Vulvovaginal candidiasis (VVC) is a vulvovaginitis commonly diagnosed in gynecology care. In recent years, the taxonomy of the most important pathogenic Candida species, such as Candida albicans have undergone significant changes.
AimsThis study examined the prevalence of C. albicans, Candida africana, and Candida dubliniensis in vaginal specimens from 210 pregnant women suffering from vulvovaginitis or having asymptomatic colonization.
MethodsPhenotypic and molecular methods were used for the identification of the species.
ResultsDuring the studied period, 55 isolates of Candida or other yeasts were obtained from specimens collected from 52 patients suffering from vulvovaginitis (24.8%). C. albicans was the predominant Candida species in 42 isolates (80.7%), either alone or in combination with other species of the genus (5.7%, n=3). Additionally, nine isolates of C. albicans (50%) were obtained from asymptomatic patients (n=18). C. dubliniensis was the causative agent in 2 (3.8%) cases of VVC, and was also isolated in one asymptomatic patient. Molecular assays were carried out using specific PCR to amplify the ACT1-associated intron sequence of C. dubliniensis. The amplification of the HWP1 gene also correctly identified isolates of the species C. albicans and C. dubliniensis. No C. africana was isolated in this work. Some C. albicans isolates were either homozygous or heterozygous at the HWP1 locus. The distribution of heterozygous and homozygous C. albicans isolates at the HWP1 locus was very similar among patients suffering from VVC and asymptomatic patients (p=0.897).
ConclusionsThe presence of C. albicans and C. dubliniensis, and the absence of C. africana in pregnant is noteworthy.
La candidiasis vulvovaginal (CVV) es una vulvovaginitis comúnmente diagnosticada en la práctica ginecológica. En los años recientes la taxonomía de Candida albicans ha sufrido cambios significativos.
ObjetivosEn este estudio se examinó la prevalencia de C. albicans, Candida africana y Candida dubliniensis en las secreciones vaginales de 210 mujeres embarazadas con vulvovaginitis o colonización asintomática.
MétodosSe usaron métodos fenotípicos y moleculares para la identificación de las levaduras.
ResultadosUn total de 55 aislamientos de Candida u otras levaduras se recuperaron a partir de muestras de 52 pacientes con vulvovaginitis (24,8%). La especie C. albicans fue predominante como especie única (42 aislamientos; 80,7%) o asociada con otras especies del género (5,7%, n=3). Nueve aislamientos de C. albicans (50%) se recuperaron de las pacientes asintomáticas (n=18). Se aisló C. dubliniensis en dos casos de CVV (3,8%) y en una paciente asintomática. Los estudios moleculares por PCR que amplifican la secuencia del intrón asociado al gen ACT1 confirmaron la presencia de esta especie. La amplificación del gen HWP1 confirmó la presencia de C. dubliniensis y C. albicans, y la ausencia de C. africana. Los aislamientos de C. albicans fueron homocigóticos o heterocigóticos en el locus HWP1; las pacientes con CVV y aquellas asintomáticas mostraron para esta especie una distribución similar de aislamientos homocigóticos o heterocigóticos en el locus HWP1 (p=0,897).
ConclusionesDestacamos la presencia de C. albicans y C. dubliniensis y la ausencia de C. africana en las CVV o en la colonización asintomática de las secreciones vaginales de las gestantes.
Vulvovaginal candidiasis (VVC) is caused by abnormal yeast growth in the mucosa of the female genital tract and is one of the most common conditions diagnosed in women seeking gynecological care.9 The distribution of the different Candida species identified in women suffering from VVC varies widely depending on the locations as well as the populations studied.2 It was reported that 80–90% of VVC cases are caused by Candida albicans whereas 10–20% are due to other species of the genus.2,25 However, in recent years, the taxonomy of the most important Candida species such as C. albicans, Candida parapsilosis and Candida glabrata, has undergone significant changes due to the description of new closely related species and therefore they are, nowadays, recognized as ‘cryptic species complexes’.4,6,7,20,21,28,31–34Candida dubliniensis is a yeast species that is closely related to C. albicans,28 and has been found in vaginal samples.1,28,35 The very close phenotypic and genotypic relationship between C. dubliniensis and C. albicans has led to the misidentification of isolates of C. dubliniensis as C. albicans.5,7 Recently, several phenotypic methods for the identification of C. dubliniensis and its differentiation from C. albicans have been reported.12,27–29 However, phenotypic variation in C. dubliniensis can result in difficulties in accurate identification.12,28,30Candida africana was first isolated from patients in Africa and Germany.33 In 2001, it was proposed as a new Candida species that produces a germ tube but no chlamydospores and is able to assimilate xylose and grow in hypertonic broths.7,23,34C. africana has been reported as a cause of VVC in African, German, Spanish, and Italian patients.16,21,22,33C. africana and C. dubliniensis strains are often misidentified as C. albicans. Therefore, rapid nucleic acid-based methods to identify C. dubliniensis, C. albicans and C. africana have been performed using polymerase chain reaction (PCR) assays.8,20
In the present study we evaluated the presence of C. albicans and the closely related species C. africana and C. dubliniensis in clinical samples collected from patients suffering from VVC and having asymptomatic colonization by Candida species.
Materials and methodsClinical C. albicans and non-C. albicans isolatesThis study was carried out in the Laboratory of Microbiology of “Ramón Sardá” Maternity Hospital in Buenos Aires, Argentina, during the period of August 2012 to January 2013. Two hundred and ten immunocompetent pregnant women attending the “Ramón Sardá” maternity hospital to be examined for vaginal secretion, regardless of the presence or absence of vulvovaginitis symptoms, were enrolled. The characterization of the vaginal discharge was performed at the time of the visit when the sample was taken according to the method proposed by Odds et al.17 The specimens were collected using sterile cotton-tipped swabs with the aid of a disposable vaginal speculum. The swabs were placed into a tube filled with saline solution prior to direct microscopic examination on a wet slide. Specific yeasts were initially phenotypically identified using conventional methods, such as the color of colonies grown on chromogenic medium CHROMagar Candida (CHROMagar Company, Paris, France) at 37°C for 48h, and the micromorphological studies on milk agar plates supplemented with 1% Tween 80.13 All the isolates that grew as light-green colonies were examined for chlamydospore formation on Staib agar as a species-specific characteristic of C. dubliniensis.12,27 Finally, all the yeasts were identified using a standard system, API ID32C (bioMérieux, Marcy l’Etoile, France).
Molecular identification of C. albicans, C. dubliniensis and C. africanaDefinitive species identification and discrimination of all members of the C. albicans species complex was performed by the amplification of the hyphal wall protein 1 (HWP1) gene20 using a single yeast colony presumptively identified as C. albicans or C. dubliniensis. The primers CR-f (5′ GCT ACC ACT TCA GAA TCA TCA TC 3′) and CR-r (5′ GCA CCT TCA GTC GTA GAG GAC G 3′) (Genbiotech, Argentina) and the PCR mixture were previously described.20 The PCR reaction conditions were as follows: denaturation at 95°C for 5min, 30 cycles of denaturation at 94°C for 45s, primer annealing at 53°C for 60s and extension at 72°C for 90s; followed by a final extension at 72°C for 10min in a Minicycler DNA thermal cycler (TMJ Research Inc., USA). The PCR products were separated and visualized on a 1.3% (wt/vol) agarose gel. The CR-f/CR-r primers amplified a DNA segment of approximately 941bp for C. albicans, 569bp for C. dubliniensis and 700bp for C. africana.20 Positive control isolates C. albicans ATCC 90028 and C. dubliniensis from the Center of Mycology of the Faculty of Medicine, University of Buenos Aires (GenBank accession number FJ8116791.1) were tested in the same manner.
Molecular identification of C. dubliniensisWe also decided to identify C. dubliniensis using the ACT1 gene-PCR based method. The PCR reaction was performed using specific primers from the ACT1 gene intron of C. dubliniensis employing primers DUBF (5′ GTA TTT GTC GTT CCC CTT TC 3′) and DUBFR (5′ GTG TTG TGT GCA CTA ACG TC 3′) (Genbiotech, Argentina),8 using a single yeast colony presumptively identified as C. albicans or C. dubliniensis. The PCR reaction conditions were as follows: denaturation at 94°C for 3min, 30 cycles of denaturation at 94°C for 45s, primer annealing at 55°C for 45s and extension at 72°C for 45s, followed by a final extension at 72°C for 10min in a Minicycler DNA thermal cycler (TMJ Research Inc.). The PCR products were separated and visualized on a 1.3% (wt/vol) agarose gel. The primers DUBF/DUBR amplified a DNA segment of approximately 288bp. The positive control isolate of C. dubliniensis from the Center of Mycology of the Faculty of Medicine, University of Buenos Aires (GenBank accession number FJ8116791.1) was tested in the same manner.
Statistical methodsThe statistical analysis of the data was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software Inc., USA). Statistical significance was set at p<0.05.
ResultsTwo hundred and ten immunocompetent pregnant women aged 10–42 years were evaluated. The median gestation time was about 24.8±6.8 weeks. Altogether, 61.9% (130 patients) were asymptomatic while 38.1% (80 patients) were symptomatic. Vaginal discharge (100%, 80/80), vulvovaginal itching (46.2%, 37/80), vulvovaginal burning sensation (22.5%, 18/80) and dysuria (6.2%, 5/80) were among the most common described symptoms. None of them reported dyspareunia. There were statistically significant differences in vaginal discharge characteristics between symptomatic and asymptomatic patients (p-value<0.0001). While white vaginal discharges were predominant among symptomatic patients, clear vaginal secretions were most common in the asymptomatic ones.
During the study period, 55 isolates of Candida or other yeasts were obtained from specimens collected from 52 patients suffering from VVC (24.8%).2
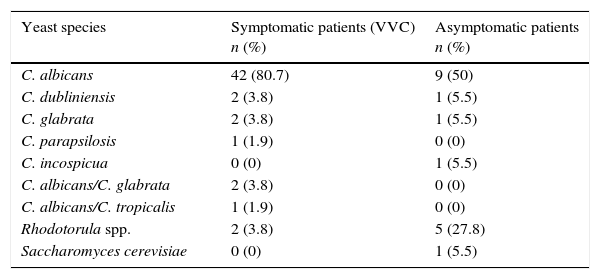
Table 1 shows the species distribution of yeasts in symptomatic (patients suffering from VVC) and asymptomatic patients (colonization). C. albicans was the predominant Candida species (42 strains; 80.7%) and was associated with three non-C. albicans (5.7%) isolates in three specimens. Additionally, nine isolates of C. albicans (50%) from asymptomatic patients (n=18) were also obtained by culture. Non-C. albicans Candida species and other yeasts were more frequent in the asymptomatic group (p=0.0038).
Yeast isolates from symptomatic (n=52) and asymptomatic (n=18) patients.
| Yeast species | Symptomatic patients (VVC) n (%) | Asymptomatic patients n (%) |
|---|---|---|
| C. albicans | 42 (80.7) | 9 (50) |
| C. dubliniensis | 2 (3.8) | 1 (5.5) |
| C. glabrata | 2 (3.8) | 1 (5.5) |
| C. parapsilosis | 1 (1.9) | 0 (0) |
| C. incospicua | 0 (0) | 1 (5.5) |
| C. albicans/C. glabrata | 2 (3.8) | 0 (0) |
| C. albicans/C. tropicalis | 1 (1.9) | 0 (0) |
| Rhodotorula spp. | 2 (3.8) | 5 (27.8) |
| Saccharomyces cerevisiae | 0 (0) | 1 (5.5) |
Forty-four isolates were presumptively identified as C. albicans on the basis of the color of the colonies on CHROMagar Candida, the micromorphological studies on milk agar plates supplemented with 1% Tween 80,13 and the identification codes in the API ID32C system (bioMérieux, France). Furthermore, three isolates produced typical clustered chlamydospores at the tip of the hyphae on agar milk 1% Tween-80,13 as well as abundant pseudohyphae and chlamydospores on Staib agar.27 These results together with the carbohydrate assimilation tests using a commercially available API kit suggested that these three isolates were C. dubliniensis. Two of these isolates (3.8%) were implicated as the causative agent of VVC, whereas the remaining one was isolated from an asymptomatic patient. None of the isolates were identified as C. africana.
Molecular identification of C. dubliniensisA PCR reaction was carried out among the 57 isolates showing the characteristic green colony color on CHROMagar Candida, for the molecular identification of C. dubliniensis using the primers DUBF and DUBFR. Three of these isolates were identified as C. dubliniensis.
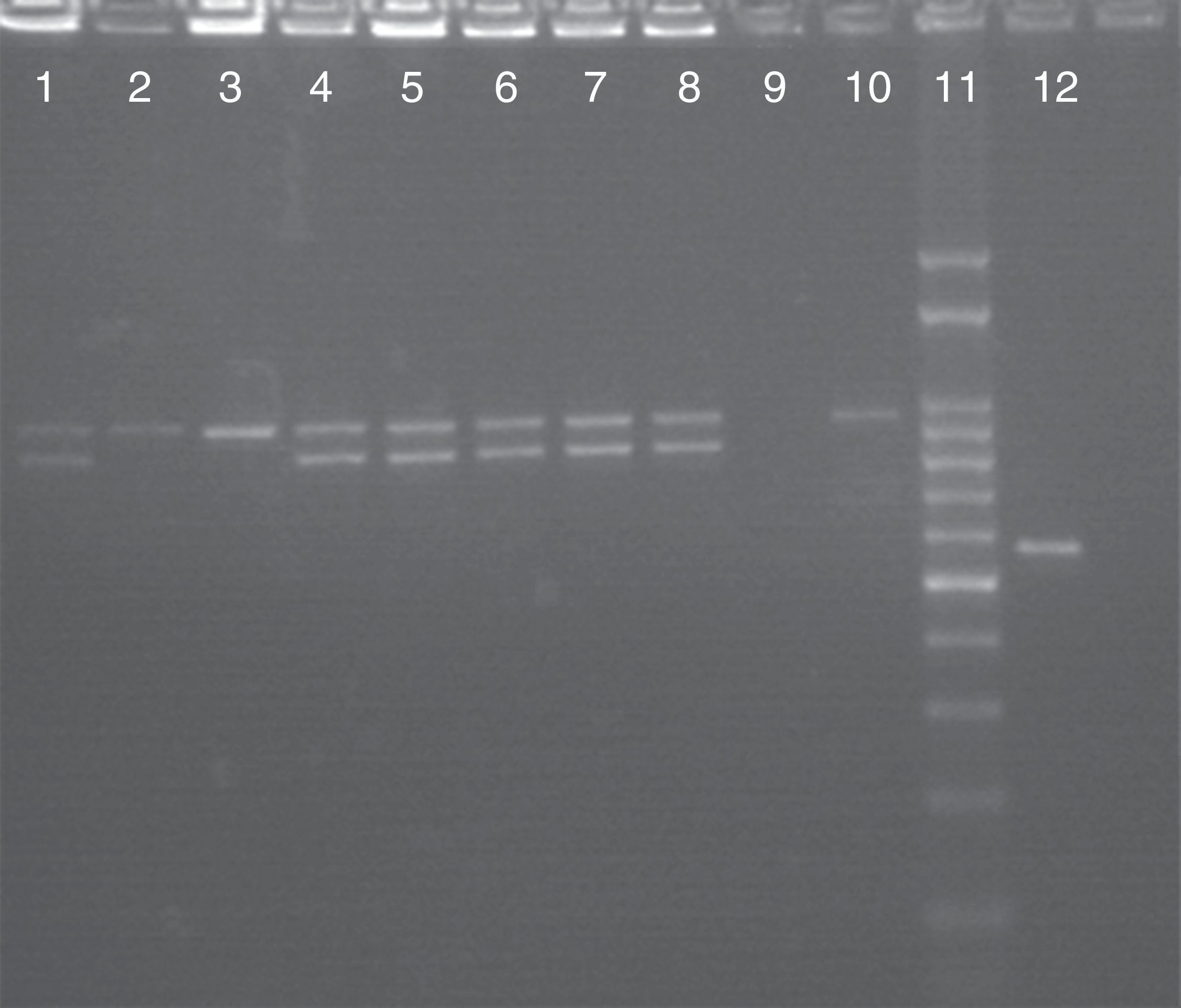
Molecular identification of C. albicans, C. africana and C. dubliniensisAmong the 57 isolates presumptively identified as C. albicans on the basis of colony appearance on CHROMagar Candida (green colonies), the use of HWP1 gene amplification verified that 54 of them were C. albicans and 3 were C. dubliniensis, confirming the above results. No C. africana isolates were found in this study. Fig. 1 shows a 941 bp-DNA fragment as expected for C. albicans and a 569 bp-fragment for C. dubliniensis. Some C. albicans isolates were heterozygous and produced two DNA fragments: one of 839bp and the other one of 941bp. Nevertheless, we found no statistically significant differences in the rate of heterozygous and homozygous C. albicans isolates at the HWP1 locus when the symptomatic (VVC) and asymptomatic groups of patients were compared (p=0.897).
Molecular identification of C. albicans and C. dubliniensis by using CR-f/CR-r primers. Lanes 1–8: PCR products of C. albicans; lane 9: negative control; lane 10: C. albicans ATCC 90028; lane 11: 100bp molecular size marker; lane 12: positive control isolate C. dubliniensis, Center of Mycology of the Faculty of Medicine, University of Buenos Aires (GenBank accession number FJ8116791.1).
Colonization refers to the presence of Candida in the vagina that is not associated with any sign of disease. In patients who are asymptomatic carriers of yeasts, the results of microscopic examinations of vaginal secretions tend to be negative; colonization is usually identified on the basis of a positive culture for yeasts.2,26 In contrast to asymptomatic colonization, VVC is defined as an infection of the vulva and the vagina, which is characterized by signs and symptoms of inflammation in the presence of Candida spp. and in the absence of other infectious etiology.2,26 In the current study, the prevalence of VVC in pregnant women was practically 25% (52/210). Garcia-Heredia et al. obtained similar results to those described,10 although higher percentages were reported.11,19 Recent studies have demonstrated that the prevalence of C. albicans accounts for 40.5–85% of cases14,36 and these differences may reflect the population analyzed in such studies. This study also shows that C. albicans was the most frequent yeast isolated from vaginal secretions in both symptomatic and asymptomatic patients. However, there is a statistical trend reflecting a high frequency of non-C. albicans species in asymptomatic women.
These findings suggest that vaginal colonization by yeasts is associated with non-C. albicans species and that the evolution of the symptoms of VVC would depend, among other factors, on the replacement of such species by C. albicans.15 In addition, C. albicans was a commensal agent in asymptomatic women (50%). In this case, the development of VVC could depend on changes in the vaginal environment.26
In VVC, C. albicans was present in all the associations between two Candida species even when their frequency was low. Typically, although a single species is identified, two or more species have been found in the same vaginal culture in a very small number of women (2–5%).2 As it is shown in Fig. 1, C. albicans isolates produced either one or two DNA fragments of the HWP1 gene (941bp or 839bp and 941bp, respectively). The 839 bp-DNA fragment is considered a novel allele of the HWP1 gene. The only 941bp-DNA fragment demonstrates that C. albicans isolates were homozygous at the HWP1 locus, while the two remaining DNA fragments indicate heterozygosity.24 Although no correlation was found between asymptomatic and symptomatic pregnant women with respect to the presence of homozygous or heterozygous C. albicans isolates at the HWP1 locus, further studies are needed to elucidate an eventual pathogenic role of these homozygous or heterozygous strains in the adherence processes.
C. dubliniensis shares so many phenotypic similarities with C. albicans that it may easily be misidentified as such.5,7 This Candida species appears to be most commonly associated with recurrent episodes of oral candidiasis in HIV5 and non-HIV infected individuals.12 In addition, there have also been reports of recovery of C. dubliniensis isolates from vaginal and fecal samples. Hence, the prevalence values of VVC caused by C. dubliniensis are scarce, ranging from 0.17 to 2.44%.1,35 As previously reported, the micromorphology on Staib agar12 was concordant with the molecular identification by PCR using specific primers from the ACT1-associated intron sequence of C. dubliniensis8 and the HWP1 gene,20 and was useful for selecting presumptive C. dubliniensis isolates. However, DNA-based methods continue to be the most effective to allow the best discrimination between the two species.3
C. africana is a yeast particularly able to cause vaginitis. Although most C. africana isolates recovered from clinical samples worldwide were from vaginal samples,16,21,22,24,32,33 Odds et al.18 described its recovery from blood in Chile (South America). Interestingly, this Chilean strain could represent the first isolation of C. africana from a different clinical sample, suggesting that this fungus can also be associated with a broader clinical spectrum.22 However, the results of the HWP1 gene amplification assay revealed that no C. africana isolate was recovered from our vaginal specimens, which may be partly due to the fact that C. africana generally has a low incidence in vaginal samples18,24 and probably a larger number of VVC cases are necessary in order to isolate this Candida species in our country. Molecular biological tools are essential for the reliable identification of C. africana and C. dubliniensis, species closely related to C. albicans.
Conflict of interestThe authors declare no conflict of interest.