The aim of this study is to evaluate the effects of castration and subsequent losartan administration on the fibrosis-related parameters in the corpora cavernosa of castrated rats.
Material and methodsTwenty-four male rats were divided into four equal groups. Group 1:sham surgery plus vehicle (0.9% NaCl) (control:con), group 2:sham surgery plus losartan (con+los), group 3: castration plus vehicle (castration:cast) and group 4:castration plus losartan (cast+los). After four weeks of oral losartan treatment, corporal levels of transforming growth factor-beta (TGF-β), thrombospondin-1 (TSP-1), alpha-actin, beta-actin and fibronectin were investigated by ELISA kits. Changes in the collagen and smooth muscle content were evaluated by histological analysis with Masson trichrome staining.
ResultsInitial and post-treatment body weights of rats were similar among groups. Castration significantly increased the expression of TGF-β, TSP-1 and fibronectin and resulted in a significant decrease in alpha-actin levels in the corpora cavernosa. Administration of losartan reduced the levels of TGF-β, TSP-1 and fibronectin in castrated rats. Alpha actin levels also increased after losartan treatment. Beta-actin levels were not significantly different among 4 groups. The levels of all markers were similar in group 1 and 2. Rate of fibrosis was significantly higher in castrated rats and treatment with losartan reduced this rate.
ConclusionCastration increased the expression of fibrosis-related markers in the corpora cavernosa of rats. Administration of losartan significantly attenuated those changes and exerted an antifibrotic effect.
El objetivo de este estudio es evaluar los efectos de la castración y la posterior administración de losartán en los parámetros relacionados con la fibrosis en los cuerpos cavernosos de ratas castradas.
Material y métodosVeinticuatro ratas macho se dividieron en 4 grupos iguales. Grupo 1: cirugía simulada más vehículo (0,9% NaCl) (control:con); grupo 2: cirugía simulada más losartán (con+los); grupo 3: castración más vehículo (castración:cast) y grupo 4: castración más losartán (cast+los). Después de 4 semanas de tratamiento oral con losartán se analizaron los niveles de factor de crecimiento transformante beta (TGF-β), trombospondina-1 (TSP-1), alfa-actina, beta-actina y fibronectina mediante kits de ELISA. Cambios en el colágeno y el contenido de músculo liso se evaluaron mediante análisis histológico con tinción con tricrómico de Masson.
ResultadosLos pesos corporales iniciales y posteriores al tratamiento de las ratas fueron similares entre los grupos. La castración aumentó considerablemente la expresión de TGF-β, TSP-1 y fibronectina, y dio como resultado una disminución importante de los niveles de alfa-actina en los cuerpos cavernosos. La administración de losartán redujo los niveles de TGF-β, TSP-1 y fibronectina en ratas castradas. Los niveles de alfa-actina también aumentaron después del tratamiento con losartán. Los niveles de beta-actina no fueron muy diferentes entre los 4 grupos. Los niveles de todos los marcadores fueron similares en los grupos 1 y 2. La tasa de fibrosis fue mucho mayor en las ratas castradas y el tratamiento con losartán redujo esta tasa.
ConclusiónLa castración aumentó la expresión de marcadores relacionados con la fibrosis en los cuerpos cavernosos de las ratas. La administración de losartán atenuó considerablemente esos cambios y ejerció un efecto antifibrótico.
Erectile dysfunction (ED) is the recurrent inability of a man to attain or maintain penile erection sufficient for sexual activity.1 It is a major health problem that seriously affects the life quality of men and their partners. Epidemiological studies revealed varying prevalence rates of 20–50% in men aged between 40 and 70 years with a steep age-related increase.2,3 The pathogenesis of ED is accepted to be multifactorial, including psychogenic, neurogenic, vascular and endocrinological factors.4 Among them, androgen deficiency is one of the most important reasons of ED.
It is generally accepted that androgens have a pivotal role in the erectile physiology. They regulate the penile erectile responses by maintaining an adequate amount of nitric oxide (NO), innervation of corpus cavernosum smooth muscle (CCSM) cells, endothelial cells and penile vessels.5–7 Experimental studies revealed that castration resulted in a decrease in smooth muscle content and an increase in collagen and other fibrosis-related mediators such as transforming growth factor beta (TGF-β), thrombospondin-1 (TSP-1) and fibronectin in the corpus cavernosum.8,9 Corporal fibrosis is the common process underlying most ED cases and associated with the loss of CCSM cells and the increase in the production of extracellular matrix (ECM) proteins.10
Angiotensin II (Ang II), a bioactive octapeptide, is the main substance of renin-angiotensin system and thought to be one of the modulators of erectile function by regulating CCSM contractility and tone.11 Ang II promotes the synthesis of reactive oxygen species in endothelial and vascular smooth muscle cells, which is thought to be related to Ang II-induced smooth muscle contraction and subsequent erectile dysfunction.12 It also stimulates increased TSP-1 expression resulting in enhanced TGF-β activation and ECM protein synthesis.
Previously, it has been shown that losartan, an Ang II type 1 receptor antagonist, counteracted the fibrotic effects of Ang II and resulted in recovery of erectile function in a bilateral cavernous nerve injury model.13 However, the effect of losartan on penile fibrosis has not been studied in a castrated rat model yet. Therefore, the objective of this study is to investigate the effect of castration on cavernosal tissue structures and the change by the administration of losartan on those structures in a castrated rat erectile dysfunction model.
Material and methodsThe study protocol was approved by Kahramanmaras Sutcu Imam University Animal Research ethics committee. Twenty-four male Wistar-Albino rats (10–12 weeks old) weighing between 250 and 300g were used for this study. They were placed in plastic cages, three rats per cage, in a constant temperature-controlled room on a 12/12-h light/dark cycle. The rats were allowed to eat standard rodent chow and water ad libitum.
All experiments comply with the ARRIVE guidelines and carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The rats were divided into 4 groups of 6 rats in each: Group 1:sham surgery plus vehicle (distilled water) (control group:con), group 2: sham surgery plus losartan (control +losartan:con+los), group 3: castration plus vehicle (castration:cast), group 4: castration plus losartan (cast+los). Castration procedure was performed as reported previously.14 The animals were anesthetized with an intraperitoneal injection of a mixture of ketamine (50mg/kg) and xylazine (10mg/kg). Next, a scrotal midline incision was created. Spermatic cords were seperated; vasa deferentia and associated vasculature were identified. Then, those structures were ligated and testicles were removed bilaterally.
From the day of surgery, rats were started to be treated daily with distilled water (2mL) in the vehicle groups. Losartan was prepared by dissolution in distilled water and administered by oral gavage at a dose of 40mg/kg per day.13 All treatments were done for 4 weeks. At the end of this period, rats were reanesthesized; penectomies were performed and blood samples from the heart were obtained to evaluate the levels of alpha actin, beta actin, TGF-B, TSP-1 and fibronectin both in the penile tissues and systemic circulation. Histopathological analyses of cavernosal tissues to determine the collagen and smooth muscle content were also performed.
Biochemical analysis of tissue samplesThe levels of alpha-actin (MyBiosource, Inc, USA Cat No: MBS9311473), beta-actin (MyBiosource, Inc, USA Cat No: MBS2502112), thrombospondin 1 (MyBiosource, Inc, USA Cat No: MBS2023972), TGF-β1 (RayBiotech, Inc. USA Cat No:ELH-TGFb1-1) and fibronectin (MyBiosource, Inc, USA Cat No: MBS494167) in corporal tissue samples were measured by a quantitative sandwich enzyme immunoassay technique (ELISA) using commercial kits according to the manufacturers’ instructions. First of all, the corpora cavernosa of the penile tissues were dissected from other structures such as urethra, corpus spongiosun and tunica albuginea. Then cavernosal tissues were weighed, blotted on a filter paper, and homogenized with three volumes of ice-cold 1.15% KCI. The levels of these parameters in the samples were measured in the supernatant obtained from centrifugation at 14,000rpm. The tissue samples were stored at −20°C until analysis.
Measurement of testosteroneWhole blood was obtained from the heart of the rats. Samples were centrifuged at 1580×g for 10min at room temperature. Serum testosterone levels were determined by electrochemiluminescence immune assay with Siemens ADVIA Centaur XP Immunoassay system (USA). Level of testosterone was reported as ng/ml.
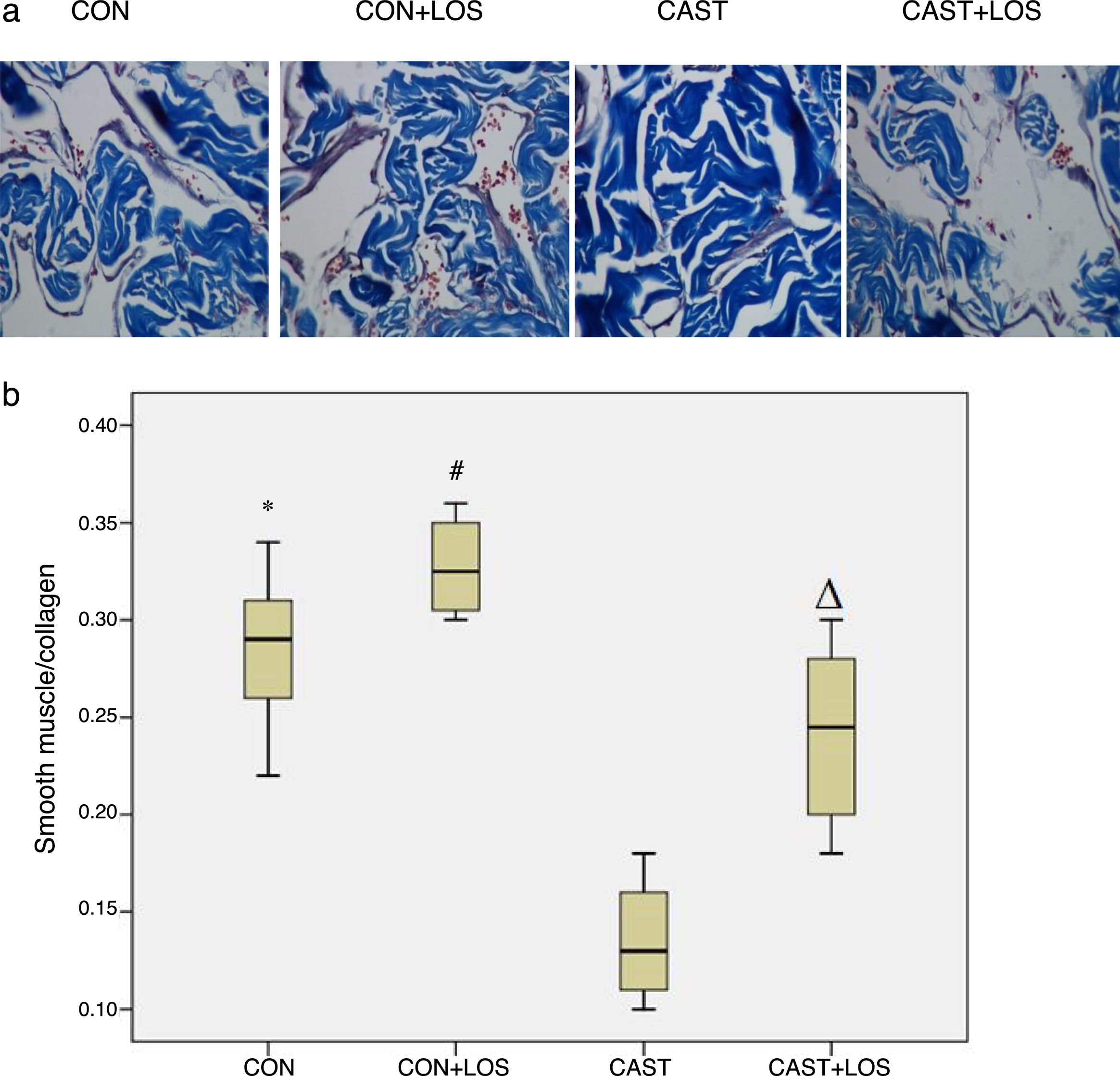
Histological analysisParaffin-embedded cavernosal samples were sliced into 4-μm sections and mounted on glass slides, followed by deparaffinization and rehydration. Staining with Hematoxylin–eosin (HE) was performed to assess the structural changes in the corpus cavernosum. In order to investigate the ratio of smooth muscle to collagen (SM/C), sections were stained with Masson's trichrome (MT) using a MT staining kit (Sigma-Aldrich Inc., St. Louis, MO, USA). The areas of smooth muscle (red stain) and collagen (blue stain) were evaluated in 200× magnification images of the tissues by using an Olympus Biological Microscope (BX50; Olympus, Tokyo, Japan) and Image Pro Plus 6.0 software (Medica Cybernetics, Silver Spring, MD, USA).
Statistical analysisThe results were presented as mean±standard deviation for all continous variables. Comparisons among groups were carried out by one-way ANOVA test and Bonferroni correction was used for post hoc analyses involving multiple comparisons. A p value of <0.05 was considered as statistical significance in all cases. SPSS® 21.0 for Windows was used for data analysis.
ResultsThe initial and post-treatment body weights and total serum teostosterone levels of rats were given in Table 1. There were no significant differences in the initial body weights of rats among groups. Castration resulted in a non-significant decrease in the body weights of the rats when compared to sham groups. Testosterone levels were significantly reduced in castrated rats.
Comparison of the body weights and testosterone levels of rats among groups.
| CON | CON+LOS | CAST | CAST+LOS | |
|---|---|---|---|---|
| Initial weight (g) | 260.10±7.35 | 265.70±8.61 | 256.70±7.85 | 262.40±8.55 |
| Final weight (g) | 341.20±9.84 | 353.50±10.57 | 316.10±9.23 | 319.70±10.12 |
| Total testosterone (ng/ml) | 8.07±0.53* | 8.15±0.61** | 0.61±0.05 | 0.63±0.06 |
CON: control, CON+LOS: control+losartan, CAST: Castration, CAST+LOS: Castration+losartan.
Data were presented as mean±SD for n=6 rats per group.
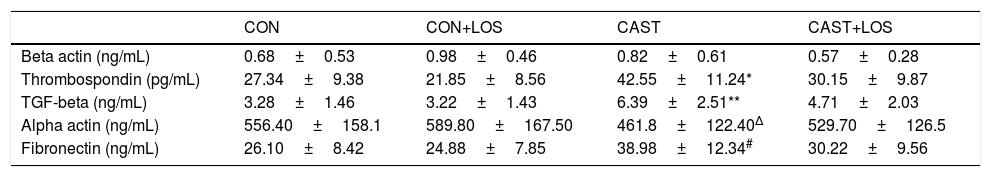
The changes in the levels of biochemical parameters among groups were presented in Table 2. Expression of TGF-β significantly increased in castrated rats (group 3) with respect to group 1 (p=0.031) and administration of losartan significantly decreased its expression (p=0.039). Also, it was found out that the levels of TSP-1 were higher in group 3 when compared to group 1 (p=0.028) and losartan treatment resulted in a significant decrease in thrombospondin expression (p=0.037). Fibronectin expression was also found to be elevated in group 3 when compared to group 1 (p=0.03). Administration of losartan significantly decreased fibronectin expression (0.023). Alpha actin levels were lower in group 3 than group 2 (p=0.016) and losartan treatment significantly increased its levels (p=0.025). Masson's trichrome staining showed the area of smooth muscle (redstain) to be reduced and the area of collagen (blue stain) increased, in the CC of Castration group compared with those of other groups. The smooth muscle/collagen (SM/C) ratios in Castration group were significantly lower than those in other groups. Masson's trichrome staining showed the area of smooth muscle (redstain) to be reduced and the area of collagen (blue stain) increased, in the CC of Castration group compared with those of other groups. The smooth muscle/collagen (SM/C) ratios in Castration group were significantly lower than those in other groups. Masson's trichrome staining showed the area of smooth muscle (redstain) to be reduced and the area of collagen (blue stain) increased, in the CC of Castration group compared with those of other groups. The smooth muscle/collagen (SM/C) ratios in Castration group were significantly lower than those in other groups. Masson's trichrome staining exhibited the area of smooth muscle (red stain) to be decreased and collagen (blue stain) increased in group 3 when compared to other groups (Fig. 1a). The ratios of smooth muscle to collagen (SM/C) were significantly lower in group 3 than those of other three groups (Fig. 1b). Beta-actin levels did not differ among the study groups. There were no significant differences for any parameters between group 1 and 2. Also, all parameters of group 1 and 4 were found to be similar.
Comparison of the corporal tissue levels of the fibrosis-related markers of rats among groups.
| CON | CON+LOS | CAST | CAST+LOS | |
|---|---|---|---|---|
| Beta actin (ng/mL) | 0.68±0.53 | 0.98±0.46 | 0.82±0.61 | 0.57±0.28 |
| Thrombospondin (pg/mL) | 27.34±9.38 | 21.85±8.56 | 42.55±11.24* | 30.15±9.87 |
| TGF-beta (ng/mL) | 3.28±1.46 | 3.22±1.43 | 6.39±2.51** | 4.71±2.03 |
| Alpha actin (ng/mL) | 556.40±158.1 | 589.80±167.50 | 461.8±122.40Δ | 529.70±126.5 |
| Fibronectin (ng/mL) | 26.10±8.42 | 24.88±7.85 | 38.98±12.34# | 30.22±9.56 |
CON: control, CON+LOS: control+losartan, CAST: Castration, CAST+LOS: Castration+losartan.
Data were presented as mean±SD for n=6 rats per group.
(a) Masson's trichrome staining of the corpora cavernosa of the groups. (b) The smooth muscle/collagen (SM/C) ratios of groups were presented through box plots. CON: control, CON+LOS: control+losartan, CAST: Castration, CAST+LOS: Castration+losartan. Data were expressed as mean±SD (n=6 rats per group). Comparisons between groups were performed by one-way ANOVA test followed by Bonferroni correction. * denotes p<0.05 when compared with CAST; # denotes p<0.01 when compared with CAST; Δ denotes p<0.05 when compared with CAST.
In the present study, the effects of losartan on fibrosis-related parameters in cavernosal tissues of castrated rats were investigated. At the end of the four week period, it was found out that castration resulted in a significant increase in the expression of TSP-1, TGF-β, fibronectin and a decrease in alpha actin. Collagen/smooth muscle ratio also significantly increased in case of castration when compared to sham group which was established by Masson trichrome staining. Administration of losartan significantly attenuated those changes and reduced collagen content in castrated rats. Our findings corroborated the fibrotic changes in the corpus cavernosum after castration and that losartan treatment had an antifibrotic effect.
CCSM cells play pivotal role in the regulation of erectile physiology and castration-related pathological changes such as reduced cavernosal smooth muscle and endothelial cell content and increased apoptosis which may impair erectile responses.14 It has also been demonstrated that androgen deficiency induces ED through promotion of corporal fibrosis which is characterized by the increase in TGF-β and TSP-1 expression, loss of CCSM cells and the accumulation of ECM proteins including collagen.8 Similarly, our histopathological assessments revealed that the collagen/smooth muscle ratio was increased in case of androgen deficiency.
Ang-II, the principal vasoactive substance of the renin-angiotensin system, is locally produced and secreted from penile endothelial and smooth muscle cells.15 It increases NADPH oxidase-dependent ROS production, followed by stimulating RhoA/Rho kinase-signaling pathway, and decreasing the eNOS activity by the activation of the angiotensin type-1 receptors (AT1R). Those receptors are mostly expressed in smooth muscle cells and responsible for the regulation of vasoconstriction, proliferation and inflammation. These responses lead to an increase in the cavernosal smooth muscle contraction and the cavernosal tissue damage, resulting in the development of ED.16,17
Ang-II also activates the expression of TSP-1 which further enhances TGF-β formation resulting in increased ECM protein synthesis in CCSM cells.18,19 TSP-1 is a significant mediator of the fibrotic complications of diabetes mellitus associated with stimulation of the renin-angiotensin system.19 TGF-β is a well-known fibrogenic cytokine that is present in the corpus cavernosum and acts on both the Smad dependent and independent pathways. A recent experimental study showed that TGF-β expression was increased in the pathological process of corporal fibrosis in case of castration.8 Consistent with those reports, we determined that TSP-1 and TGF-β expression were increased in castrated rats. In addition, fibronectin expression was elevated and alpha actin level was reduced in case of castration.
Since Ang-II mostly exerts its functions via AT1 receptors, it may be postulated that administration of losartan, an AT1 receptor blocker, can ameliorate the anti-erectile and profibrotic effects of Ang-II. Previous experimental studies have shown that losartan treatment reversed and normalized interstitial fibrosis, and decreased the expression of EMPs and TGF-β in vascular SM cells.20,21 In a diabetic rat model, it was described that losartan ameliorates the profibrotic/anti-erectile effects of Ang-II by down-regulating renin-angiotensin system.22 Another study reported that intracavernous injection of Ang II caused smooth muscle contraction which subsequently resulted in loss of spontaneous erections through the activation of AT 1 receptors, and this contraction was blocked by losartan.15
Canguven et al. reported that the administration of losartan improved erectile functions via antifibrotic mechanisms in a bilateral cavernosal nerve injury ED model.13 They noted that cavernosal nerve injury significantly increased TGF-β, TSP-1 and fibronectin levels. After one-week treatment with losartan, the levels of these markers were reduced and recovery of erectile functions associated to those changes. As a clinical perspective, they suggested that losartan can be beneficial in the preservation of erectile functions in men who underwent radical prostatectomy. More recently, Li et al. reported that a 4-week treatment with losartan restored smooth muscle/collagen ratio in the corpora cavernosa of streptozocin-induced diabetic rats.23
To our knowledge, present research is the first study investigating the effect of losartan administration on the fibrotic changes in castrated rats. Our findings were consistent with the aforementioned reports. We demonstrated that castrated rats had greater fibrosis e.g. higher expression of TGF-β, TSP-1 and fibronectin, significantly increased collagen deposition and reduced expression of alpha actin. We also observed that administration of losartan resulted in a decrease in the expression of fibrosis-related biochemical markers and reduced collagen content which means that losartan exerted antifibrotic effects in castrated rats. The increase in the alpha actin levels after losartan treatment may be related to increased number or improved functioning of endothelial cells which are thought to induce expression of alpha-actin.23 Also, reduced TGF-β expressions or very low levels of Ag II may be associated with the elevated alpha actin levels. However, further molecular studies should be conducted in order to suggest such a relationship in the castration models. Although erectile responses were not measured in the present study, increased alpha smooth muscle actin amounts together with decreased TGF-B may help to preserve erectile functions by several molecular mechanisms including inhibition of SMAD signaling pathways.13,24
When the beneficial effects of losartan on erectile functions were considered in diabetic men and experimental ED models,13,24,25 one can suggest that losartan combined with PDE-5 inhibitors may help to preserve erectile functions in men who underwent androgen deprivation therapy for prostate cancer. However, further clinical studies are mandatory to offer such a treatment option.
Major limitation of our study is the lack of functional evaluation of erectile responses. Inability to measure nitric oxide levels, Smad pathways and apoptotic markers are the other limitations of our study. In addition, a study period of four weeks may not be long-enough to explore the effects of both castration and losartan on penile tissues. Additional castration studies investigating the changes in erectile responses after the administration of losartan may help to support its previously proven erectogenic effects.
ConclusionsCastration resulted in an increase in the fibrosis-related biochemical markers and collagen content in the corpus cavernosum. Thereafter, treatment with losartan significantly attenuated those fibrotic changes. It can be concluded that administration of losartan might decrease the extent of fibrosis and this study may provoke further studies which investigate its effects on erectile functions in castrated rat ED model.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestThe authors declare that they did not have conflict of interests.
We would like to acknowledge animal and biochemistry laboratory staffs for their effort during the study.