To evaluate the relationships between oxidative stress (OS) biomarkers and total antioxidant capacity (TAC) in blood serum and seminal plasma, and their associations with semen quality and serum reproductive hormone concentrations in potential subfertile men.
Material and methodA cross-sectional study was conducted on men (n=122) attending an infertility clinic in the Murcia Region (Southern Spain) between 2012 and 2013. Concentrations of malondialdehyde (MDA), nitric oxide (NO) and TAC were measured in blood and semen. Follicle-stimulating hormone, luteinising hormone, testosterone, prolactin and oestradiol concentrations were measured in serum. Semen analyses were performed according to World Health Organization criteria. Correlation analysis and multiple linear regression models were performed, controlling for important covariates.
ResultsThere was a significant inverse association between serum MDA concentrations and all sperm parameters, except for seminal volume. Serum TAC concentrations were positively related to sperm count and motility. A positive association was observed between seminal plasma NO levels and the percentage of morphologically normal sperm. With regard to reproductive hormones, serum MDA concentrations were positively related to FSH and LH levels, and TAC inversely associated with FSH levels.
ConclusionsOur results suggest that oxidative stress may be associated with semen parameters and reproductive hormone levels in male partners of couples seeking infertility treatment. However, further studies are needed to confirm and extend these findings, in particular, with regard to serum reproductive hormones.
Evaluar las correlaciones entre marcadores de estrés oxidativo (OS) y capacidad antioxidante total (TAC) en suero sanguíneo y plasma seminal, y sus asociaciones con calidad seminal y hormonas reproductivas en varones potencialmente subfértiles.
Material y métodoEstudio transversal realizado en varones (n=122) que acudían a un servicio de infertilidad de Murcia entre 2012-2013. Las concentraciones de malondialdehído (MDA), óxido nítrico (NO) y TAC se midieron en sangre y semen. Se analizaron los niveles séricos de las hormonas foliculoestimulante, luteinizante, testosterona, prolactina y estradiol. Los análisis espermáticos se llevaron a cabo siguiendo las normas de la Organización Mundial de la Salud. Se utilizaron análisis de correlación y modelos de regresión lineal múltiple ajustando por covariables importantes.
ResultadosSe mostró una asociación inversa significativa entre las concentraciones séricas de MDA y todos los parámetros espermáticos, excepto el volumen seminal. Las concentraciones séricas de TAC se relacionaron positivamente con el recuento y la movilidad espermática. Los niveles de NO en plasma seminal se asociaron directamente con el porcentaje de espermatozoides morfológicamente normales. Con respecto a las hormonas reproductivas, las concentraciones séricas de MDA se asociaron positivamente con los niveles de FSH y LH, y las de TAC se asociaron inversamente con los niveles de FSH.
ConclusionesNuestros resultados sugieren que el estrés oxidativo estaría asociado con los parámetros seminales y hormonales en varones de parejas que consultan por problemas de infertilidad. Sin embargo, son necesarios más estudios para confirmar estos hallazgos, en particular con respecto al papel de las hormonas reproductivas.
Male factor infertility accounts for approximately 25% of consultations to infertility clinics and is a contributing factor in an additional 30%–40%.1 In recent years oxidative stress (OS) has been identified as an underlying etiological factor in the mechanism leading to sperm dysfunction and male infertility.1–3 Several animal and human studies have revealed generation of reactive oxygen species (ROS) by spermatozoa and leukocytes.2 A number of studies have also reported associations between pathological levels of ROS, male infertility and impaired seminal quality,3 including sperm concentration, motility, and morphology.4–8 ROS react with and modify lipids, carbohydrates, proteins and DNA and may result in cytotoxicity and dysfunction.9,10 For example, ROS reacts with polyunsaturated fatty acids present in the plasma membrane of the spermatozoa,3 producing lipid peroxidation – measured by malondialdehyde (MDA) levels – and changes in fluidity and integrity of the membrane, which leads to a decrease of sperm motility and changes in sperm morphology.6–9 Another ROS, the nitric oxide (NO) has been negatively correlated with sperm function, including motility10 or vitality.11 Nevertheless, there is a balance between ROS production an antioxidant scavenging activities in the male reproductive tract.12 Total antioxidant capacity (TAC) is a well-known tool to assess the overall antioxidant status. Low levels of TAC have been found in seminal plasma of infertile men,2,12 while high levels were positively associated to traditional criteria for good semen quality.13 Little is known about the role of OS markers in potential subfertile men from Southern Spain.
The role of reproductive hormones on male fertility is well documented,14 and the main pathways refer to hormonal imbalance affecting spermatogenesis. However, there are reasons to believe that sex hormones may have another pathway through which male fertility may be hampered. There is increasing evidence that hormones can modulate the antioxidant system, at least in some disorders or conditions, independently of whether there is hormone excess or deficiency.15 However to what extent sex hormones play a role in OS in adult men is still unknown and controversial.16 The aim of this study was to evaluate the correlations between OS biomarkers (MDA and NO) and TAC, both in blood serum and seminal plasma, and their associations with semen quality and serum reproductive hormone concentrations in men attending to an infertility service in Southern Spain.
Material and methodsStudy populationThis is a cross-sectional study. Men attending infertility services of the “Virgen de la Arrixaca” University Hospital in the Murcia Region (Southern Spain), 25–38 years old, were included in the current study between December 2012 and July 2013. Written informed consent was obtained from all subjects, and the study was approved by The Research Ethics Committee of the University of Murcia.
Participants were male partners of infertile couples seeking infertility evaluation and potential management, and were recruited when attending the Andrology laboratory to schedule a semen analysis. All men were informed of the purpose of the study and were invited to participate. To be included in the study, men had to be able to speak and write Spanish, have no vasectomy, infections and contagious diseases or previous oncology treatment. On the day of attendance, men underwent an andrological examination, provided a semen sample, a blood drawn and completed epidemiological questionnaires on lifestyle and general health. Of the 220 men who were offered participation in the study, 162 accepted (74%). Of these, 141 returned for semen analysis, and 122 agreed to have a blood drawn and delivered the questionnaires. Then, this analysis is based on the 122 (55%) men for whom data on complete variables were available.
Hormonal analysesBlood serum was obtained between 8:00 and 9:00a.m. by venipuncture and centrifugation. Hormone levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), testosterone (T) and estradiol (E2), were determined. E2/T and T/LH ratios were also calculated. The determinations were performed using immunoelectroluminiscence (Roche Diagnostic Corporation, Indianapolis, IN, USA). Intra- and interassay coefficients of variation (CVs) were the following: for FSH 2.6 and 3.6%, LH 0.8 and 2.0%, PRL 0.8 and 1.8%, T 2.8 and 3.2%, and E2 2.1 and 2.8%, respectively. In this study, assay sensitivities were 0.10IU/L for FSH and LH, 1.0μIU/mL for PRL, 0.025ng/mL for T and 5.0pg/mL for E2.
Semen analysisThe sample collection was performed following the recommendations of the fifth edition of the Manual for the examination and processing of human semen.17 Briefly, men had been asked to abstain from ejaculation for at least 48h before sample collection. Ejaculation abstinence time (h) was recorded as the time between current and previous ejaculation as reported by the study subject. For volume assessment a glass-graduated cylinder was used. The spermatozoa were classified as either motile or immotile17 to report the percentage of motile spermatozoa [(progressive and not progressive (P+NP)]. Sperm concentration was determined with a hemocytometer (Neubauer, Hauser Scientific Inc., Horsham, PA, USA). We also calculated the total sperm count [volume×sperm concentration (TSC)] and total motile count [sperm concentration×volume×percentage of motile sperm (TMC)]. Smears for morphology were made, air-dried, fixed, Papanicolaou stained and assessed using strict criteria. All analyzes were carried out by the same specialized biologist at the Andrology laboratory of “Virgen de la Arrixaca” University Hospital.
Oxidative stress analysesBlood serum and seminal plasma were obtained as previously described and were immediately frozen at −80°C.
Total antioxidant capacity (TAC) measurementTAC was measured with the colorimetric method using Randox total antioxidant status kit (Randox Laboratories Ltd, UK). The frozen seminal plasma or blood serum was thawed by placing the vials in a water bath at 37°C for 20min and immediately assessed for its antioxidant capacity. Twenty microliters of sample was added to 1mL of the reconstituted chromogen, ABTS-metmyoglobin (10mL vial with 10mL of phosphate-buffered saline buffer). Twenty microliters of Trolox (6-hydroxyl-2,5,7,8-tetramethylchroman-2-carboxylic acid) at a concentration of 1.71mmol/L was used as the standard. Whereas 20μL of deionized water was used as a blank. One milliliter of chromogen was added to the standard and blank samples. With spectrophotometer adjusted at a wavelength of 600nm, the initial absorbance (A1) was read. Two hundred microliters of H2O2 (250μmol/L) was then added to all tubes, and absorbance (A2) was read exactly after 3min. The difference between A2 and A1 (ΔA) was calculated. The TAC of the sample was then calculated by the following formula: TAC=Concentration of the Standard×(ΔA Blank−ΔA Sample)/(ΔA Blank−ΔA Standard). The results were expressed as mmol/L.
Malondialdehyde (MDA) measurementMDA was measured in blood serum and seminal plasma with the commercial kit MDA-586TM (OxisResearch, CA, USA). This assay is based on the reaction of a chromogenic reagent (N-methyl-2-phenylindole) with MDA at 45°C. Two hundred microliters were used for measurement, according to the manufacturer's instructions and a calibration curve was prepared using the provided MDA standard. All samples were processed in triplicate, and the absorbance was measured at 586nm with a spectrophotometer. The results were calculated using a linear regression with the standard data, and expressed as μM. Imprecision of the method, evaluated on low and high concentration controls, was <10%.
Nitric oxide (NO) measurementNO was determined only in seminal plasma using a colorimetric assay kit (Biovision Research, CA, USA). NO is rapidly oxidized to nitrite and nitrate which were used to quantify NO production. Briefly, in the first step nitrate was converted to nitrite by nitrate reductase. In a second step, griess reagents were used to convert nitrite to a deep purple azo compound. The amount of the chromophore (azochromophore) was measured at 540nm with a spectrophotometer and expressed in μM. The detection limit of the assay is approximately 1μM.
Statistical analysesDescriptive statistics are presented using untransformed data. In preliminary analyses, FSH, LH and PRL distributions closely approximated normality and were used untransformed, whereas the distributions of the remaining hormones (E2 and T), semen quality parameters and OS parameters (TAC, MDA and NO) concentrations were skewed right and transformed to the natural log (ln) for statistical analyses. Pearson correlation coefficients were calculated to assess bivariate relationships between OS parameters concentrations, reproductive hormone levels and semen quality parameters. We then used multiple linear regression analysis to control for appropriate covariates, including age, body mass index (BMI), smoking status (current vs. former/never smoker), season of the year (winter vs. spring, summer or autumn), presence of varicocele (yes/no), cryptorchidism (yes/no) prolonged disease (yes/no), taken any medication (yes/no), recent fever (yes/no) and, in the case of the models for semen quality parameters, were also controlled for time of sample collection (min) and ejaculation abstinence period (hours). Time of blood drawn was also taken into account for reproductive hormone analyses. When inclusion of a potential covariate resulted in a change in the β-coefficient of <10%, the variable was not retained in final models. Statistical analyses were performed with the statistical package IBM SPSS 19.0 (IBM Corporation, Armonk, NY, USA). All tests were two-tailed and the level of statistical significance was set at 0.05.
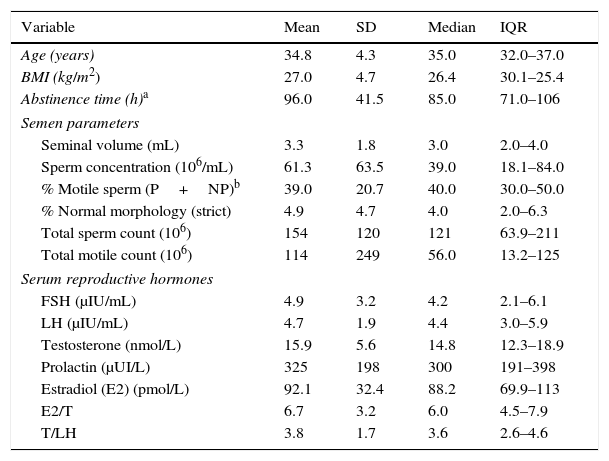
ResultsThe general characteristics of the study subjects including physical examination, sperm quality and serum reproductive hormone levels are presented in Table 1. Participants were (range) 25–38 years old [median age 34.8 with standard deviation (SD): 4.3], with a mean BMI of 27.0 (SD: 4.8)kg/m2, all Caucasian and 24% were smokers. Twenty-seven percent were found to have a varicocele. All participants presented serum reproductive hormone levels within the normal range, with a mean (SD) FSH, LH, T, E2 and PRL of 4.9 (3.2)IU/L, 4.7 (1.9)IU/L, 15.7 (5.6)nmol/L, 92.1pmol/L (32.4) and 325 (198)mUI/L, respectively. With regard to semen quality parameters, mean (SD) sperm concentration was 61.3 (63.5)×106/mL, and mean (SD) TSC was 154 (120)×106.
General description of participants.
| Variable | Mean | SD | Median | IQR |
|---|---|---|---|---|
| Age (years) | 34.8 | 4.3 | 35.0 | 32.0–37.0 |
| BMI (kg/m2) | 27.0 | 4.7 | 26.4 | 30.1–25.4 |
| Abstinence time (h)a | 96.0 | 41.5 | 85.0 | 71.0–106 |
| Semen parameters | ||||
| Seminal volume (mL) | 3.3 | 1.8 | 3.0 | 2.0–4.0 |
| Sperm concentration (106/mL) | 61.3 | 63.5 | 39.0 | 18.1–84.0 |
| % Motile sperm (P+NP)b | 39.0 | 20.7 | 40.0 | 30.0–50.0 |
| % Normal morphology (strict) | 4.9 | 4.7 | 4.0 | 2.0–6.3 |
| Total sperm count (106) | 154 | 120 | 121 | 63.9–211 |
| Total motile count (106) | 114 | 249 | 56.0 | 13.2–125 |
| Serum reproductive hormones | ||||
| FSH (μIU/mL) | 4.9 | 3.2 | 4.2 | 2.1–6.1 |
| LH (μIU/mL) | 4.7 | 1.9 | 4.4 | 3.0–5.9 |
| Testosterone (nmol/L) | 15.9 | 5.6 | 14.8 | 12.3–18.9 |
| Prolactin (μUI/L) | 325 | 198 | 300 | 191–398 |
| Estradiol (E2) (pmol/L) | 92.1 | 32.4 | 88.2 | 69.9–113 |
| E2/T | 6.7 | 3.2 | 6.0 | 4.5–7.9 |
| T/LH | 3.8 | 1.7 | 3.6 | 2.6–4.6 |
| Percent (%) | |
|---|---|
| Current smokers | 24.1 |
| Presence of varicocele | 27.0 |
| Have had | |
| Recent fever, ≥38°C at least 24h in the last 3 months | 4.4 |
| Cryptorchidismc | 3.9 |
| Taken any medicationd | 31.3 |
| Prolonged diseasee | 10.2 |
SD=standard deviation; IQR=interquartile range: 25th–75th percentile.
Ejaculation abstinence: period calculated as difference between time of current ejaculation and self-reported time of previous ejaculation.
Cryptorchidism [not born with both testicles in scrotum (irrespective of spontaneous descend, treatment or still cryptorchid)].
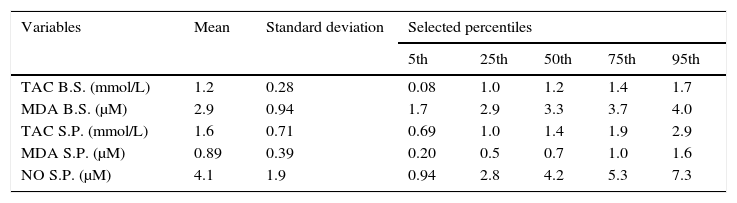
The seminal plasma and blood serum concentrations of OS biomarkers and TAC are shown in Table 2. With respect to the correlations between MDA and TAC concentrations in both body fluids, positive significant correlations were observed between TAC levels in blood serum and seminal plasma [(r)=0.27; 95%CI 0.09; 0.43] and MDA concentrations in blood serum and seminal plasma [(r)=0.25; 95%CI 0.07; 0.41]. Besides, a negative relationship between MDA and TAC levels in blood serum [(r)=−0.40; 95%CI −0.54;−0.23] was detected. No significant correlations between seminal plasma levels of NO and all the other OS parameters were found.
Summary statistics for the oxidative stress biomarkers.
| Variables | Mean | Standard deviation | Selected percentiles | ||||
|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||
| TAC B.S. (mmol/L) | 1.2 | 0.28 | 0.08 | 1.0 | 1.2 | 1.4 | 1.7 |
| MDA B.S. (μM) | 2.9 | 0.94 | 1.7 | 2.9 | 3.3 | 3.7 | 4.0 |
| TAC S.P. (mmol/L) | 1.6 | 0.71 | 0.69 | 1.0 | 1.4 | 1.9 | 2.9 |
| MDA S.P. (μM) | 0.89 | 0.39 | 0.20 | 0.5 | 0.7 | 1.0 | 1.6 |
| NO S.P. (μM) | 4.1 | 1.9 | 0.94 | 2.8 | 4.2 | 5.3 | 7.3 |
B.S.: blood serum; S.P.: seminal plasma.
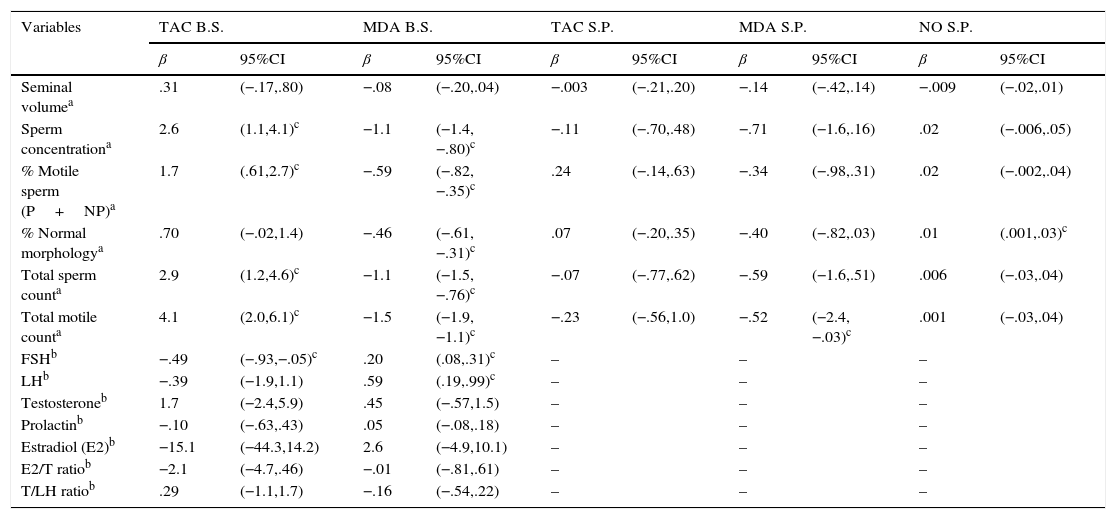
In simple pairwise correlations (bivariate analyses), without controlling for covariates, there was a significant inverse correlation between blood serum MDA concentrations and sperm parameters (except for seminal volume). Seminal plasma MDA concentrations were inversely correlated with TMC. Blood serum TAC levels were positively correlated with sperm concentration, motility, TSC and TMC. A positive correlation between seminal plasma levels of NO and percentage of normal morphology was found. With respect to serum reproductive hormones, blood serum MDA concentrations were positively correlated with FSH and LH levels, and blood serum TAC concentrations were inversely related to FSH levels. No other correlations were detected. Table 3 summarizes multivariate analysis for men's reproductive parameters and OS biomarkers concentrations. Even after taking into account important covariates (e.g. abstinence time, season, etc.) the results seen in the bivariate analysis remained.
Multivariate analysis for oxidative stress biomarker levels and men's reproductive parameters.
| Variables | TAC B.S. | MDA B.S. | TAC S.P. | MDA S.P. | NO S.P. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | β | 95%CI | |
| Seminal volumea | .31 | (−.17,.80) | −.08 | (−.20,.04) | −.003 | (−.21,.20) | −.14 | (−.42,.14) | −.009 | (−.02,.01) |
| Sperm concentrationa | 2.6 | (1.1,4.1)c | −1.1 | (−1.4,−.80)c | −.11 | (−.70,.48) | −.71 | (−1.6,.16) | .02 | (−.006,.05) |
| % Motile sperm (P+NP)a | 1.7 | (.61,2.7)c | −.59 | (−.82,−.35)c | .24 | (−.14,.63) | −.34 | (−.98,.31) | .02 | (−.002,.04) |
| % Normal morphologya | .70 | (−.02,1.4) | −.46 | (−.61,−.31)c | .07 | (−.20,.35) | −.40 | (−.82,.03) | .01 | (.001,.03)c |
| Total sperm counta | 2.9 | (1.2,4.6)c | −1.1 | (−1.5,−.76)c | −.07 | (−.77,.62) | −.59 | (−1.6,.51) | .006 | (−.03,.04) |
| Total motile counta | 4.1 | (2.0,6.1)c | −1.5 | (−1.9,−1.1)c | −.23 | (−.56,1.0) | −.52 | (−2.4,−.03)c | .001 | (−.03,.04) |
| FSHb | −.49 | (−.93,−.05)c | .20 | (.08,.31)c | – | – | – | |||
| LHb | −.39 | (−1.9,1.1) | .59 | (.19,.99)c | – | – | – | |||
| Testosteroneb | 1.7 | (−2.4,5.9) | .45 | (−.57,1.5) | – | – | – | |||
| Prolactinb | −.10 | (−.63,.43) | .05 | (−.08,.18) | – | – | – | |||
| Estradiol (E2)b | −15.1 | (−44.3,14.2) | 2.6 | (−4.9,10.1) | – | – | – | |||
| E2/T ratiob | −2.1 | (−4.7,.46) | −.01 | (−.81,.61) | – | – | – | |||
| T/LH ratiob | .29 | (−1.1,1.7) | −.16 | (−.54,.22) | – | – | – | |||
B.S.: blood serum; S.P.: seminal plasma. β-Coefficient and 95% confidence interval (95%CI).
The current study shows that high levels of oxidative stress biomarkers (serum MDA) are inversely associated with all semen parameters – except semen volume – and altered FSH and LH levels, in male partners of couples seeking infertility treatment in Southern Spain. Moreover, serum TAC concentrations were positively related to sperm count and motility, and inversely associated with FSH levels.
It has been proposed that blood OS determination is a useful tool to evaluate the sperm reproductive capacity and functional competence. Thus, in a study conducted in fertile and idiopathic infertile men significant differences in both groups as regard their OS, both in serum and seminal plasma fluids, were found.18 Previously, a study of infertile men had negatively correlated blood MDA concentrations with motility and percentage of normal morphology sperm.19 Similarly, in our current study we report that blood serum MDA concentration was associated with decreased semen quality. These results also suggest the possibility of using biomarkers of blood oxidative status for the evaluation of sperm reproductive capacity. In fact, seminal plasma MDA levels were inversely correlated with TMC, a relationship that has also been reported elsewhere.9 However, none of the other semen parameters were associated with seminal plasma levels of MDA, although a significant correlation between blood serum and semen plasma MDA concentrations was found.
We have also found a positive association between blood serum MDA concentration and FSH and LH levels. It could be hypothesized that OS created by subsets of immature spermatozoa during the maturation process might affect the production or action of protein hormones from Sertoli cells, such as inhibin B, which play a central role in spermatogenesis regulating gonadotrophin levels.20 However, we were not able to measure that hormone and, overall, in the current literature the associations between reproductive hormone concentrations and oxidative stress still remain inconclusive.
One of the mechanism for NO to induce sperm damage is inhibition of mitochondrial respiration, which leads to a lose of sperm motility.10 Several studies have reported negative associations between seminal plasma levels of NO and sperm parameters. Wu (2002) showed that there is a higher concentration of NO in seminal plasma of infertile than in fertile men,21 resulting in the inhibition of capacitation and sperm oocyte binding.11 Sperm motility seems to be one of the parameters most affected by high levels of NO, as reported elsewhere.10 However, several studies have demonstrated that NO, in physiological conditions, contributes to normal sperm function,22 and positive associations between NO concentrations and sperm quality has been shown.22 Moreover, NO also contributes to normal sperm morphology,23 as we have reported in our study, showing a positive relation between seminal plasma levels of NO and the percentage of morphologically normal sperm.
Total antioxidant capacity (TAC) is also a well-known tool for assessing the overall antioxidant status. When there is oxidative stress, free radicals are neutralized by antioxidants. Higher seminal plasma levels of TAC have been positively correlated with sperm concentration, motility and morphology.24 Several studies have also shown that seminal plasma TAC concentrations are lower in subfertile and infertile men than fertile men.2,12,13 Together with seminal OS assessment, blood antioxidant capacity has been proposed as a valuable tool to support sperm quality evaluation. For example, Shamsi and colleagues (2010) evaluated blood enzymatic antioxidants and found a positive correlation with sperm count.19 Similar to us, Benedetti and coworkers (2012) reported a positive correlation between blood TAC concentrations and sperm concentration, motility and morphology.18 In our study we found positive associations between blood serum TAC concentrations and sperm concentration, motility, TSC and TMC. Moreover, positive significant correlations were observed between TAC levels in blood serum and seminal plasma. Besides, an inverse relationship between TAC and MDA levels in blood serum were detected. This bear out the idea of using blood oxidative status as a predictive tool of sperm quality evaluation and the possibility to use dietary antioxidants to improve the clinical condition of infertile men. Besides, blood oxidative status might be useful in controlling the efficiency of antioxidant intake and its potential impact on male fertility.
However, it is not fully understood whether TAC is also under a systemic control, particularly by reproductive hormone levels. Like us, Meucci and colleagues (2003) reported that TAC was negatively correlated with serum FSH levels.25 Mancini and coworkers (2009), in a study conducted in infertile male with normal hormone levels, showed a direct correlation between seminal TAC concentrations and serum prolactin levels, suggesting that it may play a role in regulating antioxidant capacity.26 Nonetheless, other studies did not find correlations between TAC levels and any serum reproductive hormones. Appasamy et al. (2009) evaluated sperm DNA damage, TAC and serum hormone concentrations in men undergoing infertility evaluation and found no significant relationships, suggesting other mechanism for sperm dysfunction.20
Limitations of this study are common to observational studies. First, as is true for all cross-sectional designs, causal inference is limited. The sample size was relatively small, which may have limited our ability to detect subtle associations between OS parameters and hormone levels or semen quality parameters, but we found significant associations. In addition, only one semen and blood sample was collected to evaluate semen parameters and serum reproductive hormone levels, respectively. Nevertheless, several studies have shown that one sample is enough to assess semen quality in epidemiological studies27 and, equally, a single sample can be used to classify men's reproductive hormone.28
In conclusion, our results suggest that oxidative stress may be associated with semen parameters and reproductive hormone levels in male partners of couples seeking for infertility treatment. However, further studies are warranted to confirm and extent these findings, in particular, with regard to serum reproductive hormones.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article and have obtained the written informed consent of the patients or subjects mentioned in this article.
FundingThis work was supported by The Ministry of Economy and Competitiveness, ISCIII (FIS), grant no. PI10/00985 and The Seneca Foundation, Regional Agency of Science and Technology, grant no. 08808/PI/08.
Conflict of interestsThe authors declare no conflict of interest.