The aim of this study was to evaluate polymorphisms of sperm protamine genes and their effects on the result of CMA3 staining in varicocele men.
Material and methodsIn a case control study, 128 patients with male infertility due to varicocele and 128 controls were recruited. Polymorphisms of PRM1 and PRM2 genes in extracted DNA samples were assessed by PCR-SSCP and sequencing. Protamine deficiency was also indirectly estimated by CMA3 staining.
ResultNine different variants including six variants in PRM1 gene and three variants in PRM2 gene were found among varicocele patients. The results showed that sperm count, motility and morphology were significantly different between control group without gene variations and varicocele group who had several variations in their protamine genes (P<0.05).
ConclusionTherefore, PRM1 and PRM2 variations in varicocele patients are associated with the production of spermatozoa with more protamine deficiency and this is one of the possible causes of infertility due to varicocele.
El objetivo de este estudio fue evaluar los polimorfismos de los genes de la protamina espermática y sus efectos sobre el resultado de la tinción CMA3 en varones con varicocele.
Material y métodosEn un estudio de casos y controles, se reclutó a 128 varones con infertilidad por varicocele y 128 controles. Los polimorfismos de los genes PRM1 y PRM2 en muestras de ADN extraídas se evaluaron mediante PCR-SSCP y se realizó su secuenciación. La deficiencia de protamina también se estimó indirectamente mediante tinción CMA3.
ResultadoSe encontraron 9 variantes diferentes, incluidas 6 variantes en el gen PRM1 y 3 variantes en el gen PRM2 entre los pacientes con varicocele. Los resultados mostraron que el recuento de espermatozoides, la movilidad y la morfología eran considerablemente diferentes entre el grupo de control sin variaciones genéticas y el grupo de varicocele que presentaba algunas variaciones en sus genes de protamina (p<0,05).
ConclusiónPor tanto, las variaciones de PRM1 y PRM2 en los pacientes con varicocele están asociadas con la producción de espermatozoides con más deficiencia de protamina y esta es una de las posibles causas de infertilidad debida al varicocele.
Protamine is the most common protein in sperm nucleus and plays an important role in chromatin condensation and decondensation, paternal genome protection and hydrodynamic position of sperm nucleus.1 Accurate histone/protamine replacement is the main step of sperm nucleus DNA packaging and it is shown that any protamine alteration may cause idiopathic male infertility.2,3 It is also confirmed in non-human species.
Alterations in sperm protamine-like protein in reaction to environmental stress was reported in invertebrates4 as well as relationship between sperm protamine content and diminished fertility in bulls.5 Our previous studies confirmed that the rate of spermatozoa with protamine insufficiency is significantly higher among men with varicocele,6 spinal cord injury7 and alcohol consumer animals.8 Protamine deficiency is also associated with abnormal sperm penetration,9 sperm DNA fragmentation10 and subsequent embryo development.11 Furthermore, chromatin condensation and decondensation in sperm nucleus depends on protamine 1 (P1) and protamine 2 (P2) which are coded by PRM1 and PRM2 genes located on chromosome 16.1 It is reported that protamine deficiency may be due to mutations and different polymorphisms of protamine genes.12,13
On the other hand, varicocele, an abnormal dilation of the spermatic veins, which occur in 40% of subfertile men14,15 cause sperm DNA damage and less chromatin condensation.16 In our previous study, we showed that sperm protamine deficiency assessed by chromomycin A3 (CMA3) was significantly higher in varicocele patients than fertile men.6 Also, it has been reported that sperm protamine-1/2 mRNA ratio and DNA fragmentation index are associated with male infertility due to varicocele and the post-varicocelectomy protamine-1/2 mRNA ratio and DNA fragmentation index are positively correlated to the post-varicocelectomy pregnancy rate.17
With the hypothesis that variations in PRM1 and PRM2 genes may cause protamine deficiency in varicocele men, we investigate the effect of protamine genes polymorphisms on protamine defects in infertile men with varicocele.
Materials and methodsPatientsA total of 128 males with varicocele grade I, II and III attending the Research and Clinical Center for Infertility were included in this study. The varicocele diagnosis was approved by examination or through scrotal Doppler ultrasound (Ultrasonix Medical Corporation, Richmond, Canada). A population of fertile donors (n=128) with normal spermogram who had fathered within the last 12 months were considered as control group. The consent form was signed by all patients.
Semen analysisSemen samples were collected by masturbation after 2–4 days of abstinence and carried to the andrology laboratory. For each sample, routine semen analysis and Diff quick staining was performed for sperm morphology evaluation according to WHO criteria (World Health Organization, 2010).18 Azoospermic samples, patients with Diabetes and alcohol consumers were excluded from the study. All semen analyses were performed by one expert technician blinded to the study.
Sperm protamine evaluationChromomycin A3 (CMA3) staining is a fluorochrome specific for guanosine cytosine-rich sequence, which applied to evaluate the degree of mature spermatozoa protamination.6 For this purpose, smears were dried prior to fixation in Carnoys solution (methanol/glacial acetic acid, 3:1) at 4°C for 10min. Each slide was then conserved with 100μl of CMA3 (Sigma, St Louis, MO, USA) (0.25mgml−1 in McIlvain buffer; 7ml citric acid, 0.1M+32.9ml Na2HPO4·7H2O, 0.2M, pH 7.0 containing 10mm MgCl2) for 20min. After that, the slides were washed 3 times in McIlvain buffer and mounted with buffered glycerol (1:1). Bright yellow stained CMA3-reacted spermatozoa (CMA3+) and yellowish green stained non-reacted spermatozoa (CMA3−) were detected under axiplane fluorescent microscope with a 460-nm filter (Zeiss Co., Jena, Germany).
Polymorphism determinationGenomic DNA was extracted from peripheral blood samples according to the Accu Prep Genomic DNA Extraction Kit (Bioneer, South Korea) according to the manufacturer's manual.
PCR reaction were done in 25μl solution comprised 2.5μl of 10× PCR Buffer, 0.8μl (PRM1) & 0.7μl (PRM2) MgCl2, 0.8μl (PRM1) & 0.7μl (PRM2) of dNTPs, 0.6μl (PRM1) & 0.5μl (PRM2) of each forward and reverse primer, 0.1U of Taq polymerase (Cinnagene, Co., Iran) and 1.5μl (PRM1) & 1μl (PRM2) of template DNA.
For PRM1 exon 1, bp 1–209 and Exon 2, bp 301–500, genotypes were identified by Polymerase chain reaction amplification using primers 5′CACAGCCCACAGAGTTCCAC3′ (forward) and 5′CAACATTTATTGACAGGCGG3′ (reverse) and Taq PCR Master Mix (AMPLIQON, Denmark). PCR conditions compromised an initial denaturing step at 94°C for 5min, followed by 30 cycles of 95°C for 50s denaturation, 58.3°C for 50s annealing and extension 72°C for 50s, with a final extension at 72°C for 5min. For PRM2 exon 1, bp 1–381 and exon 2, bp 544–845, genotypes were determined by PCR amplification using primers 5′AGACCAGACCAACAGTAACAC3 (forward) and 5′AGCTTTATTGGGCAGGTGAC3′ (reverse). Amplification condition were initially denaturation at 94°C for 2min, followed by 35 cycles of 95°C for 30s denaturation, 63.5°C for 40s annealing and extension 72°C for 45s, with a final extension at 72°C for 5min. Reaction products were fractioned on a 2% agarose gel and visualized after ethidium bromide staining with a digital camera system (Gel Digidoc II, Kiagen, Iran).
Single strand conformation polymorphism (SSCP) method was used to screen PRM1 and PRM2 variants. All PCR products from patients and controls were analyzed by SSCP polyacrylamide gel electrophoresis. Direct DNA sequencing of samples with altered band pattern in the SSCP gel was done by a commercial company (Macrogene, South Korea).
Statistical analysisStatistical analysis was performed using SPSS v20 (IBM Corp.) software. Differences between variables with normal distribution were analyzed using Student's t-test and not normally distributed data were assessed using nonparametric Mann–Whitney U test. A P-value of <0.05 was considered statistically significant.
ResultClinical featuresThe mean age of infertile men with varicocele and normal subjects was 31.23±3.67 and 32.65±6.16 respectively (P=0.281). Duration of infertility was 4.35±2.62 in infertile men with varicocele. In the varicocele group, 25.4% of the patients defined as grade III, 74.6% presented with grade II.
Sperm concentration (83.18±47.19 vs. 92.77±81.02) and non-progressive motility (14.94±4.48 vs. 13.27±3.14) were not differing significantly between varicocele and control groups respectively (P>0.05).
Sperm parameters containing sperm total (69±11.35 vs. 58.76±13.07) and rapid motility (55.72±13.32 vs. 43.81±14.05) as well as sperm morphology (32±8.73 vs. 21.57±10.85) was significantly higher in control men than varicocele patients.
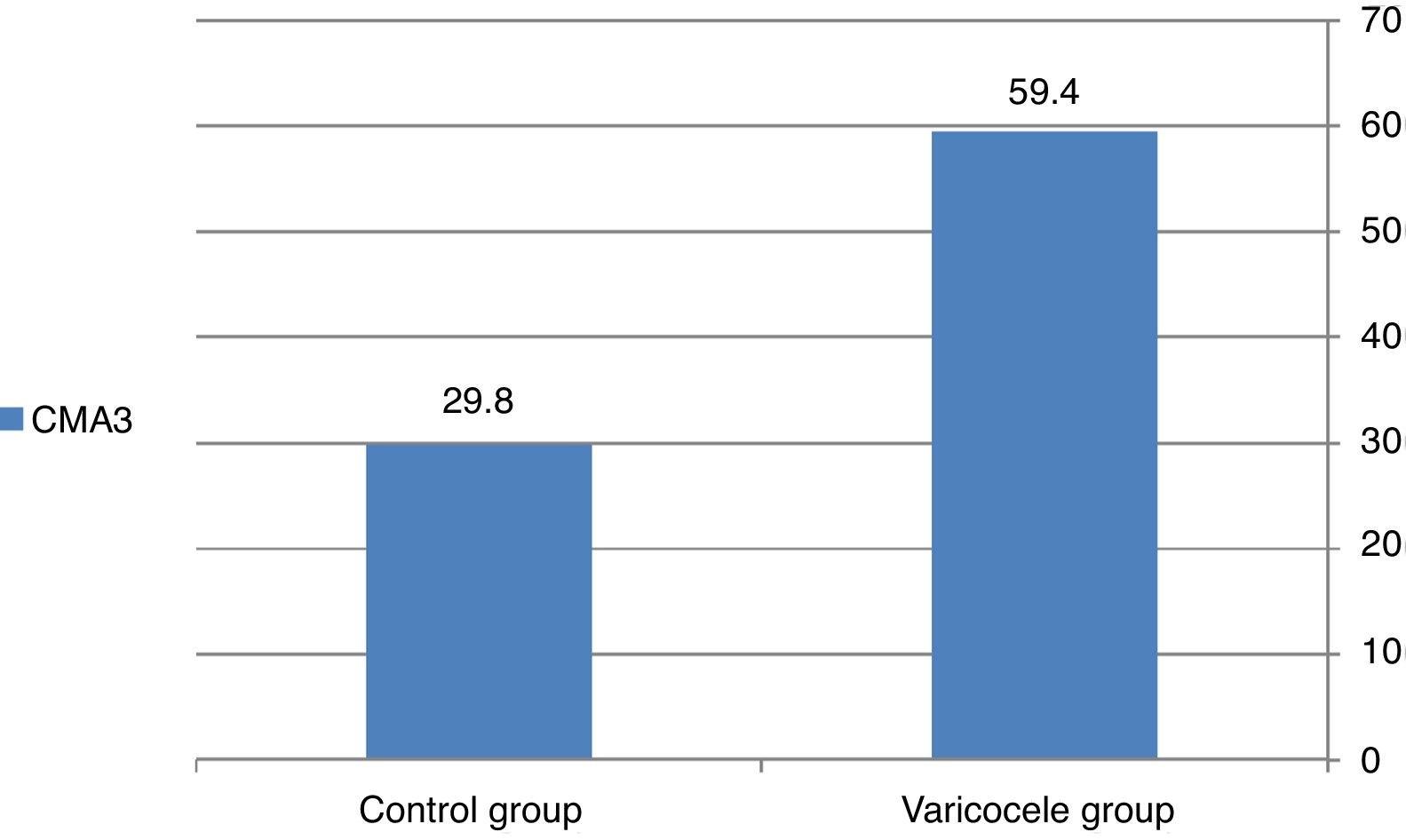
CMA3 staining resultA significant difference was detected in CMA3 staining between the two groups. The rate of reacted spermatozoa to CMA3 in the normal and varicocele group was 29.8% and 59.4% respectively (P=0.000) (Fig. 1).
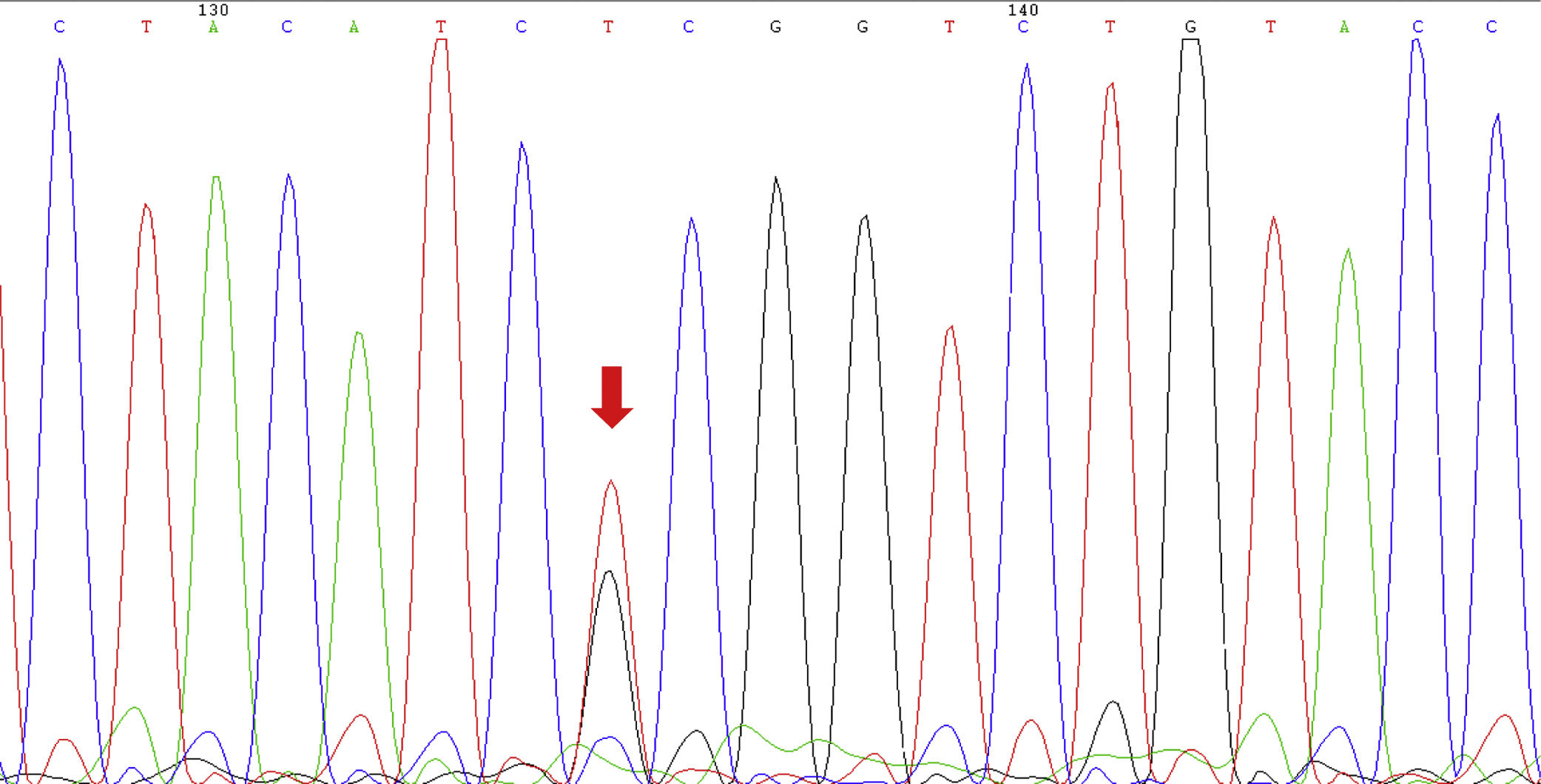
PolymorphismsNine different variants including six variants in PRM1 and three variants in PRM2 genes were found among varicocele patients. The homozygous of g.805C>T in PRM2 was the most frequent variation and was seen in all varicocele patients. Heterozygous of both g.IVS1+75T>C and g.151G>A in PRM1 with the frequency of 3% was the lowest frequent (Table 1). Two substitutions were detected in the coding region of the PRM1 gene but none of them leads to amino acid replacement. In control group, no variation was found in PRM1 and PRM2 genes (Fig. 2).
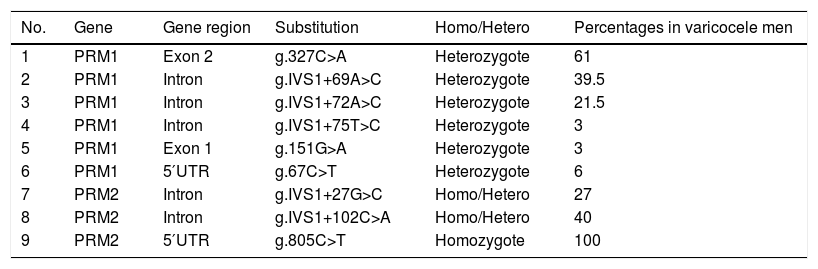
Identified polymorphisms in the protamine genes (I and II).
| No. | Gene | Gene region | Substitution | Homo/Hetero | Percentages in varicocele men |
|---|---|---|---|---|---|
| 1 | PRM1 | Exon 2 | g.327C>A | Heterozygote | 61 |
| 2 | PRM1 | Intron | g.IVS1+69A>C | Heterozygote | 39.5 |
| 3 | PRM1 | Intron | g.IVS1+72A>C | Heterozygote | 21.5 |
| 4 | PRM1 | Intron | g.IVS1+75T>C | Heterozygote | 3 |
| 5 | PRM1 | Exon 1 | g.151G>A | Heterozygote | 3 |
| 6 | PRM1 | 5′UTR | g.67C>T | Heterozygote | 6 |
| 7 | PRM2 | Intron | g.IVS1+27G>C | Homo/Hetero | 27 |
| 8 | PRM2 | Intron | g.IVS1+102C>A | Homo/Hetero | 40 |
| 9 | PRM2 | 5′UTR | g.805C>T | Homozygote | 100 |
PRM1: protamine gene I; PRM2: protamine gene II; UTR: untranslated region.
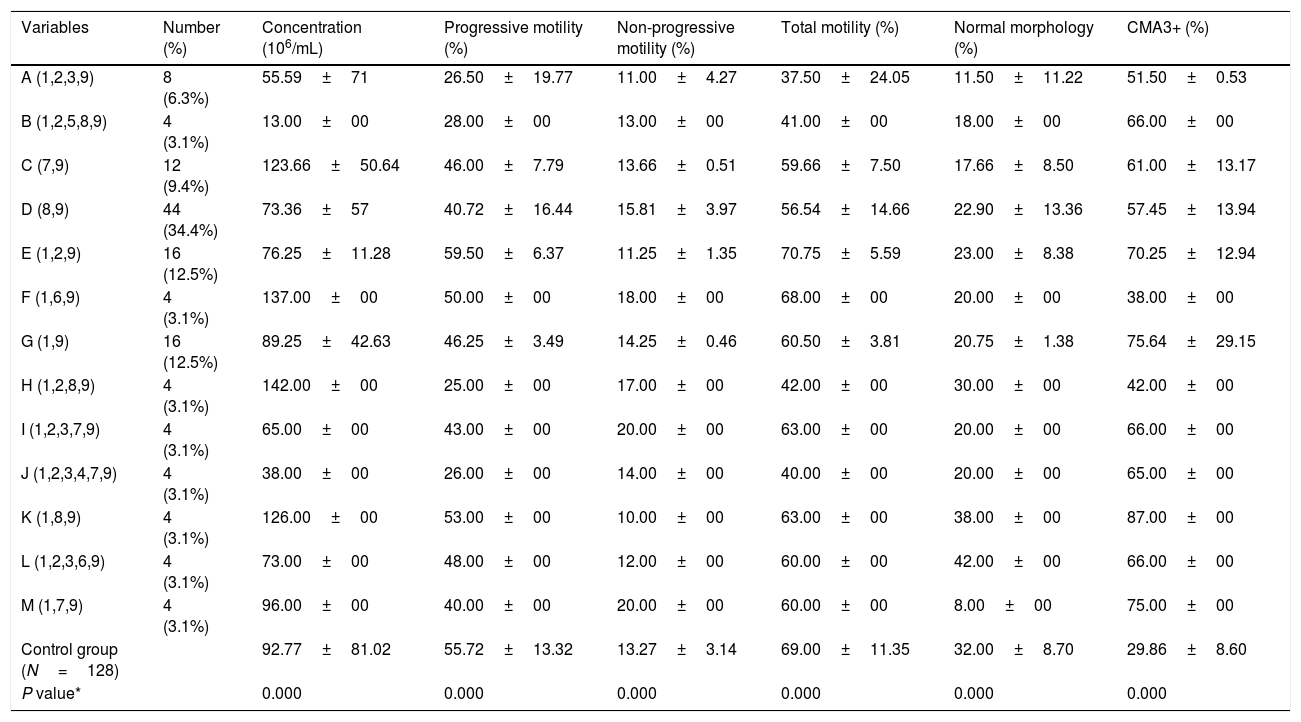
The patients were categorized to 13 groups (A to M) according to the type and number of detected nucleotide substitutions in PRM1 and PRM2 genes. Each varicocele patient had at least two (group C & G) and maximum six substitutions (group J) in protamine genes (Table 2). Regarding CMA3 staining, significant differences for variations of PRM1 and PRM2 genes were observed in varicocele group compared to the controls (P=0.0001). Semen parameters were significantly altered in varicocele patients with variations in PRM1 and PRM2 genes compared to fertile men (Table 2).
Semen parameters and protamine defects in varicocele patients according to the polymorphisms of protamine genes (I and II) mentioned in Table 1.
| Variables | Number (%) | Concentration (106/mL) | Progressive motility (%) | Non-progressive motility (%) | Total motility (%) | Normal morphology (%) | CMA3+ (%) |
|---|---|---|---|---|---|---|---|
| A (1,2,3,9) | 8 (6.3%) | 55.59±71 | 26.50±19.77 | 11.00±4.27 | 37.50±24.05 | 11.50±11.22 | 51.50±0.53 |
| B (1,2,5,8,9) | 4 (3.1%) | 13.00±00 | 28.00±00 | 13.00±00 | 41.00±00 | 18.00±00 | 66.00±00 |
| C (7,9) | 12 (9.4%) | 123.66±50.64 | 46.00±7.79 | 13.66±0.51 | 59.66±7.50 | 17.66±8.50 | 61.00±13.17 |
| D (8,9) | 44 (34.4%) | 73.36±57 | 40.72±16.44 | 15.81±3.97 | 56.54±14.66 | 22.90±13.36 | 57.45±13.94 |
| E (1,2,9) | 16 (12.5%) | 76.25±11.28 | 59.50±6.37 | 11.25±1.35 | 70.75±5.59 | 23.00±8.38 | 70.25±12.94 |
| F (1,6,9) | 4 (3.1%) | 137.00±00 | 50.00±00 | 18.00±00 | 68.00±00 | 20.00±00 | 38.00±00 |
| G (1,9) | 16 (12.5%) | 89.25±42.63 | 46.25±3.49 | 14.25±0.46 | 60.50±3.81 | 20.75±1.38 | 75.64±29.15 |
| H (1,2,8,9) | 4 (3.1%) | 142.00±00 | 25.00±00 | 17.00±00 | 42.00±00 | 30.00±00 | 42.00±00 |
| I (1,2,3,7,9) | 4 (3.1%) | 65.00±00 | 43.00±00 | 20.00±00 | 63.00±00 | 20.00±00 | 66.00±00 |
| J (1,2,3,4,7,9) | 4 (3.1%) | 38.00±00 | 26.00±00 | 14.00±00 | 40.00±00 | 20.00±00 | 65.00±00 |
| K (1,8,9) | 4 (3.1%) | 126.00±00 | 53.00±00 | 10.00±00 | 63.00±00 | 38.00±00 | 87.00±00 |
| L (1,2,3,6,9) | 4 (3.1%) | 73.00±00 | 48.00±00 | 12.00±00 | 60.00±00 | 42.00±00 | 66.00±00 |
| M (1,7,9) | 4 (3.1%) | 96.00±00 | 40.00±00 | 20.00±00 | 60.00±00 | 8.00±00 | 75.00±00 |
| Control group (N=128) | 92.77±81.02 | 55.72±13.32 | 13.27±3.14 | 69.00±11.35 | 32.00±8.70 | 29.86±8.60 | |
| P value* | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Data are presented as mean±standard deviation.
The differences were statistically significant. Between varicocele and control group; CMA3+: chromomycin A3; progressive motility (%) (grade “a”+grade “b”); non progressive motility (%) (grade “c”); total motility (%) (grade “a”+grade “b”+grade “c”); A to M: patients were divided into 13 groups according to the type and number of detected mutation in PRM1 and PRM2 genes.
It has been considered previously that defects in sperm nuclear condensation may lead to male infertility. The normal nuclear condensation recognized by a consecutive replacement of somatic histones by proteins like transition proteins and protamines, throughout spermiogenesis.19 It is shown that defects in sperm protamination can be occurred due to some reasons and it is interesting that genetic factor has an important effect.20 In the present study, we assessed the prevalence of polymorphisms of protamine genes among infertile men with varicocele. Nine different nucleotide sustitutions were found in PRM1 and PRM2 genes among varicocele patients. Whereas, there was not any variation in PRM1 and PRM2 genes in control group. According to our knowledge this is the first report on Protamine gene polymorphisms among varicocele patients. However, there are some studies assessing PRM1 and PRM2 gene mutations regarding abnormal semen parameters and male infertility.
Primarily, Iguchi et al. (2006), evaluated the infertile men protamine genes whose sperm looked like protamine knockout mice phenotypically and found a heterozygous single nucleotide polymorphism (SNP) in the PRM1 gene in 3 of 30 infertile men.21 Tanaka et al. (2003) reported three PRM1 SNPs that did not cause amino acid substitutions. In addition, these polymorphisms were similarly prevalent in sterile Japanese male cases and proven-fertile volunteers. They claim that the preservation of protamines is critical, and even a slight change in the protamine gene may cause male infertility. Moreover they indicated that introns were highly conserved in both PRM1 and PRM2 genes, and deduced that they mainly effect protamine genes expression.11
Furthermore, a PRM1 polymorphism (-190C>A) was reported which is correlated to abnormal sperm head morphology and abnormal P1/P2 ratio.22 We found that the semen samples of varicoceles with SNPs have higher rates of sperm morphological abnormalities than controls. Similarly, this SNP was found in Chinese population which is associated with teratozoospermia.23 Another study on Chinese population revealed that the PRM1 variant rs35576928 (p.R34S) (Arg>Ser) is correlated to severe oligozoospermia, however the dominant model of rs 35576928 has a protective effect on spermatogenesis.13 In general, histone H1-like plays a key role in sperm-specific chromatin compaction and comprises of higher quantities of both arginine and lysine in comparison to the somatic cells. It is assumed that phosphates were inserted between the lysine and arginine groups of histone H1 during binding to the chromatin, lead to stabilization of the protein and chromatin structures condensation.24
Moreover, a novel missense mutation (c.119G>A, p.Cys40Tyr) in PRM1 gene was detected in a man with oligoasthenozoospermia. The researchers also found the c.102G>T transversion which lead to an p.Arg34Ser amino acid change in two infertile men with oligozoospermia as well as unexplained infertility25 and these mutations were not detected in control population. In contrast, Aoki et al. (2006), mentioned no association between gene variation and both protamine deficiency and male infertility. They reported 15 SNPs in PRM1, PRM2 and transition protein genes which prevalence was similar between infertile males, fertile donors and protamine deficient patients.26
As an altered result, Tuttelmann and colleagues (2010) described that a homozygous carrier of the common haplotype ACC showed sperm concentration twice higher than men without this haplotype. They compared Caucasian patients with oligoteratozoospermia to normal controls and determined that sperm motility and morphology were not correlated to any SNP.27
Interestingly, two meta-analyses concluded that the pathogenicity of protamine gene mutations is not proven in male infertility20 and only -190C>A polymorphism can be considered as a risk factor for male infertility.28 In current study we found nine different variations in both PRM1 and PRM2 genes.
As it was specified in aforementioned studies, different polymorphisms have been detected in various populations due to male infertility. Four studies were performed in Iranian population regarding polymorphisms of protamine genes. One study showed that two previously detected SNPs in PRM121 and PRM211 which correlated with male infertility, have been entirely absent in Iranian infertile men.29 In consistence, Siasi et al. (2012), reported that SNPs in PRM1 (C321A), PRM2 (C248T) were not related to oligozoospermia and azoospermia in Iranian population.30 In the third study, the c.2190 C>A transversion in promoter region of PRM1 gene were associated significantly with increased risk of oligozoospermia.31 Another study showed that PRM1 and PRM2 mRNA copy numbers were significantly higher in normozoospermic in compared to teratozoospermic samples.32
All the above studies suggested that polymorphisms of protamine genes were rare causes of infertility in Iranian men. But, according to our results, different polymorphisms of protamine genes and protamine deficiency are frequent in varicocele patients with abnormal semen parameters.
In conclusion, current study as the first research on polymorphisms of protamine genes may be a common cause together with the presence of alterations in CMA3 staining and they should be considered for further potential study in male infertility with varicocele.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors declare that they have no conflict of interest.
Current study was financially supported by Yazd Reproductive Sciences Institute, Yazd Shahid Sadoughi University of Medical Sciences. This study was approved by ethics committee of Research and Clinical Center for Infertility, Yazd Shahid Sadoughi University of Medical Sciences.