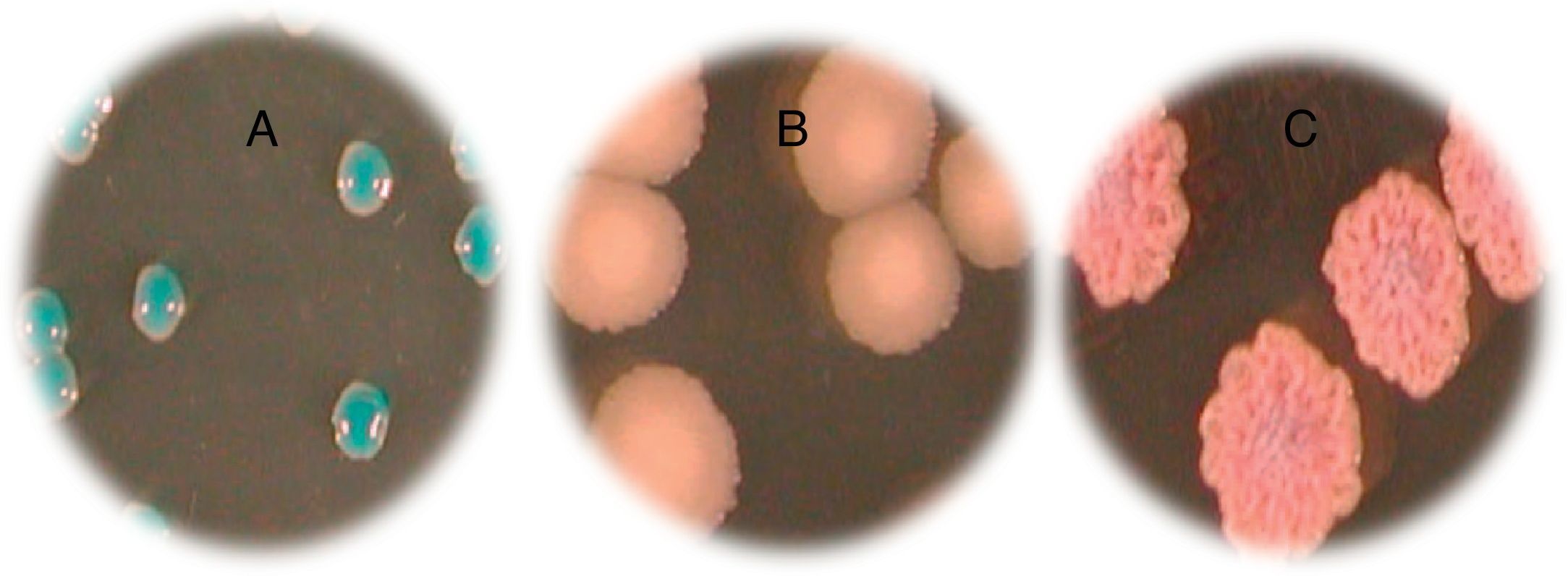
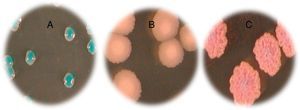
ResultsFig. 1 and Table 2 show the various species of Candida found and the frequency of the Candida isolates according to clinical specimens. Four different species were identified on Candida ID 2 chromogenic agar medium. C. albicans was found to be the dominant species isolated from vaginal mucosa (14 isolates) characterized by blue colonies, followed by white colonies of C. glabrata (12 isolates). Only one C. tropicalis isolate, with smooth light-pink colonies, and a rough and dark pink C. krusei strain were isolated on the tested chromogenic agar medium. Three of our patients had a mixed infection with two Candida species: 3 (C. albicans) and 3′ (C. krusei), 14 (C. albicans) and 14′ (C. glabrata), 15 (C. albicans) and 15′ (C. glabrata).
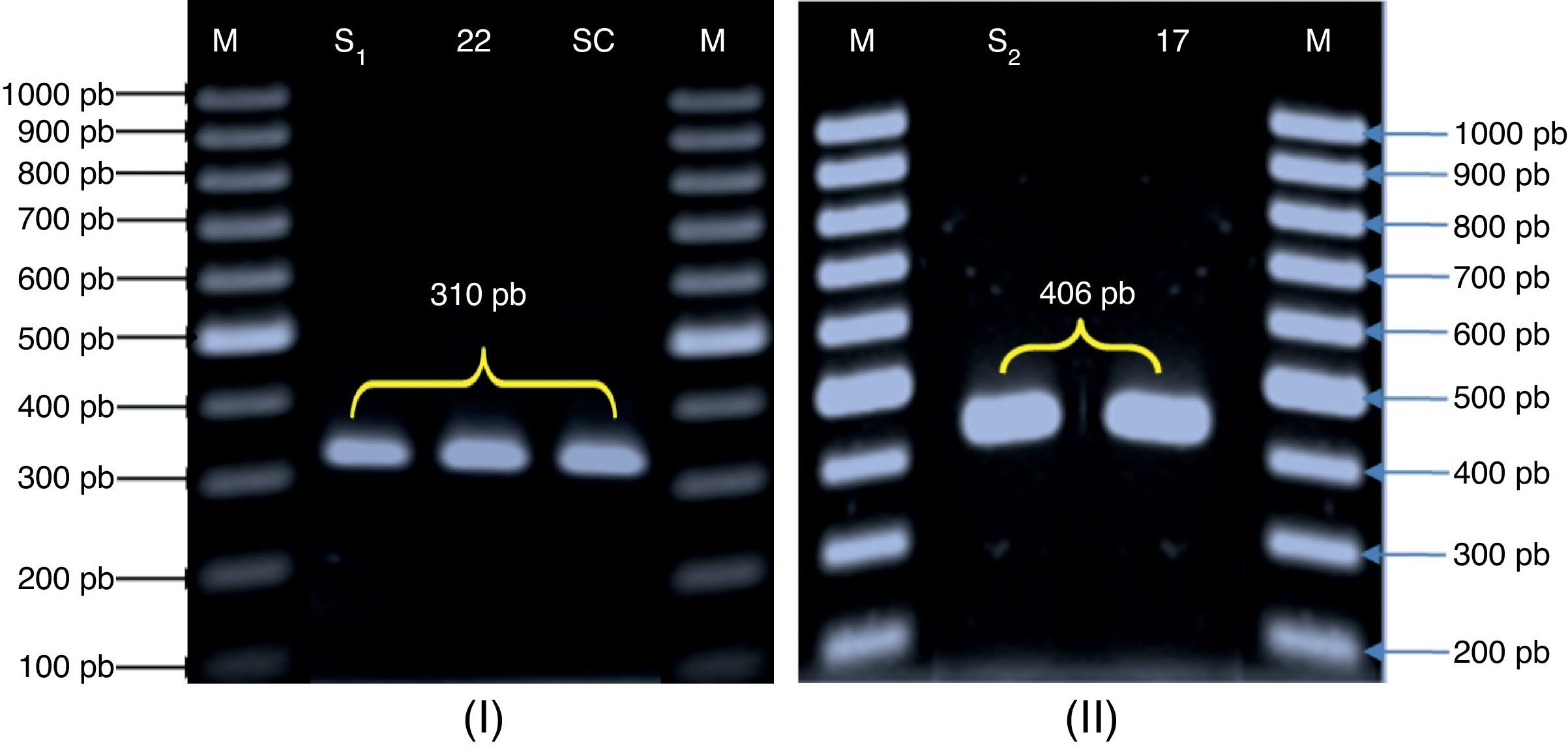
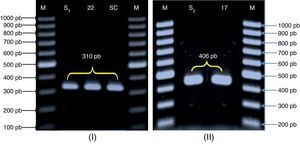
Molecular identification of the three Candida species was carried out by PCR amplification of RPS0 gene intron fragment. For C. glabrata, primers CG1 and CG216 produce a specific amplicon of 406bp (Fig. 2). We obtained the PCR product from all the 12 strains of C. glabrata (an amplicon of 406bp). Since the intron sequences are poorly conserved among microorganism strains, we decided to use this 406bp amplicon, which we termed CgRPS0-INT, to identify C. glabrata from various sources. Our results showed that these primers could specifically identify C. glabrata strains isolated from vaginal mucosa. C. glabrata ATCC 90030 reference strain produced a 406-bp region specific to this species. The second strain object of this study was C. albicans. The size of the amplicon was 310bp (Fig. 2). All strains tested were genetically confirmed to belong to C. albicans species as their relative 310-bp size fragment corresponded to the CaYST1 gene intron fragment. C. albicans ATCC 90028 reference strain produced a 310-bp region specific to this species.
Agarose gel electrophoresis (1% agarose) of the amplification products obtained for the RPS0 gene. M: molecular weight marker (100-pb DNA ladder, Fermentas, Madrid, Spain). (I): C. albicans strains. (II): C. glabrata strains; S1’: C. albicans ATCC 90028; S2: C. glabrata ATCC 90030; SC: C. albicans SC5314; 22 and 17: vaginal C. albicans and C. glabrata strains, respectively.
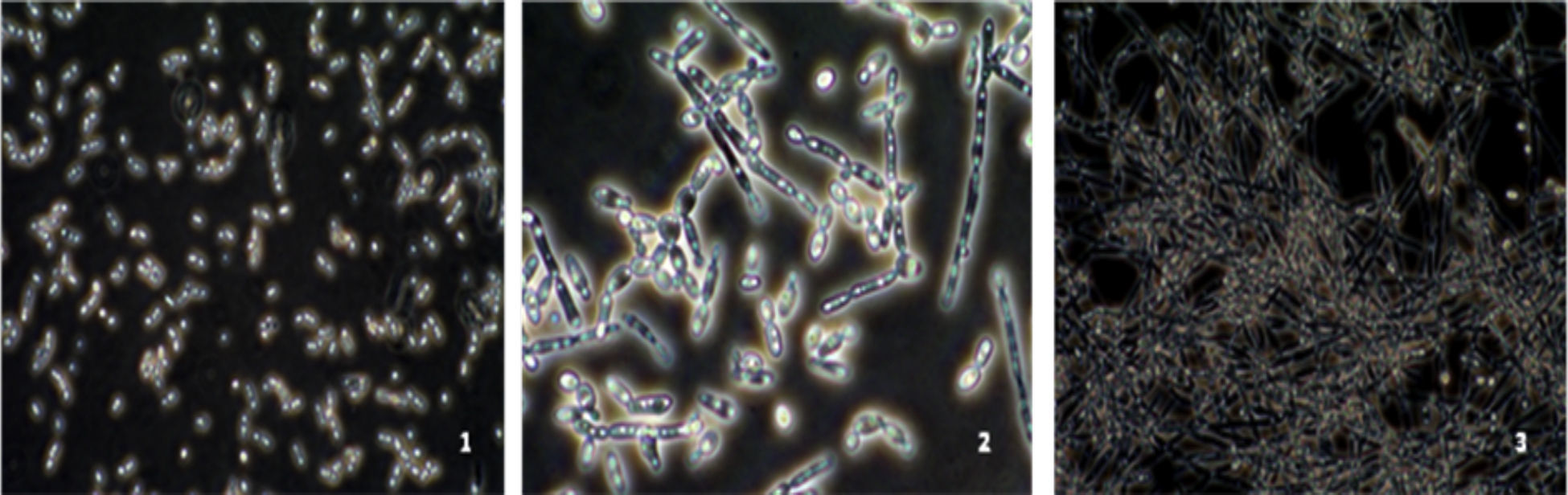
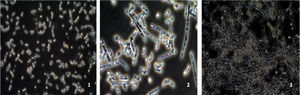
The study of the ability of different strains of C. albicans to form mycelia in Lee medium showed that after 2h of incubation, six C. albicans isolates (strains 20,7, 3,22,19 and10) were unable (−) to form a germ tube, seven strains were weakly able (+) to form a germ tube and one vaginal C. albicans isolate (strain 24) and the reference strain C. albicans SC5314 were strong mycelium producers (Fig. 3).
Microscopic observation of the filamentation range of C. albicans strains on Lee medium (×40). (1): C. albicans strain (non-filamentation producer) (strain 7); (2): C. albicans strain (moderate-filamentation producer) (type strain ATCC 90028) and (3): C. albicans strain (strong-filamentation producer) (strain 24).
The hydrophobicity test based on the measurement of the adhesion of Candida cells to the cyclohexane (hydrocarbon) showed that Candida strains were able to adhere to the tested hydrocarbon to different degrees, and in fact, this is a property of the species. Indeed, optimal adhesion was observed for C. albicans (66.2%) and C. glabrata (64.8%). However, the only C. tropicalis tested strain and isolated from urine was less hydrophobic (33.9%) than the other tested species.
For phospholipase activity, out of the 34 tested strains of Candida obtained from women suffering from different vaginal infections, as well as the type strains, 9 strains (six strains of C. albicans and three strains of C. glabrata) were strongly positive (Pz<0.63) in comparison with the reference strain C. albicans ATCC 90028, and 25 strains showed no phospholipase activity. Pz values, which are inversely proportional to the nature of the activity, ranged from 0.36 to 1 (Table 2). Also, the distribution of phospholipase Pz values varied depending on the species with a maximum of 0.36 detected in strains “4” and “15” of C. albicans.
Incubation of Candida colonies for 7 days at 37°C on BSA agar showed that out of 34 Candida strains, 23 had a very high protease activity (Pz<0.69) and 11 had a very low activity (Pz=1) (Table 2). As we observed for phospholipase activity, the distribution of Pz values of the aspartylprotease varied between species.
The results obtained in our study showed no correlation between the activities of the two hydrolytic enzymes tested. In fact, only four strains of C. albicans (strains 10, 20, 22 and 23), three C. glabrata isolates (strains 2, 5 and 12) and one strain of C. tropicalis produced both enzymes under the same experimental conditions: a positive phospholipase activity and very high protease activity. The three isolates of C. krusei (3′, Roma and ATCC 6258) had a negative phospholipase activity and a very high proteolytic activity.
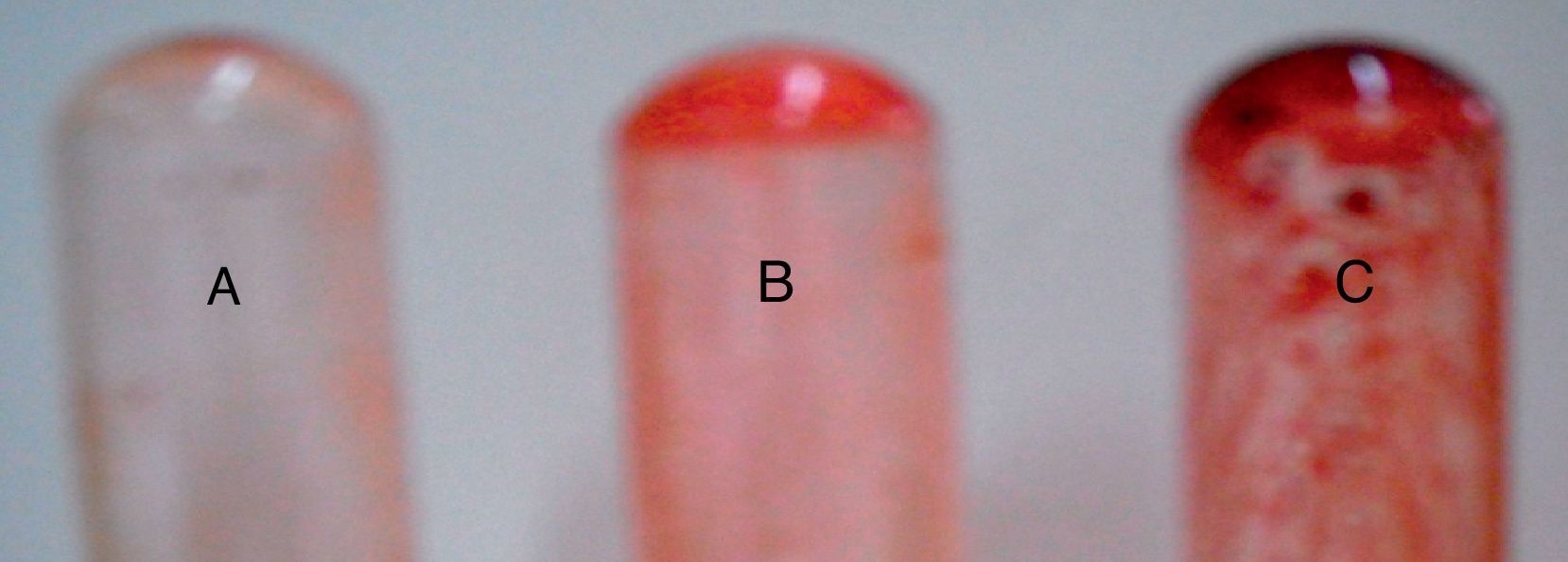
The study of the hemolytic power of vaginal Candida isolates showed that after 48h of incubation on sheep blood agar, the majority of Candida isolates had the ability to degrade hemoglobin by erythrocyte lysis. To begin with, after 24h of incubation, we observed alpha haemolysis around the inoculation site of all Candida species: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis and C. krusei. However, after 48h of incubation, the haemolysis area had enlarged and a double area could be clearly distinguished: an inner area corresponding to a beta-type haemolysis surrounded by a peripheral area corresponding to the alpha-type haemolysis. Furthermore, in the case of C. glabrata and C. albicans, we observed three types of haemolysis: gamma, beta and alpha, with a prevalence of the beta-type haemolysis in C. glabrata and an alpha haemolysis in C. albicans. In turn, the three strains of C. krusei were beta hemolytic.
The qualitative study of biofilm formation tested with the staining with safranin 1% showed that all tested Candida strains were phenotypically biofilm producers to a different degree (Fig. 4). In fact, 4/34 (11.76%) of the strains were weak biofilm producers (+), 6/34 (17.64%) were moderate biofilm producers (++) and 24/34 (70.58%) were strong biofilm producers (+++). C. albicans isolates were the strongest biofilm producers. All of these results are summarized in Table 3.
Slime production and biofilm formation of vaginal Candida strains on polystyrene microtiter plates and biomaterial, (PVC, PE and glass).
| Souche | Index of biofilm formation Safranin 1% staining | Phenotype on CRA plates | Biofilm formation on | ||
|---|---|---|---|---|---|
| PE | PVC | Glass | |||
| C. tropicalis | |||||
| 1 | ++ | Red | M | M | M |
| C. krusei | |||||
| 3′ | +++ | Pink | M | M | W |
| ATCC 6258 | + | Red | W | M | NA |
| Roma | ++ | Red with dark center | M | M | W |
| C. albicans | |||||
| 3 | ++ | Pink with dark center | M | M | NA |
| 4 | +++ | Red | W | M | NA |
| 6 | +++ | Red | M | M | M |
| 7 | +++ | Pink | M | M | W |
| 10 | +++ | Red with dark center | W | W | NA |
| 14 | +++ | Red | W | W | W |
| 15 | ++ | Red with dark center | M | M | M |
| 16 | +++ | Red with dark center | M | W | W |
| 18 | ++ | Red | W | M | S |
| 19 | ++ | Pink | M | W | W |
| 20 | ++ | Red with dark center | W | W | M |
| 22 | ++ | Red | W | M | M |
| 23 | +++ | Red | S | W | M |
| 24 | + | Red with dark center | M | M | M |
| SC5314 | +++ | Pink | W | M | NA |
| ATCC 90028 | ++ | Red with dark center | M | M | NA |
| C. glabrata | |||||
| 2 | +++ | Pink with dark center | M | M | M |
| 5 | ++ | Red | M | W | NA |
| 8 | +++ | Red with dark center | M | M | W |
| 9 | ++ | Red | M | M | NA |
| 11 | ++ | Pink | M | M | W |
| 12 | + | Pink with dark center | M | M | M |
| 13 | ++ | Red | M | M | W |
| 14′ | +++ | Red with dark center | M | M | M |
| 15′ | ++ | Red with dark center | M | W | W |
| 17 | ++ | Red | M | W | W |
| 21 | + | Red with dark center | W | W | M |
| 25 | +++ | Red | M | M | W |
| ATCC 90030 | ++ | Red with dark center | W | W | M |
| C. parapsilosis | |||||
| ATCC 22019 | +++ | Red | M | W | W |
| % of adhesion | 70.58% | 64.7% | 35.29% | ||
+: weak positive slime; ++: moderate positive; +++: strong positive; NA: Non-adhesive; W, weak adhesion; M, medium adhesion; S, strong adhesion; PE: Polyethylene; PVC: Polyvinyl chloride; CRA: Congo red agar.
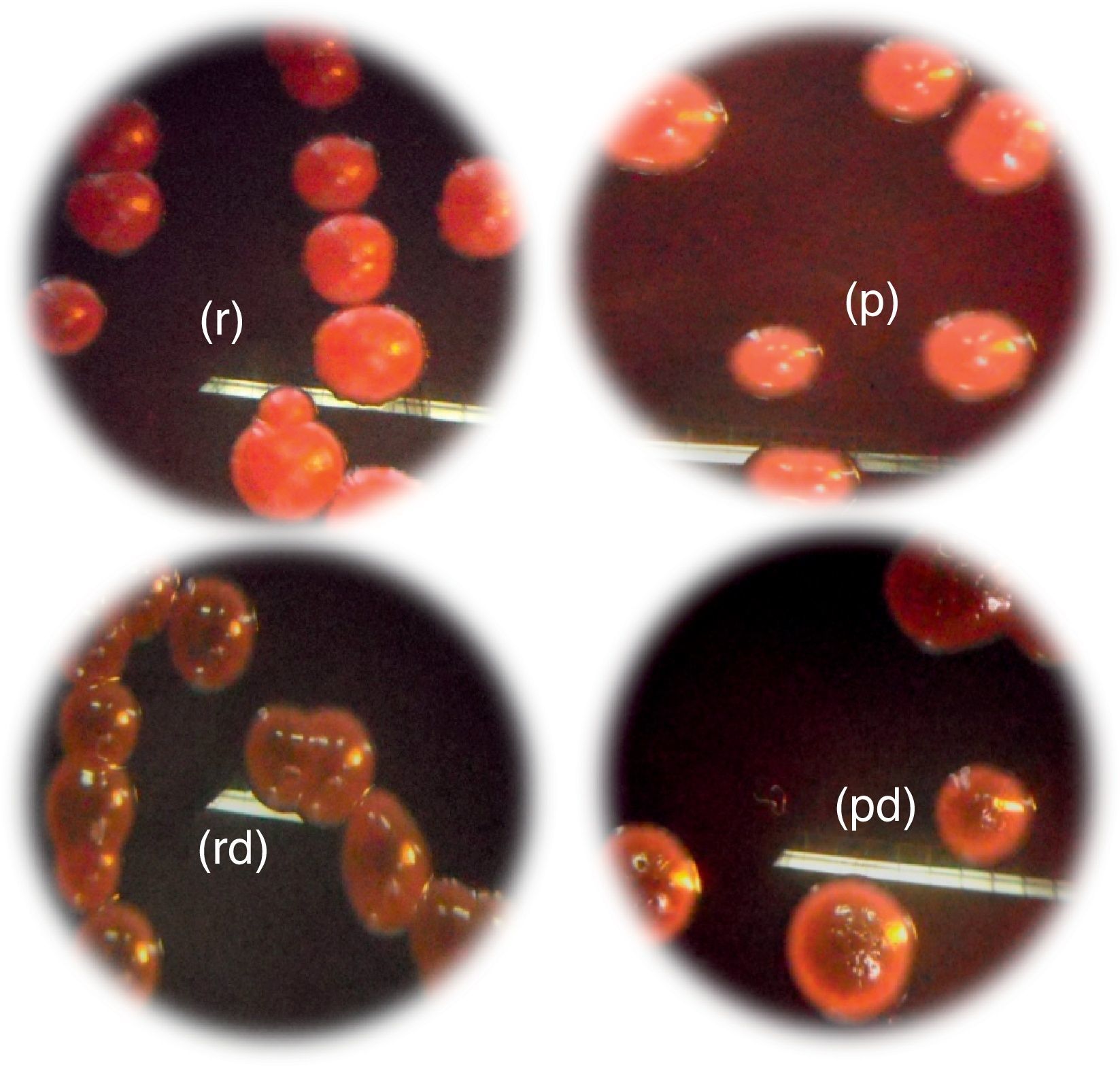
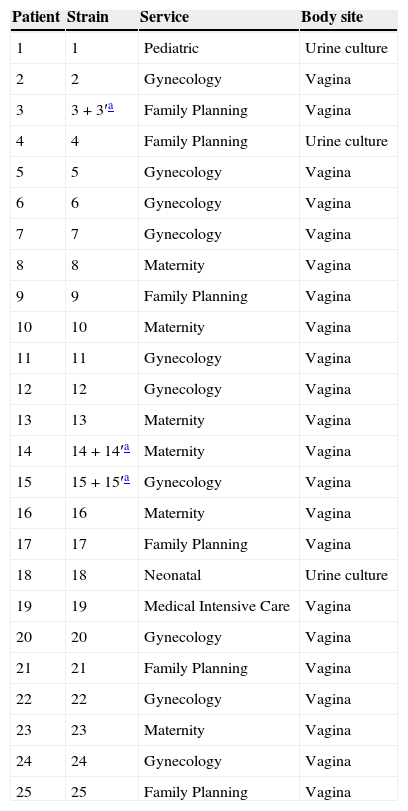
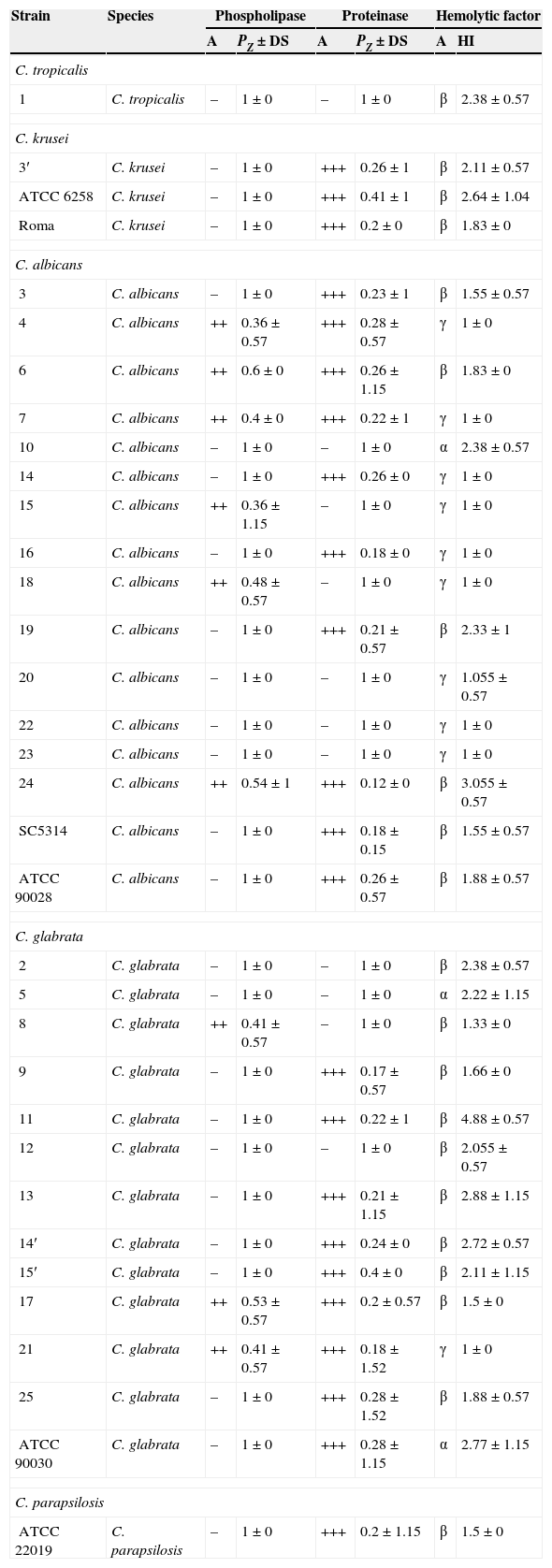
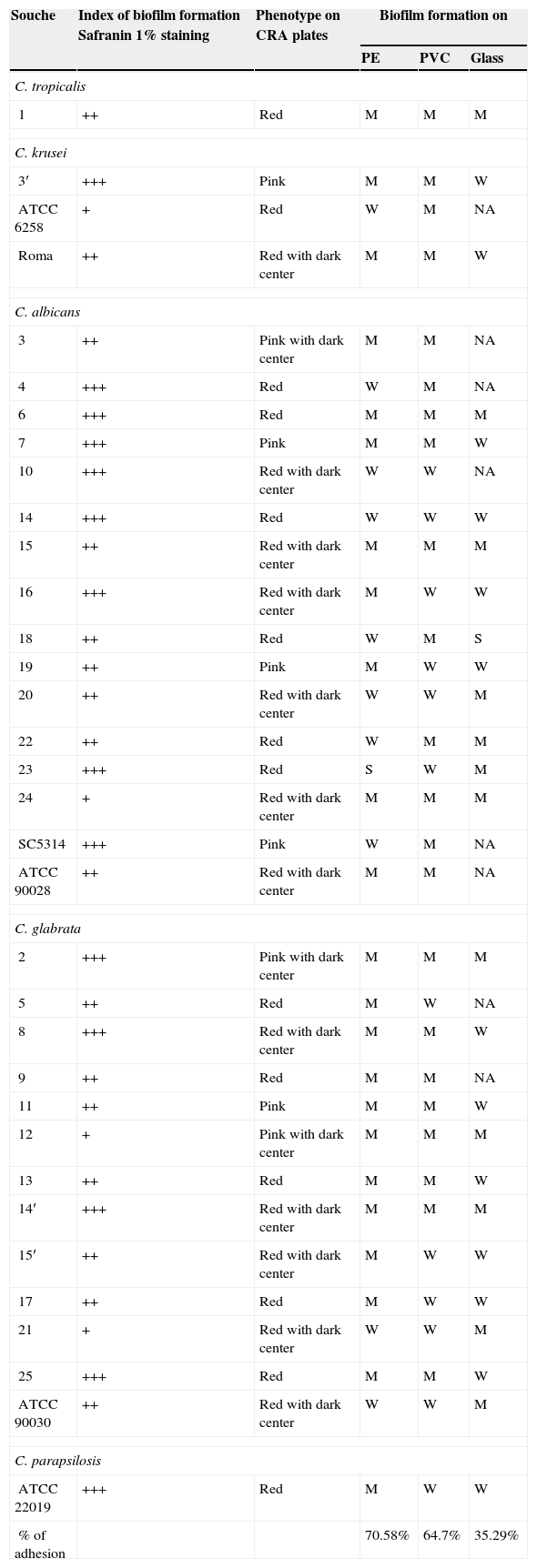
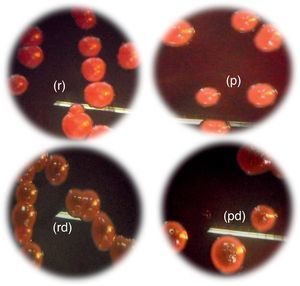
Slime production results on Congo Red Agar (CRA) plates showed that all 34 vaginal Candida strains were non-biofilm producers. Four different morphotypes were detected (14 red colonies, 12 red with darkening-at-the-center-colonies, 5 pink colonies, and 3 pink with darkening-at-the-center colonies) (Table 3, Fig. 5).
Morphotypes of Candida strains based on the colorimetric scale obtained on Congo red agar: (p), pink colonies; (pd), pink colonies with a darkening at the center; (r): red; (rd): red colonies with a darkening at the center. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Biofilm metabolic activity of each Candida strain was measured by the XTT procedure. Considerable variability was observed both between and within species. The OD492nm ranged from 0.149 for the reference strain C. albicans SC5314 to 0.929 for the clinical isolated number 4. With respect to C. glabrata, the OD492nm ranged from 0.153 for the reference strain ATCC90030 to 0.749 for the vaginal isolate number 12. When comparing C. albicans and C. glabrata, the first were a little stronger biofilm producers (OD492nm=0.420) than C. glabrata (OD492nm=0.336). Concerning C. parapsilosis ATCC22019 and C. tropicalis (an urine isolate) both presented higher OD492nm values, 0.582 and 0.857, respectively.
Three biomaterials used for the preparation of urinary catheters were used in this study to test the ability of vaginal Candida strains to form biofilm. Our results showed that 22 strains out of 34 (64.7%) were moderately adhesive to polyvinyl chloride, 24 strains (70.58%) were moderately adhesive to polyethylene, whereas only 12 (35.29%) isolates were moderately adhesive to glass slides (Table 3).
One C. albicans strain (strain 23) was strongly adhesive to PE, whereas C. albicans strain 18 was strongly adhesive to glass slides (Table 3). Finally, all vaginal Candida strains were more adhesive to the tested materials than the reference strains of C. albicans SC 5314, C. albicans ATCC 90028, C. glabrata ATCC 90030 and C. krusei ATCC 6258.
DiscussionIn this study, 28 Candida strains were isolated by endovaginal swabbing in patients attending the gynecology department of the Farhat Hached hospital in Sousse. This type of infection takes place when there is a suitable environment for Candida proliferation on the vaginal mucosa. Besides, some women could also be predisposed to this type of infection because of their hormone cycle and certain contraceptive method usage.
Previous studies have shown C. albicans to be the most frequently isolated species in vaginal infections with a prevalence of 20.1 up to 90%.1 Indeed, its prevalence in the present study proved to be 50%. In 2002, Novikova and colleagues31 demonstrated that C. glabrata was the second most commonly isolated species from vaginal infections with a prevalence of 18–37%. However, this species had a greater prevalence in our study (42.85%).
The identification of all vaginal C. albicans strains was genetically confirmed by amplifying a 310bp fragment as described previously by Baquero et al.5 A 406bp fragment, corresponding to RPS0 gene, was amplified in C. glabrata isolates. PCR approaches for the identification of Candida species are important in both epidemiological and taxonomic studies. Indeed, the RPS0 gene codes for a protein which has been extremely conserved among species and which is a component of the translational machinery.5,16 According to previous results, PCR primers based on RPS0 gene are an important tool for the identification of yeasts and fungi of clinical and environmental interest.38
The enzymatic activities have an important role in fungal pathogenesis. The secretion of phospholipase and protease has been extensively discussed in the literature.11,49
The secretion of extracellular phospholipase by Candida species was reported for the first time in 1960 by Costa et al.10 due to the growth of yeast on solid medium containing egg yolk. Our results showed that 77.52% of Candida strains were phospholipase-negative and 26.47% of C. albicans strains were phospholipase-positive, unlike the study of Farina et al.,14 that showed that 99.4% of C. albicans strains produced this enzyme.
Concerning the proteolytic activity, Naglik et al.28 showed that Candida secreted aspartyl proteinase, and that this enzyme plays an important role in Candida virulence. In turn, the hemolytic activity of Candida isolates was demonstrated by Manns et al.26 and the study of Gang et al.15 further showed the qualitative and quantitative hemolytic activity in different Candida species. Our results show that the majority of the tested strains were able to degrade hemoglobin (iron source) by producing two different types of hemolysin responsible for the erythrocyte lysis.21 We have also found that the hemolytic activity of C. glabrata is greater than that of other Candida species. This evidence was also described by Watanabe et al.48
Furthermore, we studied the hydrophobicity of 34 Candida strains by using cyclohexane. We demonstrated that several Candida isolates were able to adhere to this hydrocarbon to different degrees and that this depends on the species, the most adherent species being C. albicans (66.2%) and C. glabrata (64.8%).
In 2010, Raut and colleagues37 studied the hydrophobicity of 50 clinical Candida isolates in correlation with biofilm formation on polystyrene. They found that the hydrophobicity of the surface of Candida cells varied from 2 to 41%. They also noted that there was no correlation between the hydrophobicity of the yeast cells and their adhesion to polystyrene.
The adhesion of Candida isolates to different surfaces was tested qualitatively and quantitatively. The production of exopolysaccharides on Congo red agar showed four different phenotypes for vaginal Candida strains.
Dag et al.12 described different morphotypes: dark red (slime positive), pink with darkening at the center (low-slime producing strain) and pink and white colonies (non-slime producers strains). Congo red interacts with several polysaccharides with high affinity for chitin and glucan.39 The interaction between these molecules and Congo red limits its use to qualitatively test biofilm formation by Candida spp. strains. This can explain the scarce use of this test for the screening of biofilm formation in Candida.39
The staining with safranin 1% to qualitatively study biofilm formation showed that all Candida strains were phenotypically biofilm producers to different degrees. Cerikcioglu et al.8 suggested the use of this technique because it was simple and efficient for the determination of adhesive properties in Candida strains. This idea was also taken by Dag et al.,12 who found that the Congo red test has a low sensitivity and a high percentage of false positives.
In this work, 34 Candida strains belonging to five different species were tested for their ability to form biofilm on polystyrene plates by measuring cell viability by XTT reduction. In fact, the XTT is reduced by mitochondrial dehydrogenase of metabolically active Candida cells. The color changes during the process of biofilm formation, which indicates the amount of living cells and can be quantified by an ELISA reader.2 Our results demonstrated that vaginal C. albicans had the strongest biofilm producing strains, followed by C. glabrata. Indeed, some studies have shown that the metabolism of XTT by the adherent cells of Candida may change from one species to another, and that this metabolism is higher in C. albicans than in other species.20,25,34
Urinary catheters play an important role in increasing the risk of Candida colonization by acting as a reservoir for microorganisms.23 Therefore, concerning material adherence, we chose three materials largely used in urinary catheters: polyethylene, polyvinyl chloride and glass. The MTT reduction colorimetric method showed that the metabolic activity of Candida cells depended on the material they were adhered to. The strongest adhesion was observed for the vaginal strains of C. albicans on polyethylene and glass. Indeed, the adhesion of Candida species to inert surfaces varies with the physicochemical nature of these surfaces. Rotrosen et al.41 compared the adhesion of C. tropicalis and C. albicans to teflon and PVC catheters. The authors proved that both species adhered more readily to PVC than to teflon. Likewise, in another study, Kiremitçi-Gümüsdereliogu24 compared the adhesion of C. albicans strains isolated from urine to different materials (polypropylene, polyurethane, polyhydroxyethyl). The authors demonstrated that biofilm formation potency correlates with the physicochemical properties of the inert surface. In fact, in 1982, Samaranayake et al.42 proved that non-Candida albicans Candida species were more adherent to inert surfaces than C. albicans was. In our study, C. glabrata isolates were more adherent to polyethylene. Also Rotrosen41 found that C. tropicalis strains produced more biofilm on PVC than C. albicans. Several studies have correlated the adhesion of Candida species to different inert surfaces with the appearance of a fibrillar layer of specific adhesins on the Candida cell surface.22
In conclusion, several virulence factors of Candida strains, such as production of hydrolytic enzymes and biofilm formation, can contribute to their pathogenicity.
Conflict of interestsThe authors declare that there are no conflicts of interest in this work.
The experimental work was partially supported by a Grant PI12/01797 from the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitivity.