The mean platelet volume (MPV) is an extensively employed laboratory indicator related to platelet volume and function in inflammatory circumstances. The aim of this study was to assess the relationship between inflammation and mean platelet volume in varicocele pathophysiology.
MethodsWe conducted a recent study, which included 131 varicocele subjects and 82 healthy controls. The identification of varicocele was based on the results from both physical examination and color Doppler ultrasound. We analyzed some laboratory markers including haemogram tests in two groups.
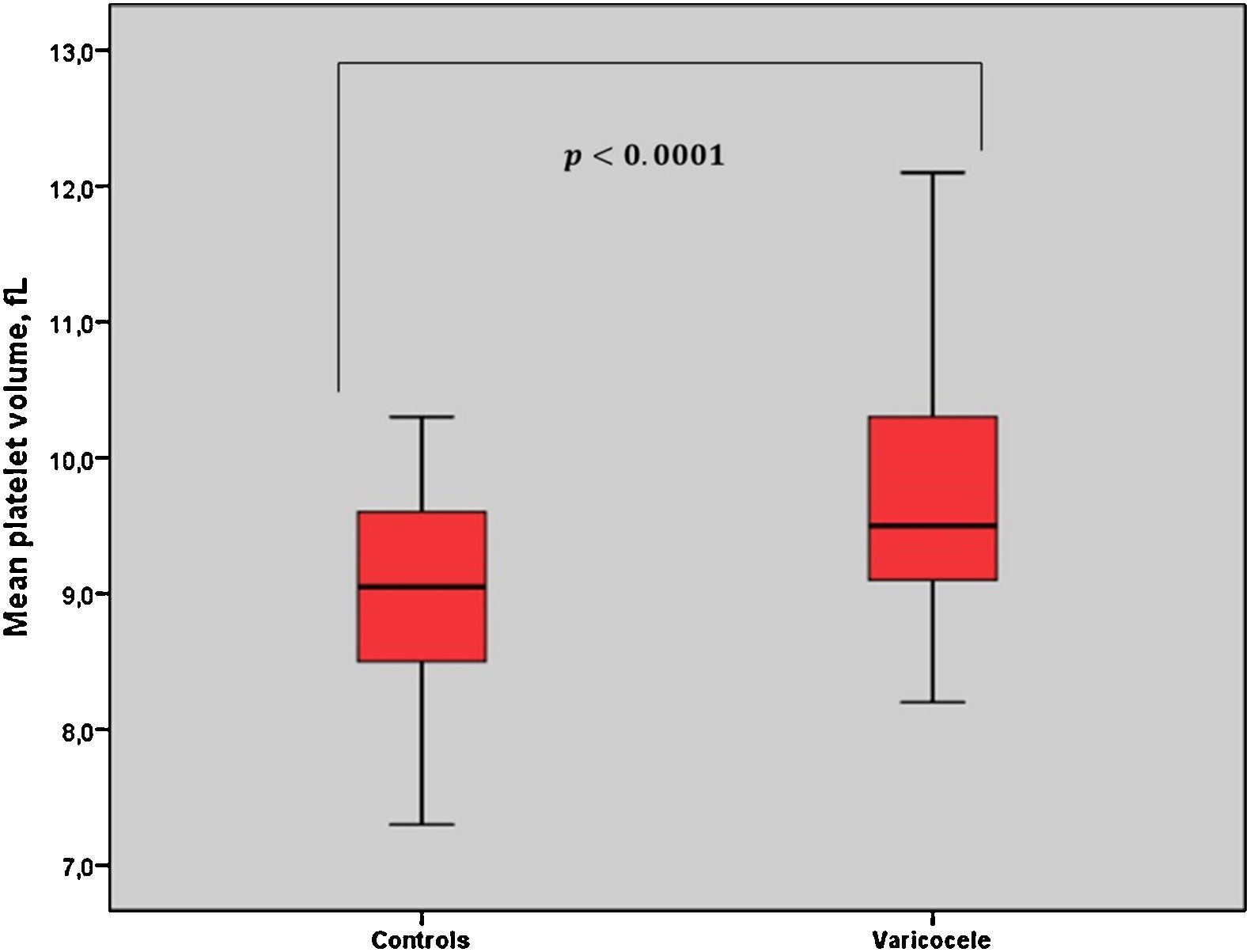
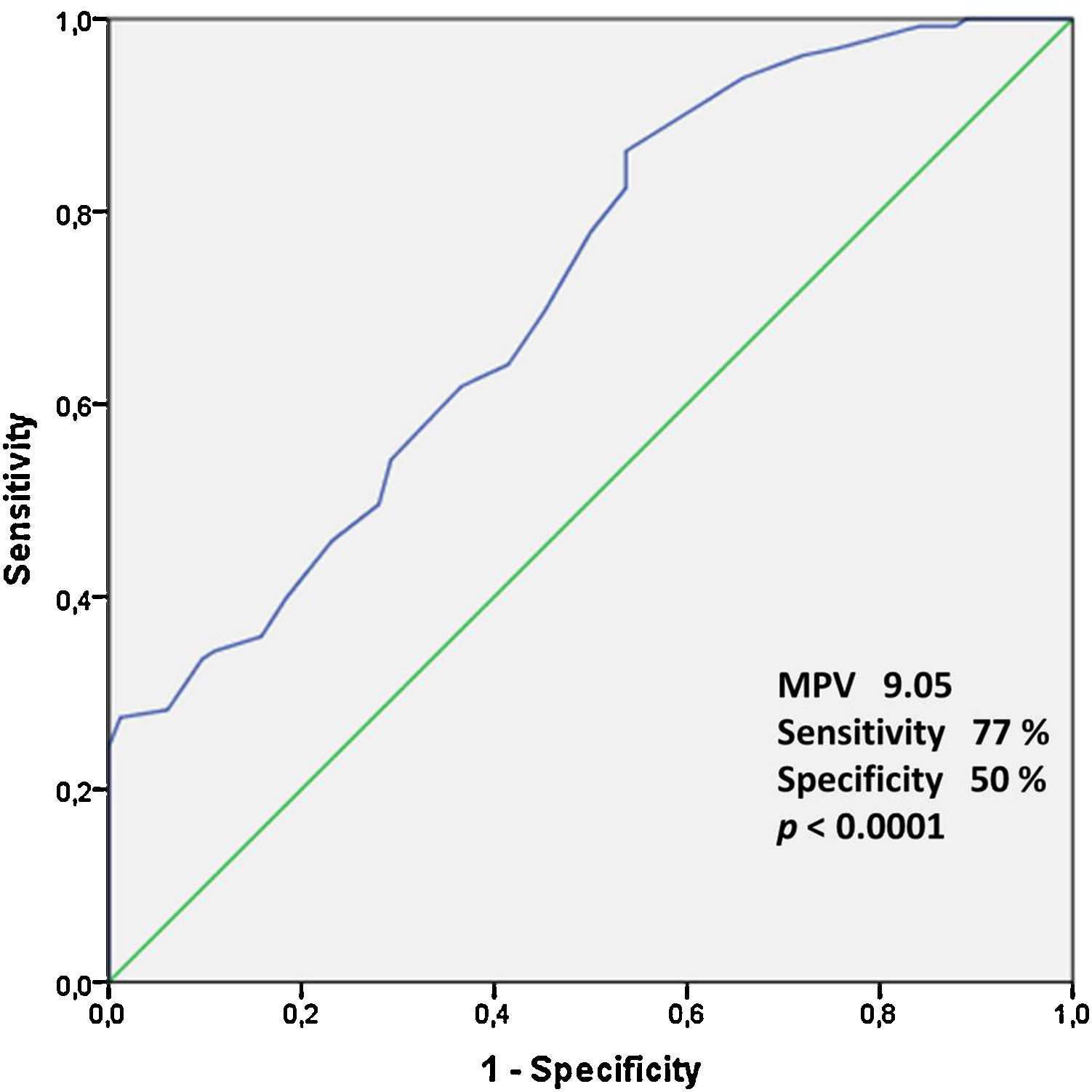
ResultsThere were no significant differences in the two groups in terms of baseline characteristics. MPV values were statistically higher in the varicocele group (9.73±0.86fL) than in the control group (9.03±0.70fL) (p<0.001). However, no significant relationship between MPV and varicocele grade was found. Furthermore, the receiver-operating characteristic curve analysis suggested the optimum MPV cut-off value for patients with varicocele as 9.05, with a sensitivity and specificity of 77% and 50%, respectively (p<0.001).
ConclusionMPV can offer information on varicocele pathophysiology. Increased MPV levels in varicocele patients may be associated with inflammation.
El volumen plaquetario medio (VPM) es un indicador de laboratorio ampliamente empleado en relación con la función plaquetaria y el volumen plaquetario en contextos inflamatorios. El objetivo de este estudio es estudiar la relación entre inflamación y volumen plaquetario medio en la patofisiología del varicocele.
MétodosSe lleva a cabo un estudio en el que se incluyen 131 casos de varicocele y 82 controles sanos. El diagnóstico de varicocele se basa en los hallazgos de la exploración física y los resultados del eco-Doppler color. Se analizan distintos marcadores de laboratorio, incluyendo hemograma, en los 2 grupos.
ResultadosNo se encuentran diferencias significativas en las características basales de los 2 grupos estudiados. El valor VPM fue más elevado en el grupo de pacientes con varicocele (9,73±0,86fL) en comparación con el grupo control (9,03±0,70fL) (p<0,001). Sin embargo, no se encontraron diferencias significativas entre el VPM y el grado de varicocele. Por otro lado, el análisis de la curva ROC sugiere que el valor de corte óptimo para el VPM para los pacientes con varicocele fue de 9,05 con una sensibilidad y especificidad del 77 y 50%, respectivamente (p>0,001).
ConclusiónEl VPM puede ofrecer información sobre la fisiopatología del varicocele. Los niveles altos del VPM pueden estar asociados con la inflamación.
Varicoceles are present in 15–20% of the normal male population and in approximately 35–40% of men presenting with infertility.1,2 Varicocele is characterized as abnormal tortuosity and dilation of the pampiniform plexus within the spermatic cord. Even though there are many studies on the aetiopathogenesis, it has not been completely explained. However, various vascular changes, segmental obliteration, including vascular wall thickening, dismemberment of the internal elastic lamina, and occasional occlusive thrombi, have been microscopically identified.2 In addition, varicoceles have been associated with other vascular pathologies in recent studies.3–5
The platelets are significantly involved in hemostatsis, vascular injury and inflammation. The size of platelets can effect their functions. Mean platelet volume (MPV) can be beneficial for forecasting functional changes and activation of platelets.6,7 Lately, increased levels of MPV were demonstrated in vascular disorders such as varicocele, testicular torsion, myocardial infarction, myocardial angina, coronary artery atherosclerosis, peripheral artery disease, cerebrovascular disease, stroke, and all of those concerning endothelial dysfunction on the footing of inflammation.8–19 The studies and the proofs acquired shows a correlation between MPV and inflammation.6,12,13 The levels of platelets are increased in the event of high-grade inflammation, leading to a decrease in the MPV level as a result of the migration of a generality of the large reactive platelets to inflammatory areas and intense consumption of these platelets. In the event of low-grade inflammation, an increment in MPV level arises as a result of the incremented reactive immature platelets.6,7
The aim of this study was to evaluate the relationship between varicocele and MPV, and was to evaluate the predictive role of the MPV in patients with varicocele. We think that the relationship may partially explain the pathophysiology of varicocele.
MethodsWe conducted a recent prospective study, which included the 131 varicocele subjects and 82 healthy controls. The varicocele was grade 1 in 38 patients, grade 2 in 39 patients and grade 3 in 54 patients. At room temperature, the diagnosis of varicocele was based on the findings from both physical examination and color Doppler ultrasound (GE Logiq P5 Ultrasound Imaging System, 12MHz probe) by 1 urologist and 2 radiologists. Varicocele was categorized based on the Dubin grading system.20 Grade I: varicose veins are palpable only during the Valsalva maneuver. Grade II: varicose veins may be palpable during rest but not visible. Grade III: visible varicose veins. We have planned a physical examination for varicose veins in places outside the scrotum. We were excluded peripheral vascular diseases from the study. MPV and other complete blood paramaters might be influenced by age, obesity, smoking, coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, metabolic syndrome, renal or hepatic dysfunction, abnormal thyroid functions, known malignancy, hematologic malignancies, inflammatory diseases, steroid intake, bone marrow or hematologic disorders, splenectomy, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura, myeloproliferative disorders, leukemia, radiation, usage of drugs, peripheral vascular disease and deep vein thrombosis.12,21 These patients and those with nonvaricocele scrotal pathologies were excluded from the study. Also, sperm parameters of the patients were evaluated. The study was accepted by the ethics committee.
Blood analysisTotal blood count, including hemoglobin (Hgb), white blood cell (WBC), platelet (Plt), MPV, and also erythrocyte sedimentation rate (ESR), and C-reactive protein parameters (CRP) were measured in both groups within 2h of sampling. Total blood count test was performed with an automatic blood count test device (Sysmex XN-1000 hematology analyzer) using blood with K-EDTA (Potassium-ethylene diamine tetraacetic acid) anticoagulation at our hospital. All samples were sent to the same laboratory and examined under controlled conditions of humidity and temperature within 2h. The optimal measuring time with K2-EDTA should be 2h after taking blood sample. Because, maximal changes in the MPV occur after the first 2h after sampling.11
Statistical analysisStatistical evaluation were carried using the SPSS ver. 15 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated as means and standard deviation (SD). The Kolmogorov–Smirnov test and sample t test was used to evaluate the statistical data. Additionally, the Mann–Whitney U test was used to investigate the differences between the independent samples; and the non-parametric Wilcoxon test was used to test for the differences between the paired samples. Spearman's correlation analysis was used to evaluate the correlation between variables in patient groups. A p value of <0.05 was considered statistically significant. Also, receiver-operating characteristic (ROC) curve analysis was used to evaluate between variables in patient groups.
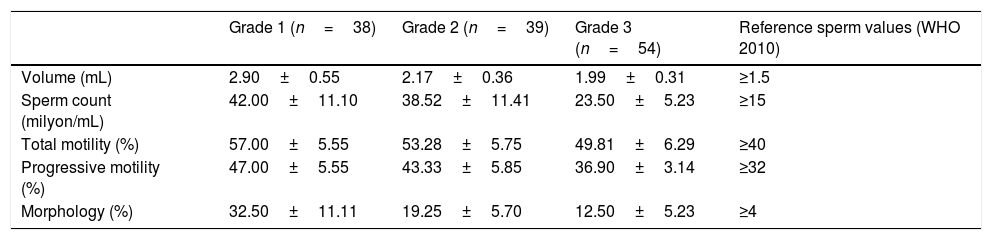
ResultsThe average patient age was 23.6±4.1 years in varicocele group and 24.5±4.4 in control group. Ninety-eight patients (74.8%) had left-side varicocele, 12 patients (9.1%) had right-side varicocele, and 21 patients (16.1%) had bilateral varicocele. The demographic and clinical features of the patients are provided in Tables 1 and 2. The seminal parameters of varicocele patients were found within normal limits (Table 3). MPV levels were statistically higher (p<0.001, Fig. 1) in varicocele group (9.73±0.86fL) than in control group (9.03±0.70fL). MPV levels were statistically higher in varicocele grade 1 (9.78±0.87fL) than in control group (p<0.001), grade 2 (9.77±0.89) than in control group (p<0.001), grade 3 (9.66±0.84) than in control group (p<0.001). However, there was no relation between MPV and varicocele grade (Table 2). The ROC curve analysis suggested the optimum MPV cutoff value for patients with varicocele as 9.05, with a sensitivity and specificity of 77% and 50%, respectively (area under curve: 0.717, 95% confidence interval=0.647–0.787, p<0.0001, Fig. 2).
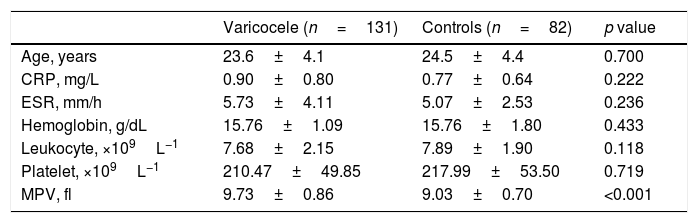
Comparison of clinical and laboratory features between varicocele and control groups.
| Varicocele (n=131) | Controls (n=82) | p value | |
|---|---|---|---|
| Age, years | 23.6±4.1 | 24.5±4.4 | 0.700 |
| CRP, mg/L | 0.90±0.80 | 0.77±0.64 | 0.222 |
| ESR, mm/h | 5.73±4.11 | 5.07±2.53 | 0.236 |
| Hemoglobin, g/dL | 15.76±1.09 | 15.76±1.80 | 0.433 |
| Leukocyte, ×109L−1 | 7.68±2.15 | 7.89±1.90 | 0.118 |
| Platelet, ×109L−1 | 210.47±49.85 | 217.99±53.50 | 0.719 |
| MPV, fl | 9.73±0.86 | 9.03±0.70 | <0.001 |
MPV: mean platelet volume; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein.
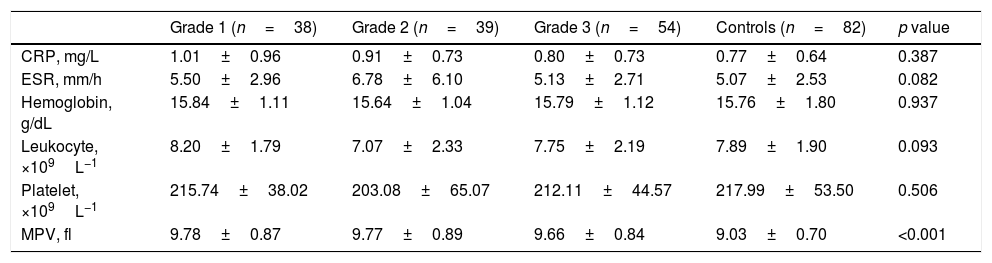
Comparison of laboratory features between graded varicocele patients and control groups.
| Grade 1 (n=38) | Grade 2 (n=39) | Grade 3 (n=54) | Controls (n=82) | p value | |
|---|---|---|---|---|---|
| CRP, mg/L | 1.01±0.96 | 0.91±0.73 | 0.80±0.73 | 0.77±0.64 | 0.387 |
| ESR, mm/h | 5.50±2.96 | 6.78±6.10 | 5.13±2.71 | 5.07±2.53 | 0.082 |
| Hemoglobin, g/dL | 15.84±1.11 | 15.64±1.04 | 15.79±1.12 | 15.76±1.80 | 0.937 |
| Leukocyte, ×109L−1 | 8.20±1.79 | 7.07±2.33 | 7.75±2.19 | 7.89±1.90 | 0.093 |
| Platelet, ×109L−1 | 215.74±38.02 | 203.08±65.07 | 212.11±44.57 | 217.99±53.50 | 0.506 |
| MPV, fl | 9.78±0.87 | 9.77±0.89 | 9.66±0.84 | 9.03±0.70 | <0.001 |
MPV: mean platelet volume; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein.
The seminal parameters of varicocele patients.
| Grade 1 (n=38) | Grade 2 (n=39) | Grade 3 (n=54) | Reference sperm values (WHO 2010) | |
|---|---|---|---|---|
| Volume (mL) | 2.90±0.55 | 2.17±0.36 | 1.99±0.31 | ≥1.5 |
| Sperm count (milyon/mL) | 42.00±11.10 | 38.52±11.41 | 23.50±5.23 | ≥15 |
| Total motility (%) | 57.00±5.55 | 53.28±5.75 | 49.81±6.29 | ≥40 |
| Progressive motility (%) | 47.00±5.55 | 43.33±5.85 | 36.90±3.14 | ≥32 |
| Morphology (%) | 32.50±11.11 | 19.25±5.70 | 12.50±5.23 | ≥4 |
A varicocele is a vascular disorder, but the fundamental pathological processes are not yet completely understood. However, various vascular changes, segmental obliteration, including vascular wall thickening, dismemberment of the internal elastic lamina, and occasional occlusive thrombi, have been microscopically identified.2 Varicocele may be part of a more general defect of the venous system, and consequently, some patients may present with varicose veins at other locations, such as the legs. There are studies showing that the relation between varicocele and other vascular diseases.3–5,22 Vascular disorders are associated with increased thrombocyte volume and increased thrombocyte activity.21,23
The platelet levels as part of the hemogram test, are significantly accompanied with hemostasis, vascular injury and inflammation. Most of the mediators required for thrombosis, atherosclerosis, coagulation and inflammation are provided by thrombocyts.6,12,13,23–25 The elevation of MPV is related with other markers of platelet activity, such as increased expression of adhesion molecules, increased platelet aggregation, increased thromboxane synthesis and thromboglobulin release. When platelets are activated they produce cytokines, like interleukin-6 (IL-6). Clinical trial results reported a strong connection between the circulating inflammatory biomarkers C-reactive protein and IL-6 and platelet gene expression.25 IL-1, IL-6, and tumor necrosis factor α (TNF-α) are among the progenitor cytokines that affect platelet production. Cytokines have been important mediators of the immunity and can be involved in numerous processes in the male genital tract including acting as immunomodulatory elements within the male gonad.26 Furthermore, considering the effects of cytokines on platelet production and activation, studies in varicocelle patients mostly investigate the cytokine level. Sakamoto et al. showed that IL-6 levels were higher in seminal plasma in varicocele patients, and a significant decrease after varicocelectomy.27 Sahin et al. reported that increased expression of interleukin-1alpha and interleukin-1beta is associated with experimental varicocele.28 Havrylyuk et al. reported that seminal plasma cytokine levels may be effective on male infertility.29
Varicocele is a vascular disorder, it leads to local and/or systemic inflammation. Varicocele may be induced by an inflammatory incident that could play a harmful role in spermatogenesis, although the exact pathophysiology of varicocele-induced harm is not completely understood. Platelets are heterogeneous in size and density within a subject. Various studies have reported the presence of great platelets to be a likely risk factor for thriving vascular disorders such as testicular torsion, varicocele, stroke, myocardial infarction, myocardial angina, coronary artery atherosclerosis, malignancy, ulcerative colitis, familial Mediterranean fever, Behçet's disease and Alzheimer's disease.7,21,23–25,30 The MPV is the most commonly used among the markers evaluating the changes in the platelet function and activation. The higher MPV values are also considered to be a useful indicator of higher thrombocyte activity. MPV demonstrate variability between the asset of low-grade and high-grade inflammation. The levels of platelets are incremented in the event of high-grade inflammation, leading to a decrease in the MPV level as a conclusion of the migration of a generality of the large reactive platelets to inflammatory areas and intense consumption of these platelets. In the event of low-grade inflammation, an increment in MPV level arises as a result of the increased reactive immature platelets.6
In the recent data, there are limited studies about examining the relation between MPV and varicocele. First, preliminary studies defined important higher MPV values in patients with varicocele than control group subjects. Moreover, positive correlations were detected between MPV and the varicocele grade as between MPV and the diameter of the left spermatic vein.8 Another study reported particularly higher MPV values in patients with varicocele than control group subjects.11 However, in this study, as in our study, there was no relation between MPV and varicocele grade. The comparatively small sample size of these two studies reduced the power of study. Coban et al.,9 also found MPV values to be significantly higher in the varicocele group than in the control group. At the same time, no significant correlation was found between MPV value and varicocele grade and spermatic vein diameter. Coban et al. demonstrated that an increased MPV level in patients with varicocele could conceivable be a vascular risk factor triggering endothelial dysfunction. In other study of Coban et al. researched the relation between preoperative and 6-month postoperative MPV values in patients whose varicocele was corrected with surgery.10 They found a significant decrease in MPV values in the postoperative period. But, it was not statistically significant.
Camoglio et al. reported that MPV is higher in adults with varicocele as reported by other studies, but this result is not true in adolescents.26 They assert that the reasons of different results could be some factors, as andrological disease or smoking status. But, We were excluded various situations affecting MPV from our study. The study of Pyo et al. is the first meta-analysis of published studies on the roles of MPV in varicocele and shows four major findings. First, there was a significant difference in MPV between varicocele patients and healthy subjects. Second, platelet count was significantly lower in varicocele patients than in healthy subjects. Third, there was no significant difference in platelet distribution width between varicocele patients and healthy subjects. Fourth, varicocele patients with low platelet count showed higher MPV level than patients with high platelet count.31 However, the correlations between pathogenesis of varicocele and MPV have not yet been fully explained, and MPV could be useful for the identification or observation of varicocele. In contrast to all these studies, Polat et al. reported that there was no difference in MPV between the varicocele patients and healthy subjects.32 They defended that an increase in MPV is generally associated with arterial vascular disease. But, the platelets are significantly involved in hemostatsis, vascular injury all, vascular diseases (arterial vascular disease, periferik venous vascular disease and deep vein thrombosis) and inflammation.6,7
Our study demonstrated MPV levels were statistically higher in varicocele patients than in normal healthy controls. Furthermore, we determined sensitivity and specificity levels for MPV in the prediction of varicocele (77% and 50%, respectively). However, there was no relation between MPV and varicocele grade. Our study supports the meta-analysis studies of Pyo et al. Acute phase reactant such as white blood cell count, CRP, and ESR are normal in patients with varicocele in our study. In the case of low-grade inflammation, an increase in MPV level arises as a result of the increased reactive immature platelets.6 In the light of these information, we think that the underlying mechanism of increased MPV in patients with varicocele is the result of low grade inflammation. The outcomes of our study suggested the role of platelet activation in the vascular etiopathogenetic foundation of varicocele. Lately, there is new evidence published that promotes the platelet activation together with vascular endothelial cells and leukocytes in microvascular dysfunction.33 When considering this evidence, etiopathogenesis of varicocele may be related with platelet activation and/or low-grade inflammation.
ConclusionThe results of this study suggested that MPV could represent an inexpensive marker that could be used in assessing low-grade inflammation in patients with varicocele in the pathophysiology of varicoceles. Furthermore, MPV may be a significant predictor in patients with varicocele. Nevertheless, comprehensive studies are required to expose this relationship.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNone.
Conflict of interestNone.
The place where the work was developed: Department of Urology, Radiology and Internal Medicine, Eskisehir Military Hospital, Eskisehir, Turkey.