It has been reported in the literature that proinflammatory interleukin-1 beta (IL-1β) is increased in cases of testicular ischemia reperfusion (I/R) damage. This information suggests that anakinra, an IL-1β antagonist, may be effective in testicular I/R damage.
ObjectiveIn our study, we investigated the effect of anakinra on testicular I/R damage induced in rats with torsion/detorsion.
MethodsThe 50mg/kg anakinra+testicular torsion/detorsion (KTD-50) and 100mg/kg anakinra+testicular torsion/detorsion (KTD-100) groups received an intraperitoneal (i.p.) injection of 50mg/kg and 100mg/kg of anakinra, respectively. In turn, the testicular torsion/detorsion (TTD) and sham operation (SOG) groups received a single dose of distilled water as a solvent 1h before ketamine anaesthesia. After the testes of the TTD, KTD-50 and KTD-100 groups were subjected to torsion and detorsion for 4h each, the rats were killed with a high-dose anaesthesia, and their testicles were removed and evaluated through biochemical, gene expression and histopathological examinations. The results were evaluated in comparison with those of the SOG group.
ResultsThe levels of malondialdehyde (MDA), myeloperoxidase (MPO) and IL-1β showed significant increases in the TTD group, which underwent torsion/detorsion, compared to the KTD-50, KTD-100 and SOG groups. Conversely, the levels of glutathione (tGSH), glutathione peroxidase (GPO) and glutathione s-transferase (GST) were found to be significantly higher in the KTD-50, KTD-100 and SOG groups than in the TTD group.
ConclusionAnakinra at a 100mg/kg dose histologically suppressed better oxidative stress and tunica albuginea, germ cell, seminiferous tubule and interstitial damage in the testicular tissue compared to a 50mg/kg dose. Experimental results indicate that anakinra might be beneficial in the attenuation of testicular I/R damage.
Se ha reportado en la literatura que citoquinas interleuquina-1 beta (IL-1β) es mayor en el daño de la isquemia reperfusión testicular (I/R). Esta información sugiere que la anakinra, que es un antagonista IL-1β puede ser eficaz en daño testicular I/R.
ObjetivoEn nuestro estudio se investigó el efecto de este medicamento en daño testicular I/R inducida en ratas con detorsion/torsión.
MétodosKTD-50 grupo recibido intraperitonealmente (i.p.) inyección de 50mg/kg y KTD-100 Grupo 100mg/kg de anakinra, mientras TTD (control) y SOG (sham grupo operación) recibieron una dosis única de agua destilada como solvente, una hora antes de ketamina anestesia. Después de que los testículos de TTD, KTD-50 y KTD-100 grupos fueron sometidas a torsión y detorsion para cuatro por cuatro horas, las ratas fueron asesinados con altas dosis de anestesia, sus testículos fueron extraídos y evaluados a través de la expresión génica, bioquímicas e histopatológicas de exámenes. Los resultados fueron evaluado en comparación con la de SCG grupo.
ResultadosLos niveles de MDA, MPO y IL- 1β mostraron incrementos significativos en el grupo TTD/torsión detorsion administrados frente a-50, KTD KTD-100 y SOG grupos. Por el contrario, los niveles de tGSH, GPO y GST resultaron significativamente más altas en KTD-50 KTD-100 y grupos SOG de TTD en grupo.
ConclusiónLa anakinra en 100mg/kg dosis mejor histológicamente suprime el estrés oxidativo y la túnica albuginea, células germinales, túbulos seminíferos apretadamente enrollados intersticial y daño en el tejido testicular en comparación con la dosis de 50mg/kg. Los resultados experimentales indican que la anakinra puede ser beneficiosa en la atenuación de los daños I/R testicular.
Testicular ischemia reperfusion (I/R) damage occurs as a result of the detorsion of torsioned testes. Despite early surgical intervention to recover the ischemic testicle, only 32% of testicles can be recovered successfully.1 This is because while testicular torsion leads to ischemic damage, detorsion results in reperfusion damage.2 Studies have shown that the damage detected in testicular biopsies following reperfusion is more severe than the damage in biopsies following ischemia alone.3 Despite the fact that even a single healthy testicle does not pose a problem for fertility, unilateral testicular torsion leads to infertility in 25% of cases, and the testicular damage, which may be seen at any age, increases as the degree and duration of the torsion increase.4 Free oxygen radicals are blamed for this I/R damage in the tissues.5 On the other hand, proinflammatory cytokines, such as interleukin one beta (IL-1β), are reported to increase following testicular I/R damage.6 Recent studies proposed that I/R damage is a complex pathological process beginning with the tissues becoming deprived of oxygen, progressing to a change in the oxidant/antioxidant balance in favor of oxidants and expanding with the inflammatory response.7 Therefore, it is thought that drugs showing antioxidant and anti-inflammatory impacts that antagonise especially the effect of IL-1β might be beneficial in testicular I/R damage. Anakinra will be investigated in the present study to determine whether it has a protective effect against testicular I/R damage. It is the first biological agent approved to block the proinflammatory effects of IL-1β in patients with rheumatoid arthritis.8 Anakinra has been reported to improve inflammatory symptoms rapidly.9
Hasturk et al. reported that the amount of malondialdehyde (MDA), which is a product of lipid peroxidation, was increased and the activities of enzymatic antioxidants were decreased in spinal cord damage experimentally induced in animals; in addition, the authors stated that anakinra prevented the increase of MDA and decrease of enzymatic antioxidants in the spinal cord.10 Another recent study reported that anakinra inhibited an increase in the IL-1β level following brain trauma, protecting the brain tissue against oxidative damage.11 Again, the protective effects of anakinra have been emphasised in renal I/R damage.12 However, no information was found in the literature regarding whether anakinra has a protective effect against testicular I/R damage. Therefore, the objective of this study is to investigate the effect of anakinra on testicular I/R damage induced in rats with torsion/detorsion through biochemical, gene expression and histopathological examinations.
MethodsAnimalsThis study included 40 Wistar albino male rats in total, weighing between 215 and 225g (6 weeks) that were supplied from Atatürk University Medical Experimental Application and Research Center. The animals were housed and fed as groups at normal room temperature (22°C) before the experiment. Our studies were conducted in accordance with ethical rules, and all stages were approved by the Ataturk University Local Ethics Committee for Animal Experiments (AUHADYEK) in document No. 75296309-050.01.04-E.1500124344 dated 27.11.2015 and by the document of Ataturk University.
Chemical agentsThe ketamine used in the experiment was supplied from the Pfizer pharmaceutical company (Turkey), the anakinra (Kineret) was supplied from Sobi (Sweden) and the xylazine (Rompun) was supplied from Bayer (Istanbul-Turkey).
Animal groupsThe experimental animals were divided into four groups: the testicular torsion/detorsion (TTD), 50mg/kg anakinra+testicular torsion/detorsion (KTD-50), 100mg/kg anakinra+testicular torsion/detorsion (KTD-100) and sham operation (SOG) groups.
Experimental procedureThe KTD-50 (n=10) and KTD-100 (n=10) groups received an intraperitoneal (i.p.) injection of 50mg/kg and 100mg/kg of anakinra, respectively, 1h before anaesthesia. Alternatively, the TTD (n=10) and SOG (n=10) rat groups were given distilled water by the same method. The surgical interventions were performed in a proper laboratory setting under sterile conditions with 50mg/kg i.p. ketamine anaesthesia, where animals were given a sniff of xylazine with adequate intervals. TTD, KTD-50, KTD-100 and SOG group animals’ scrotum area was cleaned with 10% povidone iodine solution. The scrotal space was reached by applying a vertical incision of two cm in length on the scrotum (on the midline) of the subjects. Then the right testicle in the scrotal space was taken out. Alternatively, the testicles of the SOG group were inserted into the scrotum again without being subjected to any procedure. The testes of the animals in the TTD, KTD-50 and KTD-100 groups were torsioned 720 degrees for 4h. At the end of this period, the testicles were detorsioned to provide perfusion for 4h again. All the rat groups were then killed with a high-dose anaesthesia and their testicles were removed. Biochemical, gene expression and histopathologic examinations were performed on the removed testicular tissues. The results obtained from the KTD-50, KTD-100 and TTD groups were evaluated in comparison with the results from the SOG group.
Biochemical proceduresPreparation of the samplesTissue weighing 0.2g was removed from each testicular tissue. pH=6 potassium phosphate buffer containing 0.5% HDTMAB (0.5% hegza dodecyltrimethyl ammonium bromide) for MPO assay in tissues, 1.15% potassium chloride solution for MDA and pH=7.5 phosphate buffer for tGSH were made up to 2mL and homogenised in ice. These were than centrifuged at 10,000rpm for 15min at +4°C, and the supernatant obtained was used as the analysis specimen.
Malondialdehyde (MDA) analysisThe MDA measurement was based on the method described by Ohkawa et al.13 This method is based on the spectrophotometric measurement of the absorbance of the pink colour complex formed by thiobarbituric acid (TBA) and MDA at a high temperature (95°C) and a 532nm wavelength. The amount of red colour formed was read at 532nm using 3-ml cuvettes, and the MDA amounts of the samples were determined utilising the standard chart, which was created using a prepared MDA stock solution, taking into account dilution coefficients.
Determination of myeloperoxidase (MPO) activityA pH=6 potassium phosphate buffer containing 0.5% HDTMAB (0.5% Hexa-Dexyl-Tri-Methyl-Ammonium Bromide) was prepared to determine the MPO level in the testicular tissue homogenates. The oxidation reaction with MPO mediated the H2O2, involving 4-amino antipyrine/phenol as a substrate to determine MPO activity.14
Total glutathione (tGSH) analysisA total glutathione (tGSH) analysis was conducted according to the method described by Sedlak and Lindsay.15 In this method, sulfhydryl groups of GSH form a yellow colour TNB (5-thio-2-nitrobenzoic acid) following a chemical reaction with DTNB (5,5′-dithiobis [2-nitrobenzoic acid]). The intensity of this colour is measured spectrophotometrically at 412nm.
Glutathione peroxidase assayGlutathione peroxidase (GPO) activity was determined using the method described by Lawrence and Burk.16 After tissue homogenisation, the supernatant was used for the GPO measurement. The mixture was incubated after adding monopotassium phosphate, EDTA, GSH, beta-NADPH, sodium azide and GRx. As soon as hydrogen peroxide (H2O2) was added, the chronometer was turned on, and the absorbance at 340nm was recorded every 15s for 5min.
Glutathione s-transferase activityGlutathione s-transferase (GST) activity was determined by Habig and Jakoby.17 Briefly, the enzyme's activity was assayed spectrophotometrically at 340nm in a 4-ml cuvette containing 0.1M PBS (pH 6.5), 30mM GSH, 30mM 1-chloro-2,6-dinitrobenzene and a tissue homogenate.
RNA extractionFirst, 200.0μL of the extract obtained from the fragmented tissue was placed in a MagNA Pure Compact automatic RNA isolation device (Roche). Then, a 50.0μL RNA sample was obtained through RNA isolation using the MagNA Pure Compact RNA isolation kit (Roche).
Stage of cDNA synthesisThe concentration of the RNA obtained was measured. Based on the measured RNA concentration, the RNA was either diluted or undiluted to yield 15–20ng of cDNA. Then, 10.0μL of each calibrated sample, 2.0μL of a random primer and 1.0μL of distilled water from the Transcriptor First Strand cDNA Synthesis Kit (tube No. 6) were transferred into the 0.2-PCR tube. Denaturation was then conducted in the reverse-transcription PCR instrument at 65°C for 10min. The mixture was added to the denatured RNA to form cDNA. The quantities of the substances included in the mixture used for each sample were as follows (from the Transcriptor First Strand cDNA synthesis kit): 4.0μL of the reaction buffer (No. 2), 5.0μL of RNAase (No. 3), 2.0μL of the deoxynucleotide mix (No. 4) and 0.5μL of the reverse transcriptase (No. 1). After the addition of 7.0μL of the mixture to the denatured RNA, the tube was placed in the reverse-transcription PCR instrument and subjected to an appropriate PCR program.
Determination of the IL-1β gene expressionFor each cDNA sample, gene expression of IL-1β was analysed using the Roche LightCycler 480 II Real-Time PCR instrument (Mannheim-Germany). PCR reactions were in a final volume of 20μL: 5μL cDNA, 3μL distilled water, 10μL LightCycler 480 Probes Master (Roche Diagnostics) and 2μL primerprobe set (Real-Time Ready single assay-Roche). Cycle conditions of the relative quantitative PCR (qPCR) were preincubation at 95°C for 10min, followed by 45 amplification cycles of 95°C for 10s, 6°C for 30s, 72°C for 1s, followed by cooling at 40°C for 30s. qPCR analysis and calculation of quantification cycle (Cq) values for Relative Quantification were performed with the LightCycler 480 Software, Version 1.5 (Roche Diagnostics). Relative quantitative amounts were calculated by dividing the target genes by the expression level of the reference gene. Reference gene was used for normalisation of target gene expression.
Histopathological analysisTesticular tissues obtained from the rats were fixed in a 10% formalin solution for 24h. After routine tissue processing, 4-μm thick sections were cut from the paraffin blocks and stained with Hematoxylin and Eosin (H&E). All sections were examined under an optic microscope (Olympus BX 52, Tokyo, Japan) by a pathologist who was unaware of the treatment protocols under optic microscope.
Statistical analysisThe results obtained from the experiments are expressed as mean±standard deviation (x±SD). The significance of the difference between the groups was determined using a one-way analysis of variance (ANOVA) test followed by Fisher's post hoc least significant differences (LSD) test. All statistical analyses were performed with the SPSS Version 18 statistical software, and p values<0.05 were considered significant.
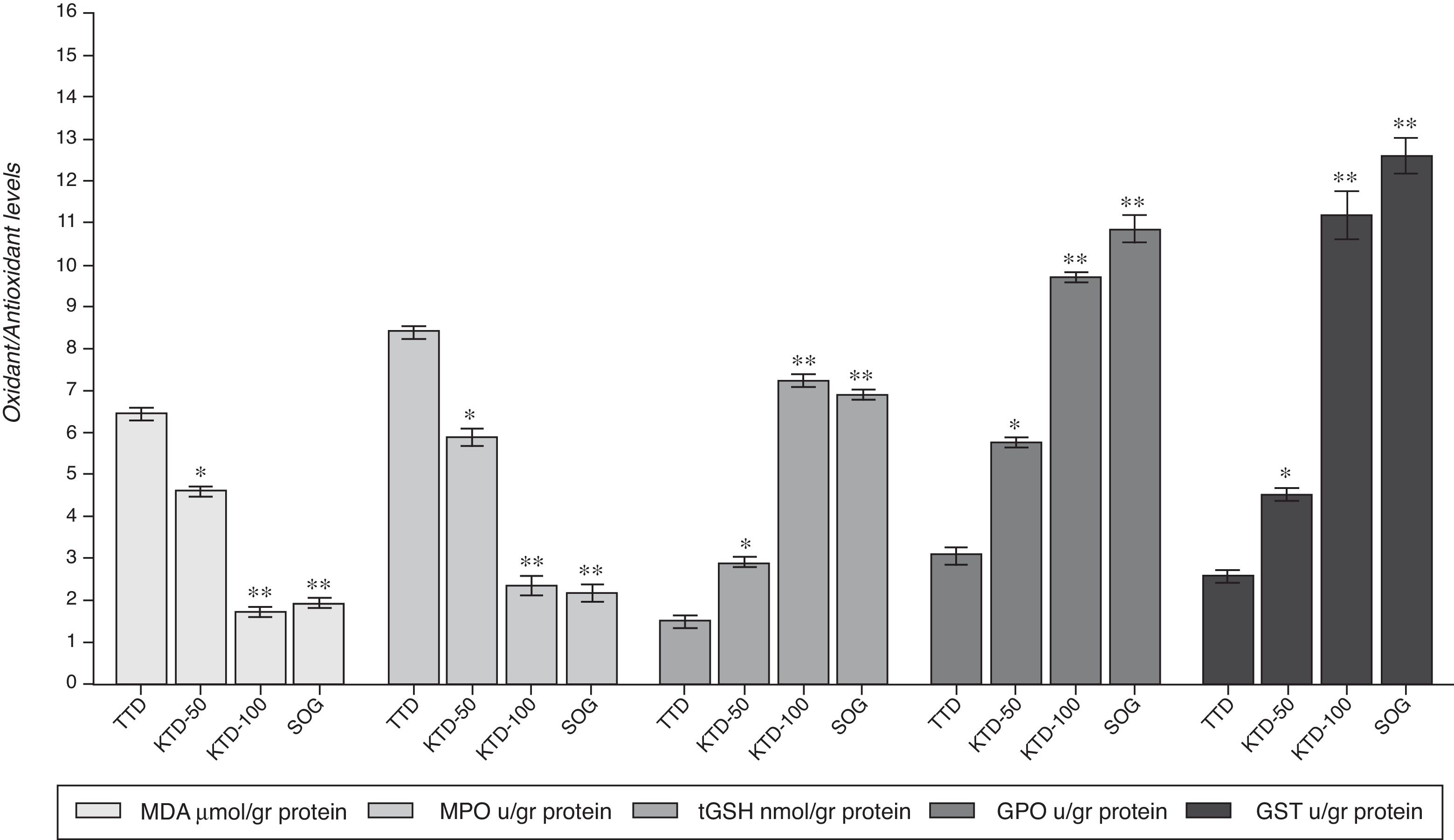
ResultsBiochemical resultsAs shown in Fig. 1, the mean amount of MDA was 6.6±0.4μmol/g protein in the testicular tissue of the TTD animal group subjected to torsion/detorsion alone. The amount of MDA was measured as 4.6±0.3μmol/g protein (p<0.05) and 1.8±0.2μmol/g protein (p<0.0001) in the KTD-50 and KTD-100 groups given anakinra, respectively, and 1.6±0.2μmol/g protein (p<0.0001) in the SOG group.
The torsion/detorsion process also significantly increased the MPO activity in the testicular tissue. The MPO activity was elevated to 8.4±0.5μmol/g protein in the TTD group and decreased to 5.9±0.3μmol/g protein (p<0.05), 2.3±0.6μmol/g protein (p<0.0001) and 2.2±0.2μmol/g protein (p<0.0001) u/ml in the KTD-50, KTD-100 and SOG groups, respectively (Fig. 1).
The torsion/detorsion event caused a decrease in the tGSH amount in the testicular tissue. The mean amount of tGSH, which is an endogenous antioxidant parameter, was found to be 1.5±0.5nmol/g protein in the TTD group. Alternatively, tGSH was measured at 2.9±0.4nmol/g protein, 7.2±0.4nmol/g protein (p<0.0001) and 6.9±1.5nmol/g protein (p<0.0001) in the testicular tissues of the rats in the KTD-50, KTD-100 and SOG groups (Fig. 1).
The torsion/detorsion process caused a decrease in the GPO activity in the testicular tissue as well. The lowest GPO activity was found in the TTD group at 3.1±0.6μmol/g protein, and the highest GPO activity was found in the SOG group at 10.8±1.7 (p<0.0001)μmol/g protein. These values were found to be 5.8±1.1 (p<0.05)μmol/g protein and 9.7±0.3 (p<0.0001)μmol/g protein in the KTD-50 and KTD-100 groups, respectively (Fig. 1).
Another parameter with decreased activity in the testicular tissue subjected to torsion/detorsion was GST. The GST activity was found to be 2.6±0.3μmol/g protein in the TTD group, while it was measured as 4.5±0.5 (p<0.05)μmol/g protein, 11.2±1.8 (p<0.0001)μmol/g protein and 12.6±1.4 (p<0.0001)μmol/g protein in the KTD-50, KTD-100 and SOG groups, respectively (Fig. 1).
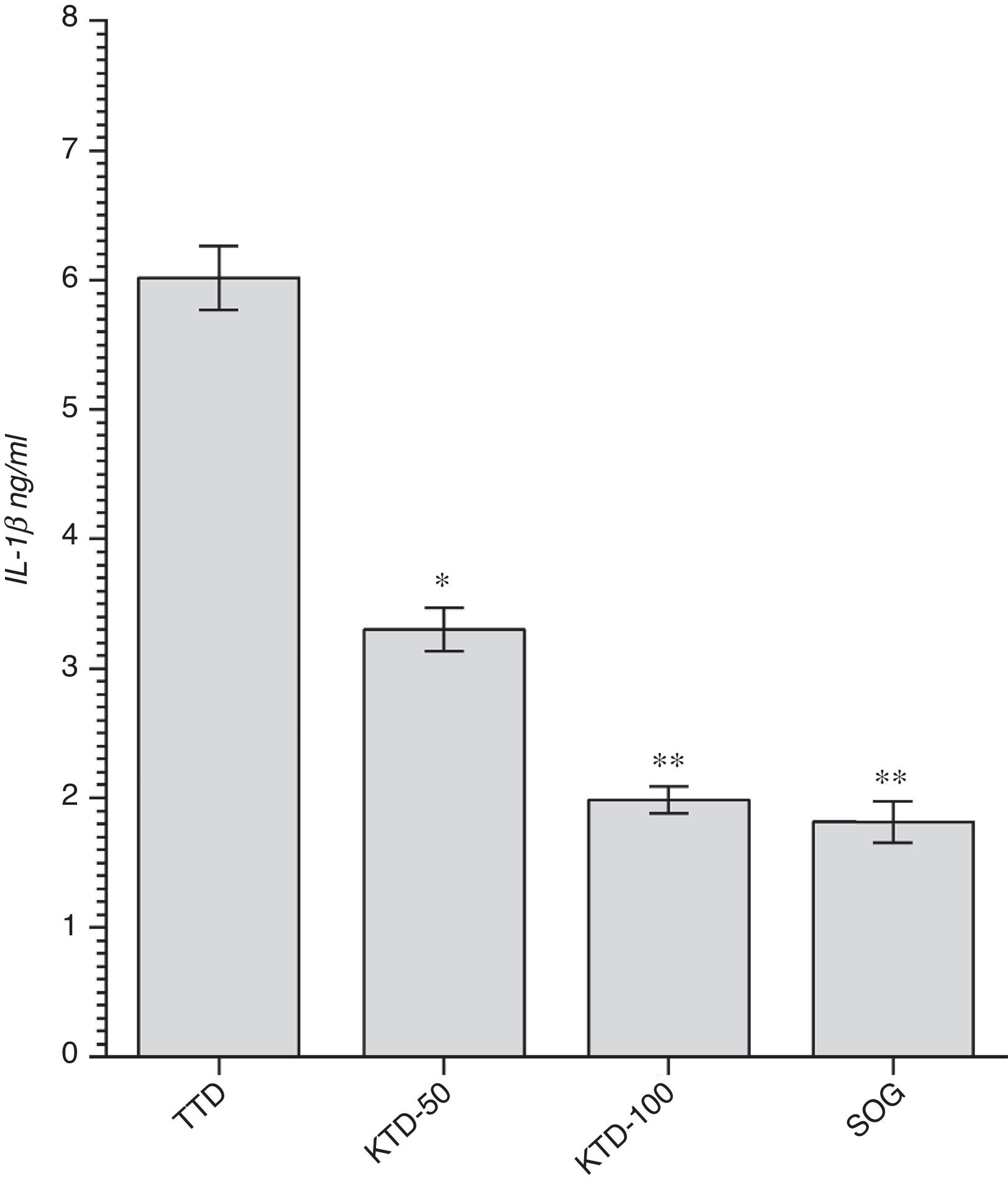
Gene expression resultsThe expression of IL-1β, which is known as a proinflammatory cytokine, was significantly increased in the testicular tissue subjected to detorsion following the torsion process. The mean expressions of IL-1β were found to be 6.0±0.8ng/ml, 3.3±0.7ng/ml (p<0.05), 2.0±0.4ng/ml (p<0.0001) and 1.8±0.4ng/ml (p<0.0001) in the TTD, KTD-50, KTD-100 and SOG groups, respectively (Fig. 2).
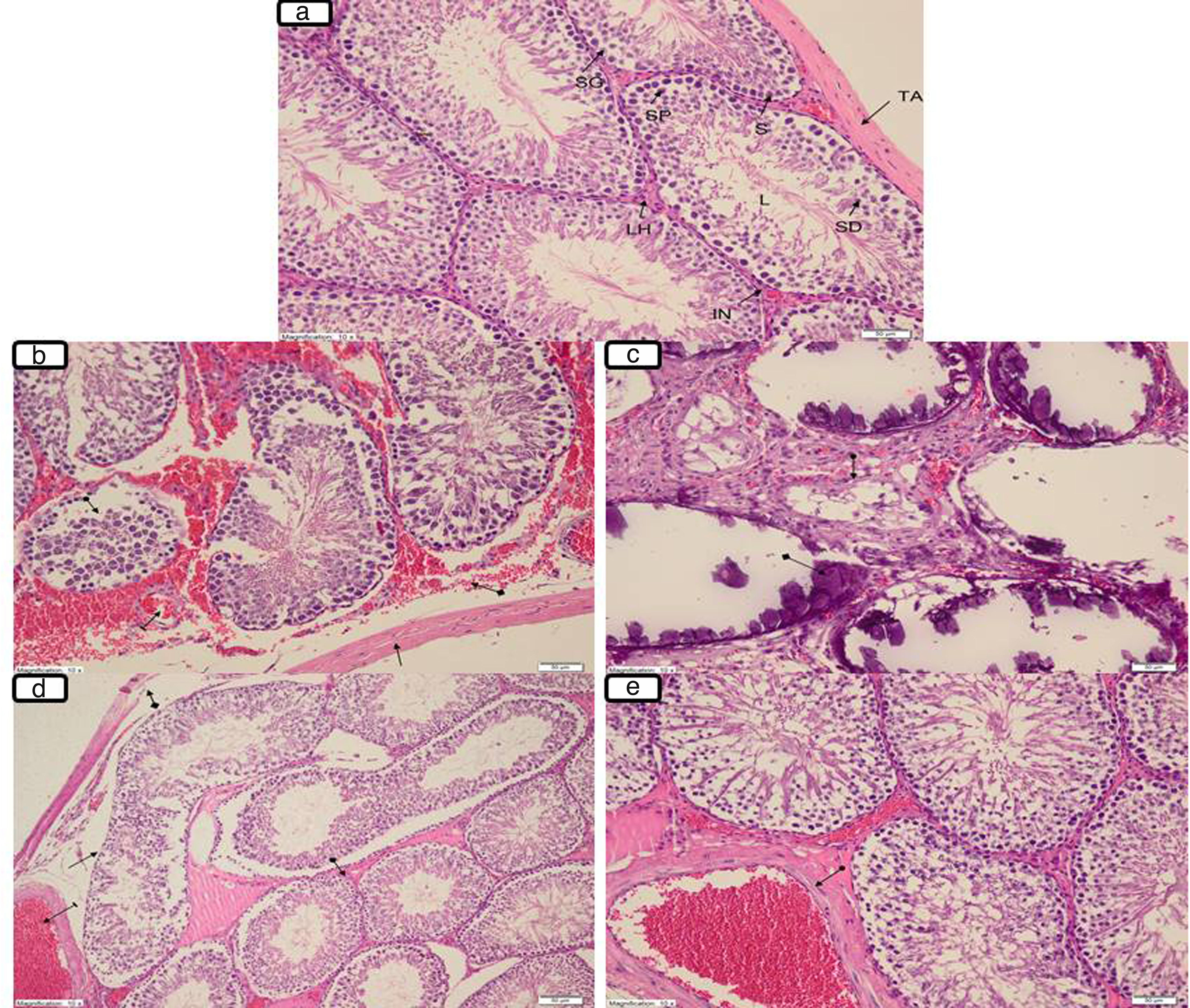
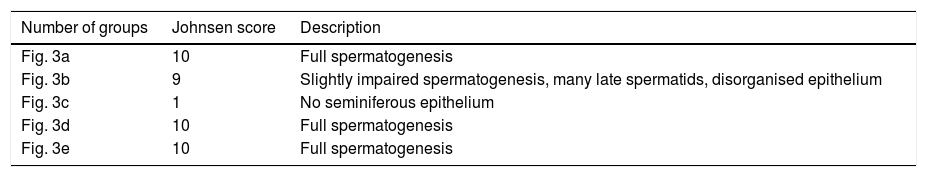
Histopathological resultsFig. 3a illustrates tunica albuginea, Leydig cells, interstitium lumen, Sertoli cells, spermatogonium, primary spermatocyte and spermatid in the testicular tissue of the SOG group (Johnsen score: 10; Multiple germinal epithelium, a large number of spermatozoa, Table 1). However, there was degeneration in the tunica albuginea (arrow), diffuse interstitial area damage involving haemorrhage and oedema (square arrow), dilated congested blood vessels (line arrow) and seminiferous tubule structures containing disorganised germ cells (circle arrow) (Johnsen score: 9; Disorganised germinal epithelium, lumen accumulation and spermatozoa present, Table 1) in the TTD group, which was subjected to the torsion/detorsion process (Fig. 3b). Again, destructive seminiferous (circle arrow) tubular structures involving dystrophic (square arrow) calcifications secondary to necrosis and necrotic destructed seminiferous tubules were monitored in the testicular tissue of this groups (Johnsen score: 1; there is no cell in the seminiferous tubule, Table 1) (Fig. 3c). Mild degeneration and a better protected tunica albuginea (square arrow), dilated congested oedema in the interstitium, dilated congested blood vessels (line arrow), mildly disorganised germ cells in the focal areas, irregular seminiferous tubular structures (arrow) and close to normal interstitium tissue (circle arrow) involving Leydig cells in most areas were observed in the KTD-50 group, which was given 50mg/kg anakinra (Johnsen score: 10; Multiple germinal epithelium, a large number of spermatozoa, Table 1) (Fig. 3d). Anakinra at 100mg/kg better protected the testicular tissue. Only the KTD-100 group exhibited dilated congested vessels (circle arrow; Johnsen score: 10; Multiple germinal epithelium, a large number of spermatozoa, Table 1) (Fig. 3e).
(a) Normal testicular histology of SOG (sham operation group); TA: Tunica albuginea, LH: Leydig cell, IN: Interstitium, L: Lumen, S: Sertoli cell, SG: Spermatogonium, SP: Primer spermatocid, SD: Spermatid. (b) The degeneration and haemorrhage at tunica albuginea (arrow) and the interstitial area with oedema (square arrow), congestion dilated blood vessels (line arrow), and seminiferous tubule structures containing disorganised germ cells (circle arrow) in the TTD group. (c) The necrotic destructed seminiferous tubules (circle arrow) and destructive seminiferous tubule structures containing dystrophic calcifications secondary to necrosis (square arrow) in the TTD group. (d) The tunica albuginea containing mild degeneration (square arrow), interstitial oedema and congestion of dilated blood vessels (line arrow), mildly disorganised germ cells in the focal areas and an irregular structure of the seminiferous tubules (arrow) and similar to interstitium tissue (circle arrow) including Leydig cells in many areas in the KTD-50 group. (e) The dilated vascular congestion (circle arrow) in the KTD-100 group (magnification 10×, H&E 200).
In this study, the effect of anakinra on I/R damage induced in the testes of rats with torsion/detorsion was investigated through biochemical, gene expression and histopathological examinations. The biochemical results indicate that the levels of oxidant parameters, such as MDA and MPO, were increased and the levels of antioxidant parameters, such as tGSH, GPO and GST, were decreased in the testicular tissue of the TTD group compared to that of the SOG group. This demonstrates that the oxidant/antioxidant balance was changed in favor of oxidants in the tissue subjected to torsion/detorsion. This event is known as oxidative stress in the literature.18,19 Studies propose that free oxygen radicals are responsible for the tissue I/R damage.5 Furthermore, MDA is reported to be a reliable indicator of lipid peroxidation caused by free oxygen radicals.20 Recent studies have reported that the torsion/detorsion process causes an increase in the amount of MDA in the testicular tissue.21
Again, in the present study, MPO activity was found to be higher in the testicular tissue of the TTD group. As mentioned above, oxidative I/R damage expands with inflammation.7 An indicator of inflammation, MPO both causes the production of toxic radicals, such as O2−, H2O2 and OH, and converts H2O2 to hypochlorous acid radical (HOCl), a potent oxidant, in the presence of the Cl ion.22,23 Sılay et al. noted an increase in the MPO activity in the damaged testicular tissue of the animals subjected to I/R.24 In our study, the MPO activity was found to be higher in the TTD group compared to the SOG group, indicating our results are consistent with the literature. The level of tGSH was found to have decreased in direct proportion to the increases in MDA and MPO in the testicular tissue subjected to the torsion/detorsion process. Working to protect against oxidative stress, tGSH is known as an important endogenous antioxidant in the body. Yıldız et al. reported that the amount of tGSH decreased in the testicular tissue, while the amount of MDA observed increased.25,26 The torsion/detorsion process caused a decrease in GPO activity in the testicular tissue, as well. GPO is a cytosolic antioxidant enzyme responsible for the reduction of hydroperoxydes and the protection of cells from oxidative stress.27 Ozbek et al. demonstrated a significant decrease in GPO activity following testicular I/R damage.28 In our study, besides GPO, GST activity also decreased. No studies were found in the literature associating testicular I/R damage with GST activity. However, GST is known as an antioxidant enzyme that inactivates oxidants.29 This information obtained indicates that the oxidant/antioxidant balance was changed in favor of oxidants in the testicular tissue of the TTD group.
The expression of IL-1β showed a significant increase in the testicular tissue of the TTD group. Shih et al. reported that besides oxidant status, the torsion/detorsion process also increases the level of IL-1β, which is an inflammation index.30 IL-1β is known to have numerous functions, including an oxidative burst of neutrophils and release of free radicals.31,32 IL-1β has been proposed to exert this effect on neutrophils via stress-dependent kinases.33,34 This suggests that I/R damage may be alleviated with antioxidant therapy. As seen from our experimental results, anakinra changed the oxidant/antioxidant balance in favor of antioxidants in the testicular tissue subjected to torsion/detorsion in a dose-dependent manner. No studies were found in the literature regarding the effect of anakinra on testicular damage induced by torsion/detorsion. However, some studies demonstrated that anakinra decreases the amount of MDA and increases the activities of enzymatic antioxidants in the spinal cord tissue with damage induced.10 In recent studies, anakinra has been reported to suppress the expression of IL-1β in tissues with increased antioxidants.11 Furthermore, the IL-1β antagonist has been demonstrated to have potential therapeutic value in the treatment of I/R damage.35 Histopathologically, degeneration in the tunica albuginea, diffuse interstitial area damage involving haemorrhage and oedema, dilated congested blood vessels, seminiferous tubules containing disorganised germ cells and necrotic destructed seminiferous tubule structures were observed in the testicular tissue of the TTD group with higher oxidant and lower antioxidant parameters. In addition, the histopathological findings were milder in the KTD-100 group with a more significantly changed oxidant/antioxidant balance in favor of antioxidants and a more significantly suppressed expression of IL-1β compared to the KTD-50 group. Degeneration in the tunica albuginea observed in the TTD group is among the pathological findings of the testicular tissue.36 In their study, Tuglu et al. demonstrated the interstitial areas involving haemorrhage and oedema histopathologically in the testicular tissue subjected to I/R.37 Again, it was reported that I/R may lead to the occurrence and necrosis of seminiferous tubules containing disorganised germ cells in the testicular tissue.38 Aldemir et al. observed such histopathological findings as seminiferous tubule oedema, congestion and haemorrhage in the testicular tissue subjected to I/R.39 This information indicates that our histopathological findings are consistent with the literature. In conclusion, the torsion/detorsion process created oxidative stress in the testicular tissue. Anakinra at a dose of 100mg/kg better prevented testicular damage caused by torsion/detorsion compared to a dose of 50mg/kg. The experimental results indicated that anakinra might be beneficial in clinical practice in attenuation of the damage that may occur during the reperfusion of torsioned testicles.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestsThe authors have no conflict of interests.