Concerns about the long-term outcomes of ankle arthrodesis, has created renewed interest in total ankle replacement over the last decade. New implants have been designed with attention to reproducing normal ankle anatomy, joint kinematics, ligament stability, and mechanical alignment.
Encouraging intermediate clinical results for second-generation arthroplasties hold promise for patients with end-stage ankle osteoarthritis. The unique physiological and mechanical characteristics of the ankle joint, however, remain a challenge. Failures of ankle implants are, to date, still higher than implants in otherjoints. To a certain extent, this may be related to the inabilityof a surgeon to adequately restore the critical stabilizing role of the ligaments, as well as to poor reproduction of the normal mechanics of the ankle joint. However, adequate patient selection, careful preoperative planning, appropriate treatment of associated disorders (for example, instability, malalignment, and osteoarthritis of adjacent joints), and minimizing perioperative complications will help to maximize the chance for a successful outcome.
The ankle is one of the most commonly injured major joints in the human body, and the joint whose cartilage undergoes the greatest amount of biomechanical stress per square inch, yet the prevalence of osteoarthritis (OA) involving the ankle is significantly lower than that of the knee or hip joints. When OA does occur, it is most frequently secondary to trauma to the joint, and symptomatic end-stage ankle OA has been shown to result in mental and physical disability at least equal to that of people experiencing end-stage hip arthritis.
During the last decade, our understanding of the epidemiology and biomechanics of ankle OA has helped in developing new treatment options for this debilitating condition. Despite dismal failings in the 1970s and 1980s, new advances in total ankle replacement (TAR) techniques and implants are now challenging arthrodesis for the label of ”gold standard for treatment of ankle OA.” A number of new TAR systems have entered in the market and are showing great promise but not without persistent concerns and unanswered questions.
Modern TAR is highly predictable in providing good-to-excellent clinical results that are as good, if not better, than ankle arthrodesis. TAR offers patients significantly improved function and decreased pain with high satisfaction rates. By conserving ankle motion, TAR approximates more of a normal gait pattern than arthrodesis. TAR also reliably decreases stresses on joints adjacent to the ankle, such as the subtalar joint. Ultimately, this minimizes the chances a patient will require an arthrodesis at those joints. For all of these reasons, TAR rather than arthrodesis is the better treatment option for the right patient.
In an attempt to successfully replace the osteoarthritic ankle, this article aims first to highlight the specific changes of the osteoarthritic ankle, and second to provide an update about the progresses in TAR over the last year. By this, it will be a summary of the first author's experience with TAR in the last 18 years.
Ankle osteoarthritisAnkle osteoarthritis is characterised by a progressing loss of normal structure and function of the articular cartilage, ending in a complete anatomical and functional joint destruction. Clinically, OA patients suffer from joint stiffness, pain, reduction of physical and sports activity, daily life limitations, and sometimes even loss of their jobs.
Prevalence of Ankle OACompared to knee and hip OA, ankle OA is rarer in its prevalence (1). However, regarding the etiology subgroups distribution, the post-traumatic etiology is much higher in ankle OA (65–80%) (2) than in knee or hip OA (9.8% and 1.6%, respectively). The reason for this high percentage distribution in favour of the post-traumatic subgroup in ankle OA is to be seen in the high incidence of fractures about the ankle in the past decades. Regression models have shown that in the next decades a further increase of ankle fractures might be registered, this given the current demographic development with the generally increasing age of our population. Moreover, changes in leisure time activities with people being more active and performing more risky sports might further increase the amount of injuries of the lower leg and ankle. Therefore, patients in ankle OA are usually younger than in knee or hip OA (3).
Etiology and Pathomechanics of Ankle OAThe predisposing factors and the pathomechanisms which lead to the degeneration of the ankle after an injury are only insufficiently known. In principle, fractures of the tibial shaft, the distal tibia, the malleoli and the talus could well be the origin of a post-traumatic OA as chronic malalignment, chondral joint damage, or chronic instability patterns (4,5). The latency time between injury and OA was found to depend on fracture type and severity, occurrence of complications in the healing process, and patient related factors, e.g. age (6). Possible reasons for discrepant results among studies include differences in judging radiographic OA signs and the ankle OA grading systems used. The observation of several authors, that there seems to be hardly or no correlation between clinical outcome and radiological OA signs (7), might additionally lead to a variable definition of ankle OA. The evaluation of the importance of risk factors iseven more complicated by the interaction of one factor with the others.
Treatment of Ankle OATreatment of the end-stage osteoarthritic ankle is often complicated by associated problems such as scarring of the thin soft-tissue envelope, stiffness, malalignment, and degenerative changes in the subtalar and talonavicular joints that may result in instability, deformity, and changes in the biomechanics of the joint(s). An isolated arthrodesis of the ankle may address the immense pain at the ankle, but may not sufficiently address the associated problems and ongoing changes in the neighboring joints. This may become particularly problematic in young patients who have a long life expectancy
Total ankle replacementIn an era of joint replacement surgery, ankle procedures have failed to achieve what has been accomplished with other joints. An example that to some extent typifies the ”ankle replacement experience” to date is that of British orthopedic surgeon John Charnley, who, frustrated by the failure of his compression arthrodesis, turned to hip arthroplasty and successfully pioneered procedures in that specialty. Decades after Charnley's failed efforts, ankle arthrodesis is still the most commonly used procedure for the painful arthritic ankle. Although unilateral ankle arthrodesis may result in acceptable function (provided that the subtalar and midtarsal joints are normal and provide a compensatory mechanism), the disadvantages are, at least in the long term, significant (8,9).
Evolution and critical Issues of Total Ankle ReplacementIn the last two decades TAR gained an increasing acceptance among foot and ankle surgeons as a valuable treatment option in patients with end-stage ankle OA. TAR has a relatively short history compared with total replacement of the hip and knee joints. Most first-generation TAR were two component prostheses with two main prosthesis designs -constrained and unconstrained (3). In most cases, cement fixation was used on both sides- talar and tibial. The predominantly unsatisfactory results and extremely high failure rate substantially delayed the further development of TAR designs and limited acceptance among foot and ankle surgeons making the ankle arthrodesis the only one reasonable treatment option in patients with end-stage ankle osteoarthritis.
First-generation TAR designs had unacceptably high complication and failure rates (10,11). More recent prostheses have had encouraging intermediate-term results because of refined surgical techniques and improved designs (12–16). The most recent, less constrained designs require less bone resection but ligament stability, and permit increased axial rotation (12,14). The intermediate-term promising results (12–16), however, remain tempered by the difficulty of perfecting the surgical technique and the troublesome complications (17).
Discouraging results of 1st generation TAR have clearly suggested that only substantial improvement in prosthetic design (e.g. improve the intrinsic stability), change of fixation principle (e.g. cementless fixation and use of biological surfaces for improved osseous integration), and improved anatomical approach (e.g. in order to avoid the perioperative complications like wound healing disturbance or infection) would help to accept TAR as an alternative treatment in patients with end-stage ankle OA. Therefore, a thoroughly analysis of the common failure reasons for the 1st generation TAR was crucial and essential for the development of the 2nd generation TAR designs. All 5 main 2nd generation TAR replacement designs - Agility, Buechel-Pappas, HINTEGRA, STAR, and TNK prostheses - have been used in patients with end-stage ankle OA with encouraging and promising mid- and long-term results (12–16,22).
Today, different ankle designs are available, which can be divided into two main groups: two- and three-component systems (23).
Adequate mechanical support and bonding between the host bone and implant are fundamental to the success of TAR. Excessive bone removal as much as 17 mm on the tibial side and as much as 7 mm on the talar side resulted in seating of the implant on soft cancellous bone that failed to support the bone-implant interface, resulting in subsidence with weight-bearing (18). The nonanatomically-shaped and the undersized tibial implants also tended to subside into the soft cancellous bone (19). The residual tibial surface obviously was too weak to support the loads imposed by the implants. From the host side, bone integrity of the distal tibia therefore should be preserved as much as possible by minimal bone resection and conservation of the cortical rim. From the prosthesis side, the components should be enlarged to obtain full coverage of the subchondral bone and adequate support through the cortical rim.
A meniscal-bearing prosthesis can achieve complete congruency over the entire range of joint motion with minimally-constrained components to enable the soft tissues still to control physiologic motion at the joint. Appropriate balancing of the ankle ligaments is, however, mandatory to obtain stability and alignment of the replaced ankle. Ligament misbalance may result in pain, increased stress at the bone-implant interface, increased PE wear, and instability (3,17,21). This implicates the need of high intrinsic stability and optimal load transmission in an ankle prosthesis, which only can be achieved with surfaces that are as anatomical as possible (3).
The Author's SolutionIn an attempt to respond to the discovered problem of early ankle designs, the main author (BH) developed the HINTEGRA ankle as a new ankle prosthesis concept (figure 1). The main innovation was a fíat resurfacing area of an anatomically shaped tibial component to use the whole resection area of tibial metaphysis for bony support and to avoid any stress shielding. The talar component is anatomically shaped, with a shorter radius on medial side. The two interfaces between the metallic components and the PE insert are parallel to provide coronal plane stability. While the early HINTEGRA ankle design has evidenced some problems of mid- to long-term stabilities of components, improvements were achieved by adding pegs to talar component and Titanium fluid on porous coat. This new three-component prosthesis requires minimal bone resection, retains the entire cortical rim of the distal tibia, has enlarged components, and has anatomic-shaped surfaces. Due to the stem less fixation to distal tibia, it allows for full weight-bearing from the very beginning after the surgery.
Indications for Total Ankle ReplacementBoth, primary (e.g. degenerative disease) and posttraumatic OA are important indications for TAR. Other common indications for TAR are systemic arthritis (e.g. rheumatoid arthritis) and secondary OA. Secondary OA has also been found to be associated with some underlying diseases and/or pathologies, such as hemophilia, hereditary hemochromatosis, gout, postinfectious arthritis, avascular talus necrosis.
Patients with bilateral ankle OA are good candidates for TAR, because bilateral ankle fusion may not be the most optimal surgical procedure in this patient cohort, given its detrimental influence on gait and functional results.
A special indication for TAR is the salvage for failed TAR (24). One of the critical issues in revision arthroplasty is the quality and amount of remaining bone stock toensure long-term stability of revision prosthesis components. Therefore, if the residual bone stock is not sufficient, ankle fusion should be performed in this patient cohort. Another special indication for TAR is the salvage for non-union or mal-union of ankle fusion. However, taking down an ankle fusion and conversion toTAR is a technically demanding procedure, which should be performed only if bone stock is sufficient and soft tissue condition are appropriate (25). If performed by an experienced foot and ankle surgeon, this surgical procedure shows promising mid-term results with low intraoperative and postoperative complications.
Contraindications for Total Ankle ReplacementThe absolute contraindications for TAR are the following: acute or chronic infections, avascular necrosis of more than one third of the talus, neuromuscular disorders, neuroarthropathy (Charcot arthropathy of the midfoot and/or hindfoot), and diabetic syndrome with polyneuropathy. Also patients with unmanageable instability and/or malalignment, which cannot be sufficiently addressed by additional procedures (e.g. corrective osteotomies) should not be considered for TAR. Highest demands for physical activities (e.g. contact sports, jumping) are another contraindication for TAR. Suspected or documented metal allergy or intolerance is a rare contraindication for TAR; however, it should be excluded preoperatively.
The relative contraindications for TAR are the following: severe osteoporosis, immunosuppressive therapy, diabetic syndrome without polyneuropathy. Patients with increased demands for physical activities (e.g. jogging, tennis, downhill skiing) should be informed about possible prosthesis failure due to increased wear and higher rate of aseptic loosening.
”Ideal candidate” for Total Ankle ReplacementBased on our clinical experience the ”ideal candidate” for TAR is/has:
- -
Middle-to-old aged
- -
Reasonably mobile
- -
No significant co-morbidities
- -
Low demands for physical activities (e.g. hiking, swimming, biking, golfing)
- -
No obesity/overweight (normal or low body mass index, however obesity is not a contraindication for TAR41)
- -
Good bone stock
- -
Well aligned and stable hindfoot
- -
Good soft tissue condition (e.g. no previous surgeries of the foot/ankle)
- -
No neurovascular impairment of the lower extremity
First, all previous medical reports (e.g. surgery reports) and imaging studies should be collected and carefully analyzed. Second, careful assessment of patients’ history should be performed with specific address of following aspects: actual pain, limitations in daily activities, sports activities, and current and previous treatments. Patients with any aforementioned absolute contraindications should be excluded. If necessary, a consultation in department of neurology and/or internal medicine should be performed prior to planning of surgery.
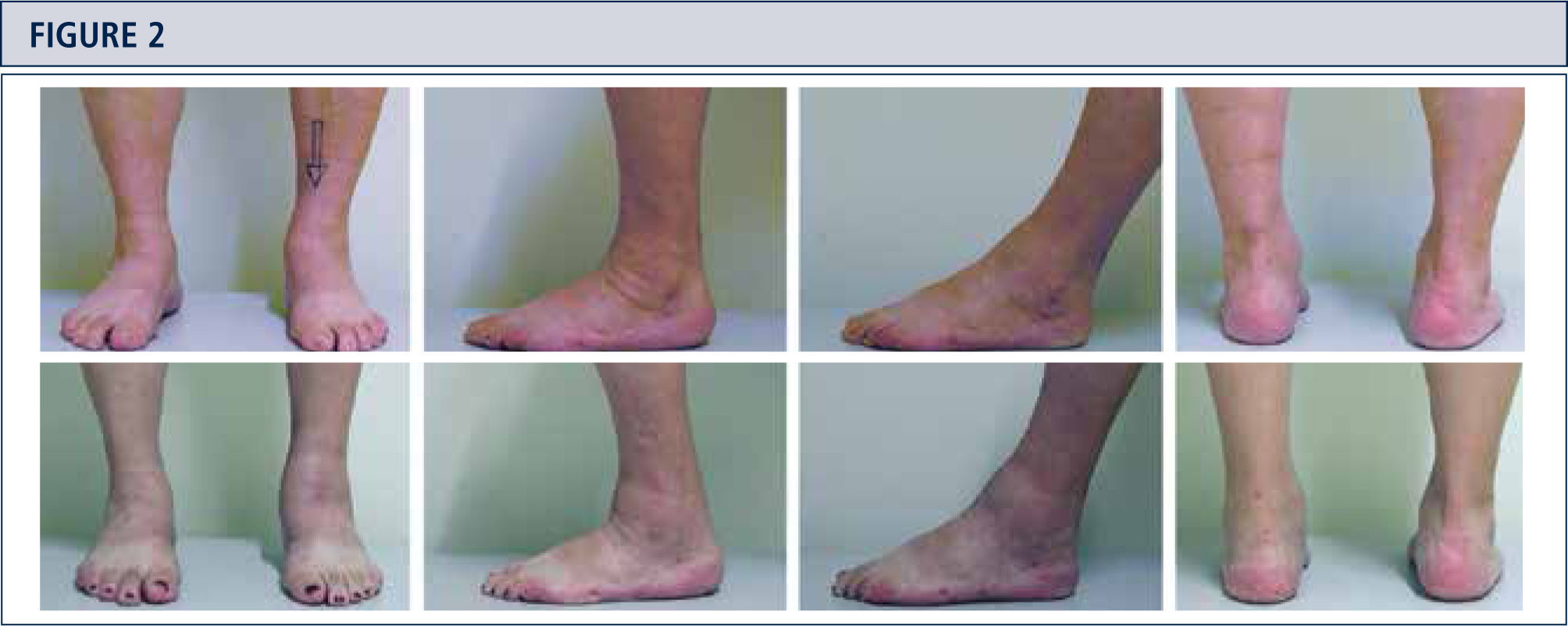
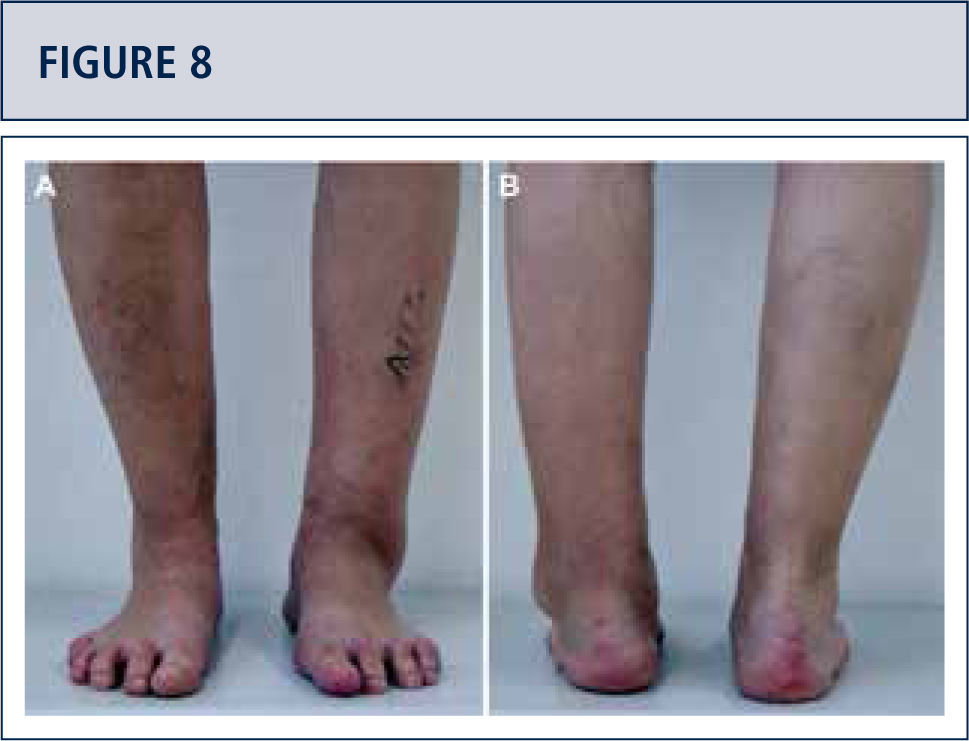
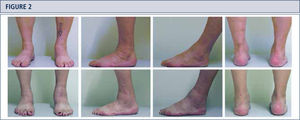
The routine physical examination includes careful inspection of the foot and ankle while walking and standing with special attention given to obvious deformities and skin and soft tissue condition (figure 2). Hindfoot stability should be assessed manually with patient sitting. Ankle alignment is assessed with patient standing. Ankle range of motion is determined with a goniometer placed along the lateral border of the leg and foot. All goniometer measurements are performed in the weight-bearing position.
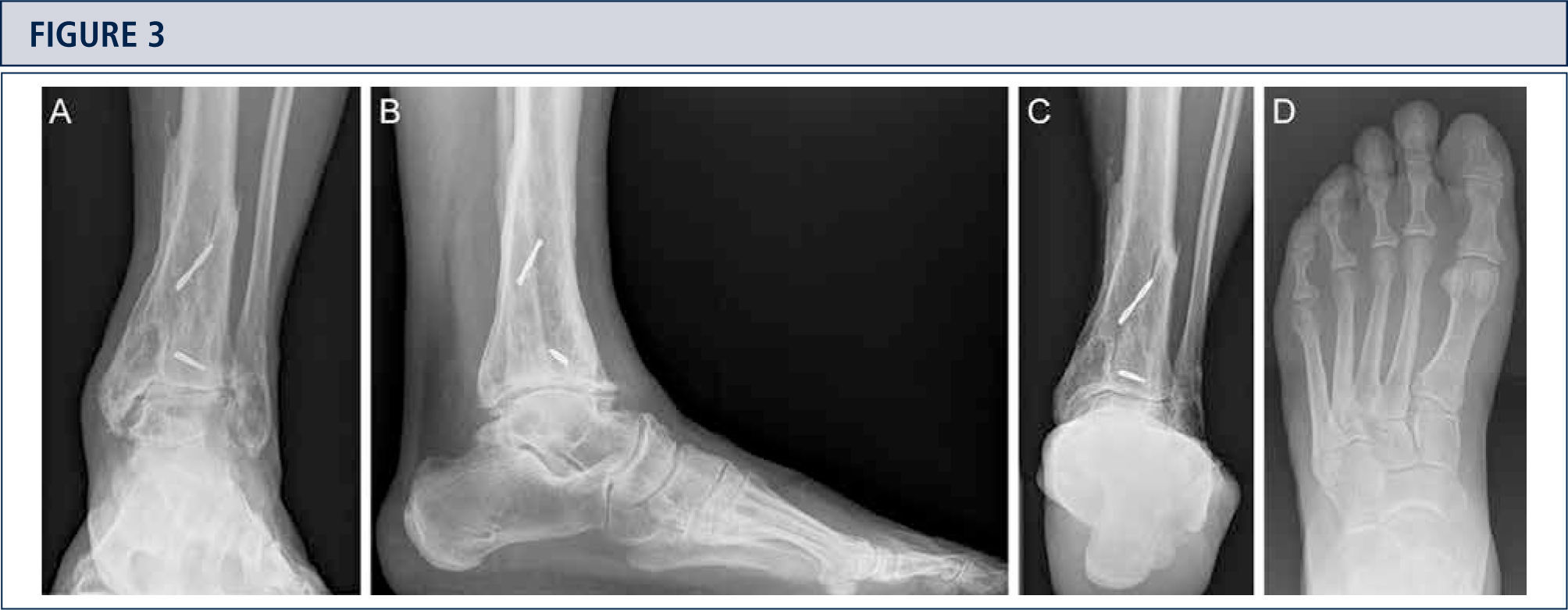
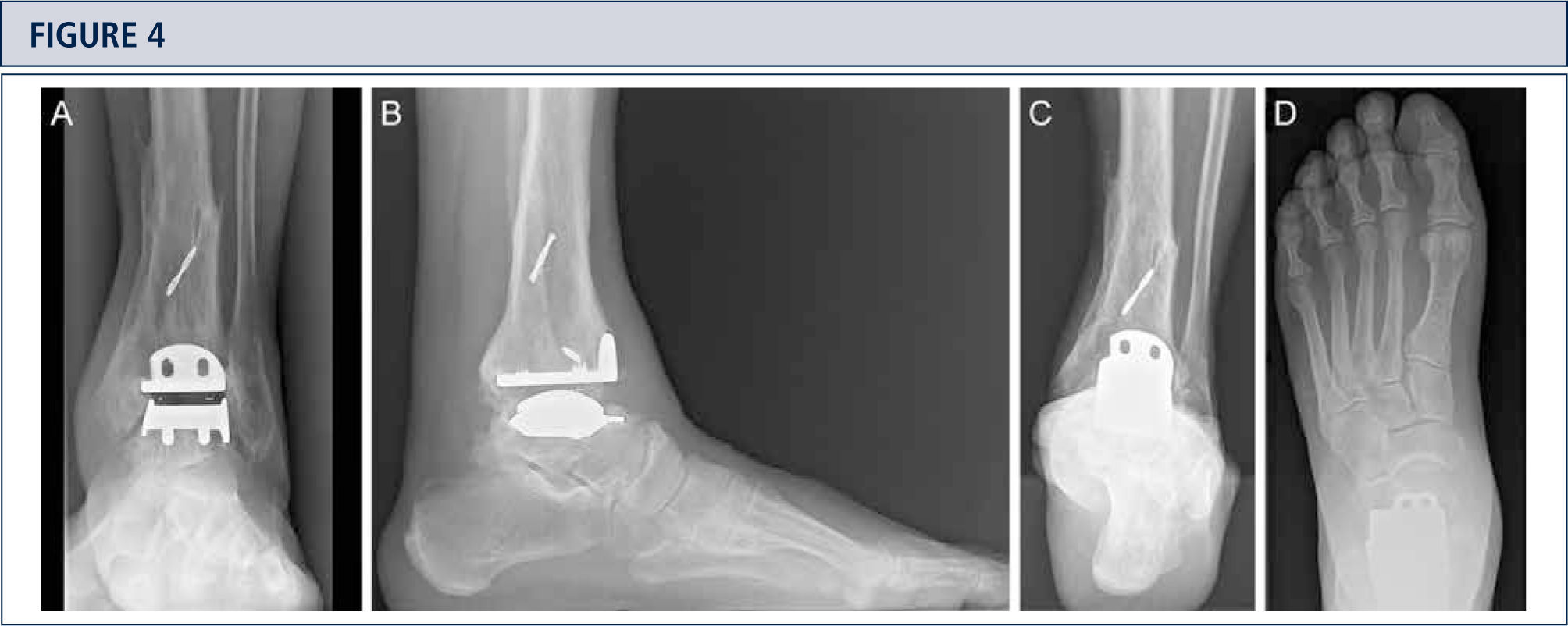
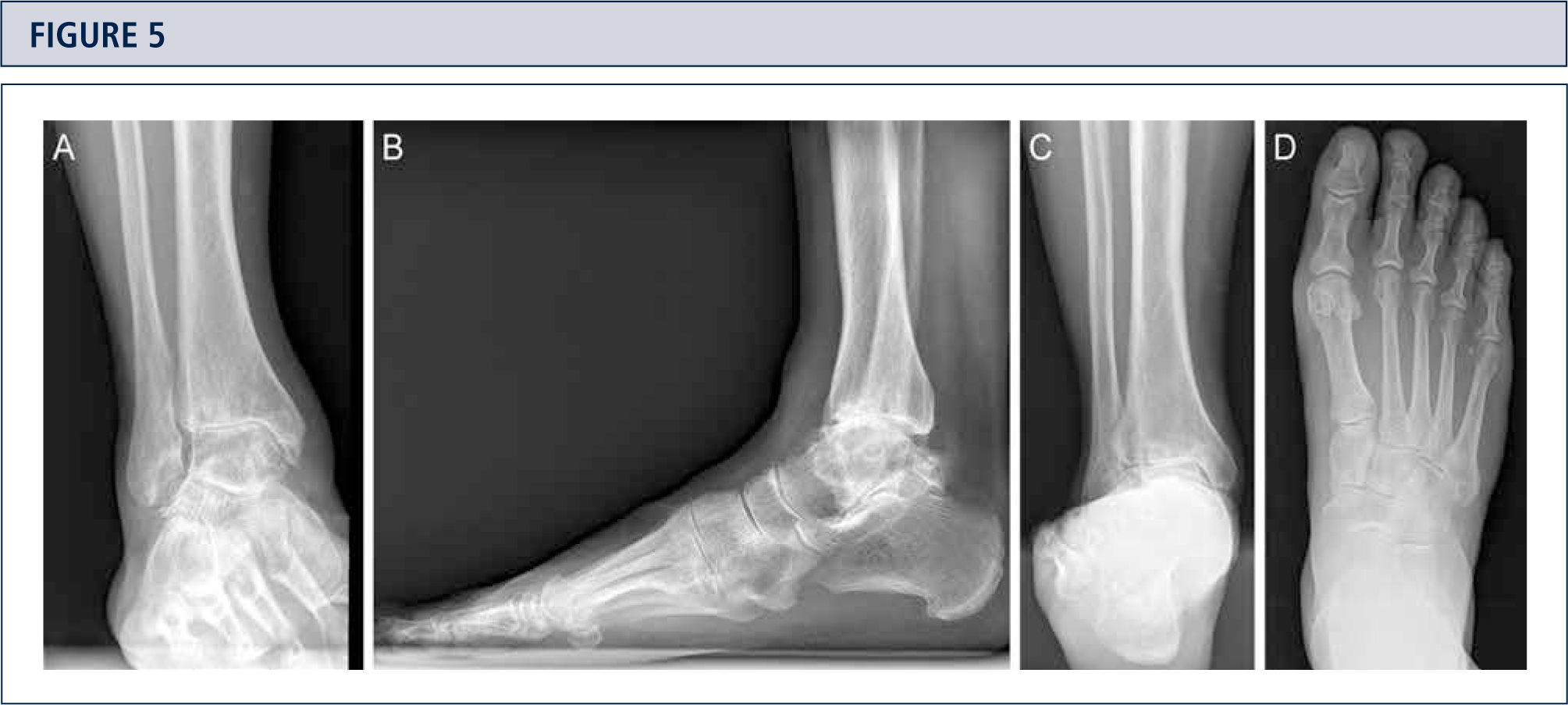
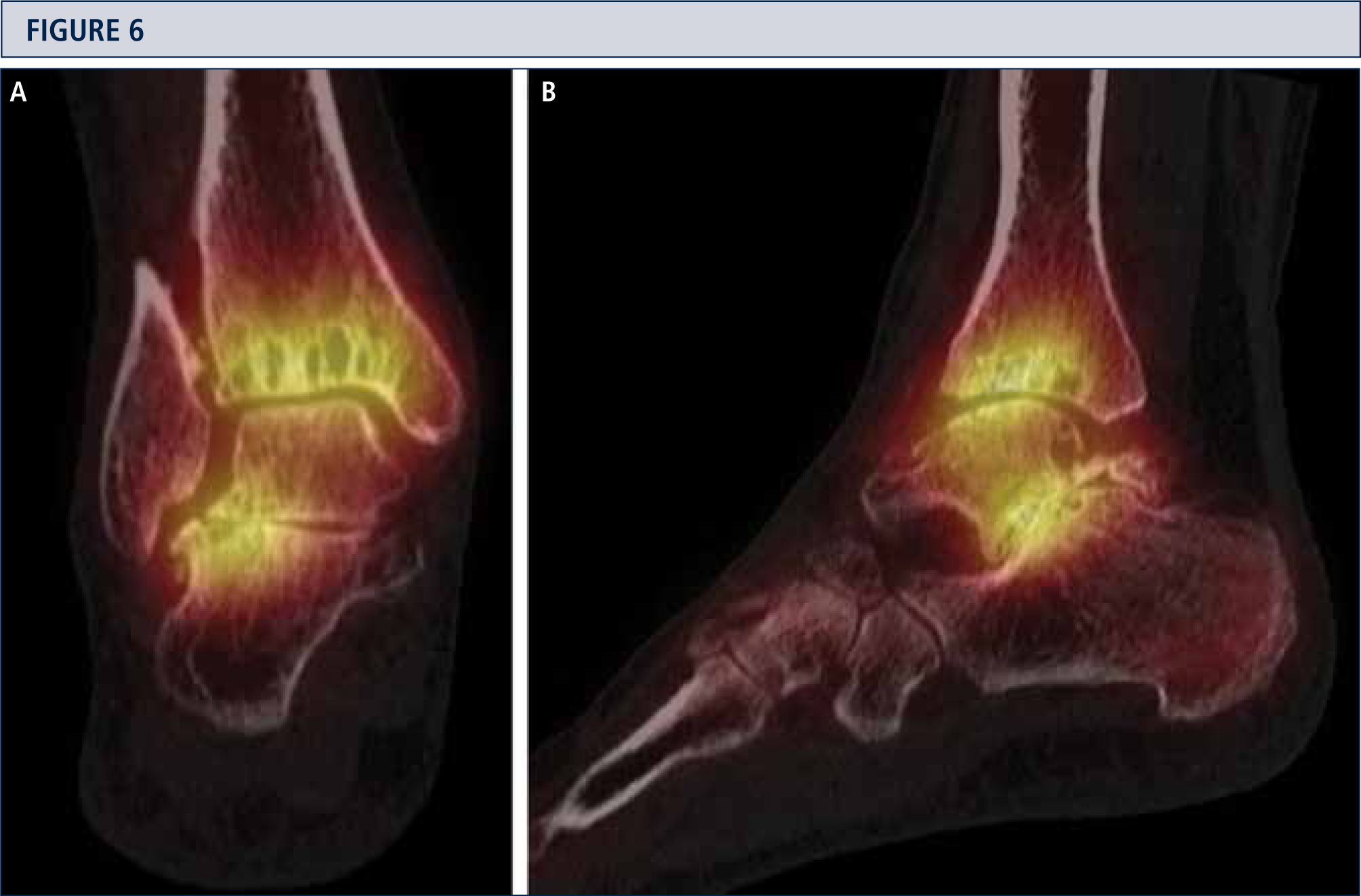
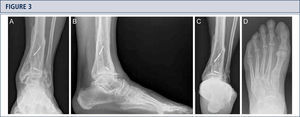
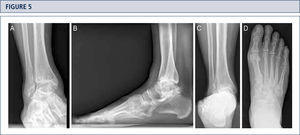
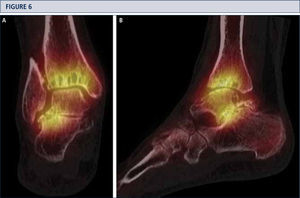
Radiographic EvaluationRadiographic evaluation of affected ankles is performed using weight-bearing radiographs including antero-posterior views of the foot and ankle and a lateral view of the foot (figure 3 A,B,D). Only weight-bearing radiographs should be used for evaluation of foot and ankle alignment and biomechanics because non-weight-bearing radiographs are often misleading. Furthermore, standing position may help to standardize the radiograph technique allowing more reliable comparison between pre- and postoperative radiographs (figure 3 and 4). Saltzman view should be used to assess the inframalleolar alignment (figure 3C). The supramalleolar ankle alignment should be assessed in coronal and sagittal plane by measurement of medial distal tibial angle and anterior distal tibial angle, respectively (26). In patients with degenerative changes of the adjacent joints single-photon-emission computed tomography (SPECT-CT) may help to evaluate the morphologic changes and their biological activities (figure 5–7) (27). We do not recommend the routinely use of magnetic resonance imaging in patients with ankle OA. However, this diagnostic tool may be helpful to assess injuries or morphologic changes of ligament structures and tendons, and to evaluate the localization and degree of avascular necrosis of talus and/ or tibia.
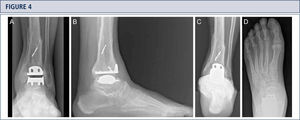
Eight-year follow-up evaluation (same patient as Fig 2): Standard weight-bearing X-rays done with the same technique under fluoroscopy allow for precise analysis. A) AP-view of the ankle; B); lateral view of the foot C), Saltzman alignment view; D) AP-view of the foot.
Preoperative evaluation for a painful end-stage OA in a 53-year old woman 19 years after a severe ankle sprain. Radiographic assessment evidences an advanced stage of ankle OA with bipolar subchondral cyst formation, a slight anterior extrusion of talus, anda peritalar instability with subsequent valgus tilt of talus with redagr to the calcaneus. A) AP-view of the ankle; B) lateral view of the foot; C), Saltzman alignment view; D) AP-view of the foot.
A SPECT-CT evidences an active process at ankle and subtalar joint, which helped to identify the source of pain. Based on this, a TAR in combination with a subtalar arthrodesis was done (same patient as Fig 5). A) coronal plane view; B) sagittal plane view.
General or regional anesthesia is used for this procedure. The patient is placed in a supine position, and a pneumatic tourniquet is applied. A single preoperative dose of a second-generation cephalosporin is administered.
An anterior longitudinal incision is made to expose the extensor retinaculum, which is then dissected along the lateral border of the anterior tibial tendon. After capsulotomy and ankle joint exposure osteophytes on the tibia and on the talar neck are removed.
The tibial cutting block is placed aligned to the tibial tuberosity as the proximal anatomical landmark and to the middle of the anterior border of the tibiotalar joint as the distal anatomical landmark. The anterior rim of the tibia serves to align the jig. Given by the resection bloc, the natural slope of the tibial plafond of 4° is maintained. Two to 3mm of the tibial plafond is resected using an oscillating saw. Careful debridement of the posterior capsule is performed and all ossification is removed if necessary. A measuring gauge is used to determine the size of the tibial component. Tibial trial component is inserted to assess corred fit and alignment. In the case of inappropriate fit of anterior shield to anterior border of tibia, it is trimmed accordingly.
The talar resection block is placed into the tibial cutting block, and maximal distraction is applied to the ankle in order to tension the collateral ligaments of the ankle joint complex. While to foot is held in neutral position, talar bloc is fixed to talus with two pins. After having done the horizontal resection cut of talus through the cutting slot using an oscillating saw, the resection bloc is removed. The 12-mm-thick spacer, accounting for the minimal thickness of the three prostheses components, is then inserted to prove whether sufficient bone resection is performed or not. Usually, the size of talar resection bloc is selected according to the measured tibial component. In the case of important undersize to the talar resection area, e.g. more than 3 mm left on the medial and lateral side, the resection bloc of one size bigger is selected. In the case of important oversize to the talar resection area, e.g. less than 1mm left on the medial and lateral side, the resection bloc of one size smaller is selected. The selected talar resection block is positioned with its two hooks to the posterior talus and parallel to the medial border of resection area, which typically results in a correct alignment to the longitudinal axis of the foot, e.g. the handle being directed along the 2nd ray of the foot held in a plantigrade position. In this position, the resection bloc is fixed to the talus with two to four pins and it serves then to perform the posterior, medial, and lateral talar resection cuts. Talar trial component is impacted. Once an appropriate fit is achieved, the anterior resection is done, and the two drill holes for the pegs are done.
Tibial and talar surfaces are checked for any cysts or defects. If present, they are debrided to vital bone and filled with bone matrix (Isotis Orthobiologics US, Integra, Plainsboro, New Jersey). Final prosthesis components are inserted with press-fit technique using a hammer and special impactor: first the talar component, then the tibial component, and finally the mobile bearing. Fluoroscopy is used to check the position of implants.
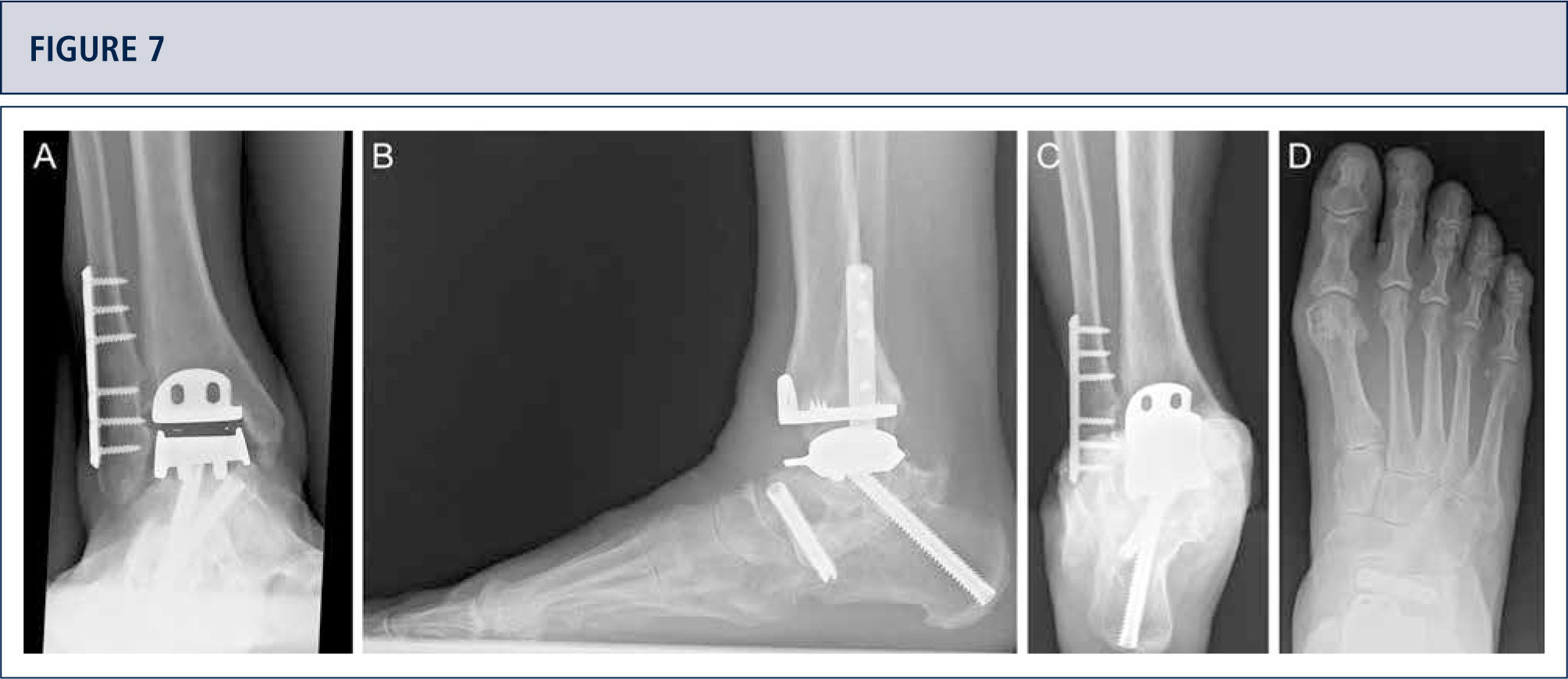
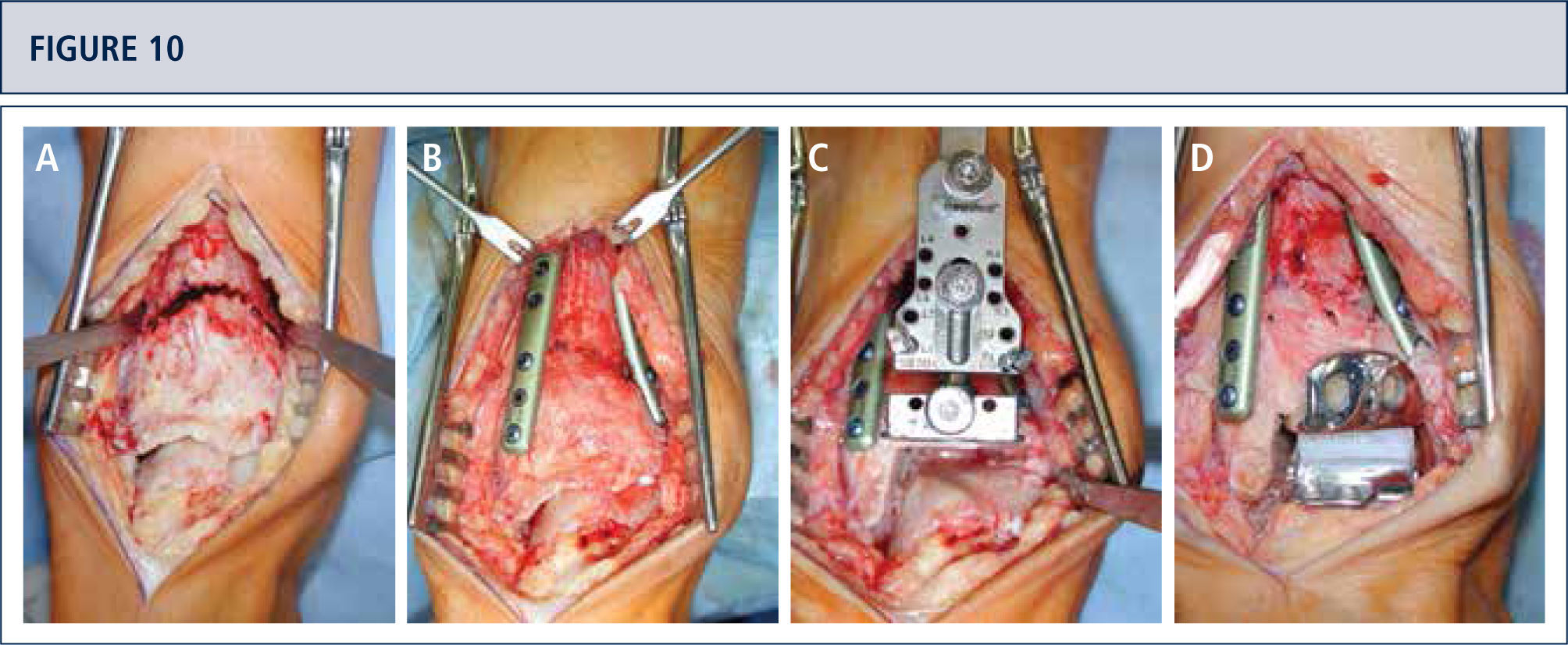
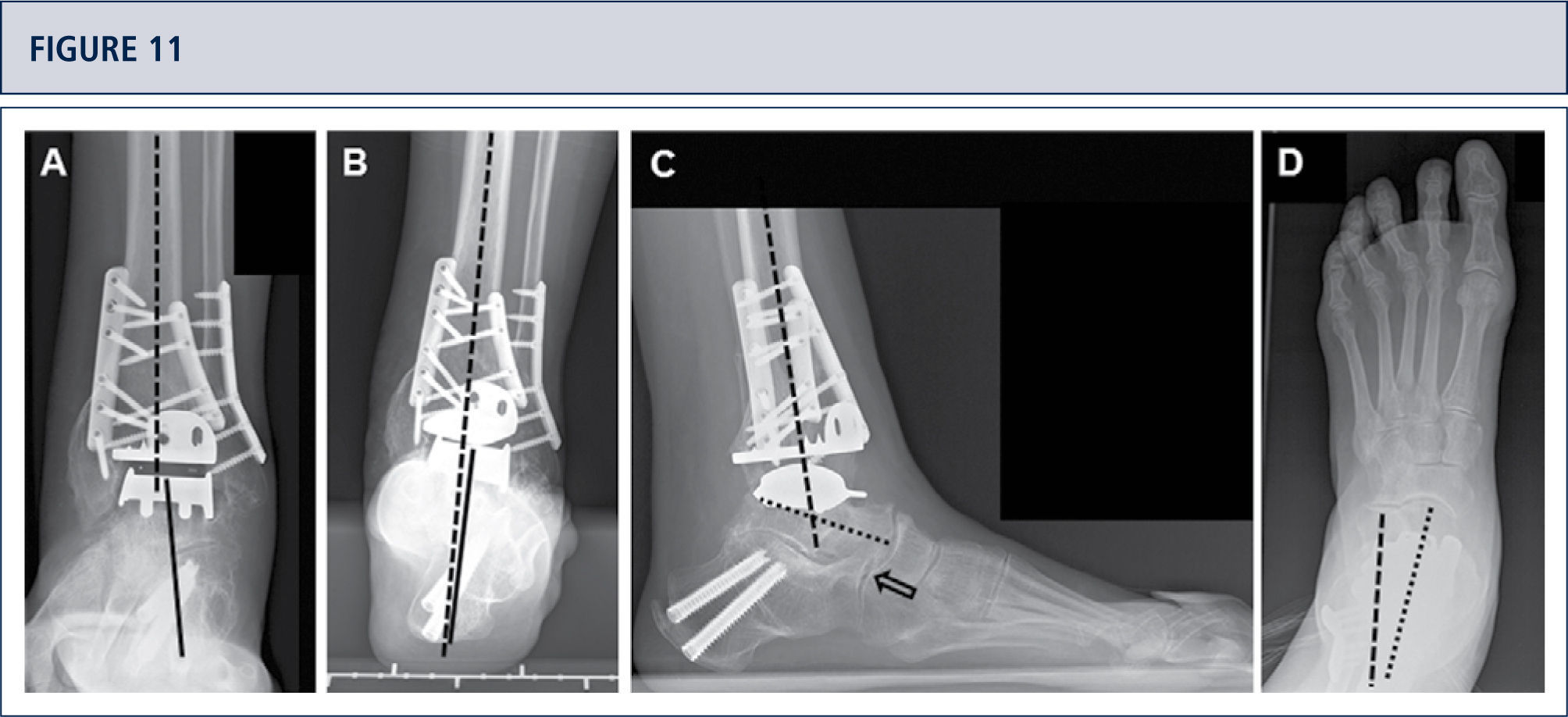
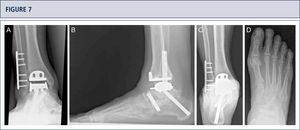
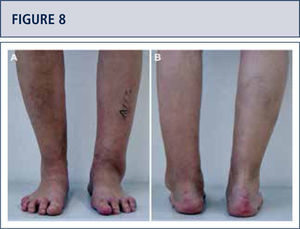
If necessary, additional surgeries are added to properly balance (figure 8–11) and stabilize the ankle (figure 7). Then, the joint is closed over a drainage drain by continuous suture of extensor retinaculum, and interrupted sutures of the skin.
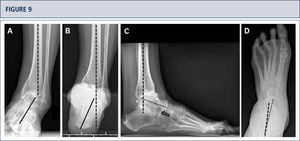
Preoperative evaluation radiographic evaluation (same patient as Fig 8). Standard weight-bearing X-rays. A) AP-view of the ankle showing a varus tilt of >30°; B) Saltzman alignment view showing a significant varus malalignment of the heel; C), lateral view of the foot showing a horizontalization of talus; D) AP-view of the foot showing a exorotation of talus with highly decreased talo-calcaneal angle.
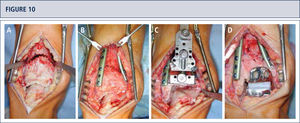
Intra-operative situs (same patient as figura 8). A) A dome osteotomy of distal tibia was made; B) After having osteomized the fíbula, the distal tibia was rotated until a neutral joint line was achieved anda double plate fixation was done; Q Resection of distal tibia using the tibial resection bloc that was aligned along the tibial axis; D) Final situation after TAR. In addition to the supramalleolar correction, a medial sliding osteotomy of calcaneus was done to properly align the heel.
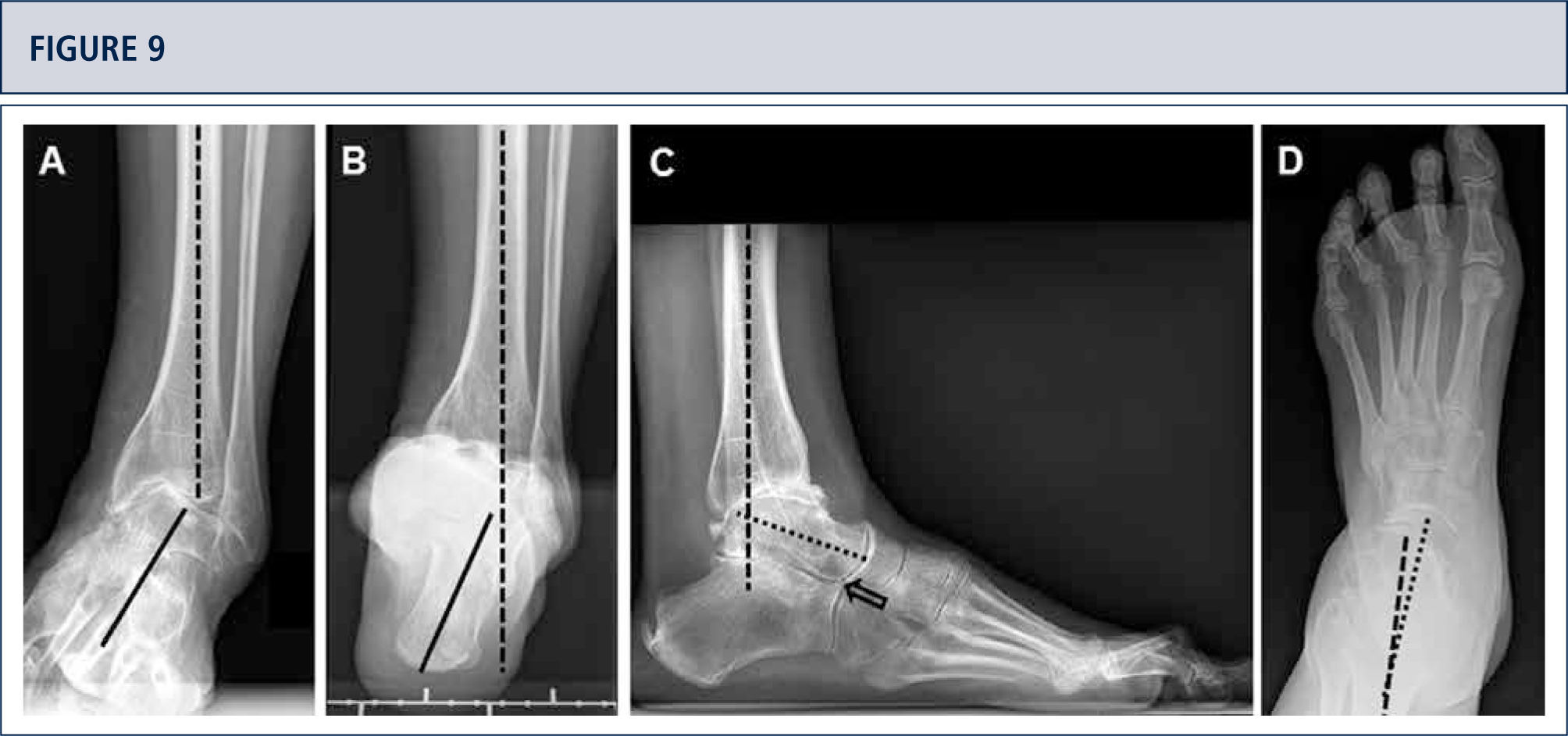
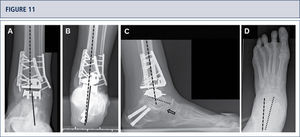
Five years after surgery, the patient was highiysatisfied with the result. Standard X-rays show a well-balanced ankle and stable implants. A) AP-view of the ankle showing a balanced talus within the ankle mortise; B) Saltzman alignment viewshowing a well aligned ankle joint complex; C), lateral view of the foot showing thea normalized position of talus (plantar flexión with overlapping of calcaneus of 20%); D) AP-view of the foot showing a normalized position of talus (talo-calcaneal angle of 28°).
After final fluoroscopic check the wound is closed in layers. A dressing is applied, and a splint is used to keep the foot in a neutral position.
TAR in Varus Osteoarthritic AnkleIn patients with incongruent tibiotalar joint the joint contracture at the medial side can be addressed by osteophytes resection of the medial malleolus. If medial contracture still persists a surgical release of the deltoid ligament can be performed.As an alternative, we prefer a flip osteotomy of the medial malleolus to lengthen and align it to the talus.
After the proximal varus correction is performed the hindfoot alignment should be proven clinically and using fluoroscopy. In patients with remaining varus position of the heel the deformity may be corrected by Dwyer osteotomy or z-shaped osteotomy of the calcaneus. In patients with progressive degenerative changesof the subtalarjointthe subtalar arthrodesis may be considered.
In patients with lateral ligamental instability anatomic repair of the lateral ligament complex using suture anchors should be performed. In patients with insufficient ligament tissues an augmentation with a free plantaris tendon graft can be considered for reconstruction of the anterior fibulotalar and calcaneofibular ligaments. Furthermore, the peroneus longus to peroneus brevis tendon transfer may provide reliable soft tissue stabilization and reduce the inversion moment arm of the first ray.
After hindfoot correction and stabilization of ankle complex in patients with remaining plantar flexed first ray a dorsi flexión osteotomy of the 1st metatarsal or medial cuneiform bone will allow to address the pronation position. In patients with varus malalignment of the hindfoot an equinus contractures is often observed leading to limited ankle dorsiflexion. PercutaneousAchilles tendon lengthening orgastrocnemius recession can be performed. The surgeons should be aware to avoid the failure of triple hemisection atthe ankle mobilization, however.
TAR in Valgus Osteoarthritic AnkleIn patients with valgus malalignment of the distal tibia of more than 5° we suggest a supramalleolar correcting osteotomy. After the supramalleolar correction the heel position should be proven clinically and using fluoroscopy. In patients with remaining inframalleolar valgus deformity a medial displacement osteotomy of the calcaneus should be performed with the aim of the neutral alignment of the heel (0°–5° of valgus). In patients with significant subtalar contracture and/or degenerative changes of the subtalar joint a subtalar arthrodesis should be performed. In the case of a advanced flatfoot deformity, a triple arthrodesis may be considered. In patients with significant ligamental instability medial and/or lateral ligament reconstruction should be performed.
Postoperative CareThe wound drain is removed after 24 hours. After two days the dressing and splint are changed. A pneumatic foot cuff is used to reduce postoperative swelling. All patients receive thromboprophylaxis with subcutaneous low-molecular-weight heparin (Fragmin, 5000IU; Pfizer AG, Zürich, Switzerland), starting 12 hours preoperatively and continuing daily for 6 weeks postoperatively. When the wound is dry – usually three to four days after the surgery – a stable walker (VACOped; OPED, Cham, Switzerland) is used for the mobilization for six weeks. In the case of additional surgeries to stabilize the hindfoot, or to fuse neighbor joints, a scotch cast is used for eight weeks. Full weight bearing is allowed as tolerated with exception of patients who underwent additionally a corrective osteotomy of distal tibia. Lymph drainage and active motion is allowed those patients who are treated with the walker.
After the patient stopped using the walker or scotch cast, the rehabilitation program is continued, including active and passive ankle motion, stretching and strengthening of the triceps surae, and proprioceptive exercises. In patients with persistent swelling we recommend compression stockings. A low level (e.g. hiking, swimming, biking, golfing) and a normal level (e.g. tennis, downhill skiing) of sports activities are allowed according rehabilitation status, usually after three and six months, respectively. Contact sports or activities excessive impact forces are prohibited, however.
Follow-UpThe first clinical and radiographic follow-up is made at 6-8 weeks to check the healing of softtissues including skin and osteointegration and position of the prosthesis components. The next clinical and radiographic follow-ups are performed at four months, one year, and then annually thereafter.
Clinical Follow-upFor appropriate analysis of the clinical outcome following parameters/scores are used. We measure the range of motion clinically with a goniometer along the lateral border of the leg and foot. To assess the postoperative pain relief all patients rate their pain on a visual analog scale (VAS) of 0 points (no pain) to 10 points (maximal pain). The American Orthopaedic Foot and Ankle Society (AOFAS) hindfoot score is calculated.The SF-36 questionnaires are used to assess the quality of life.
Patients indicate their satisfaction with the procedure using a modífied Coughlin rating for category scale: very satisfíed, satisfíed, partially satísfied, and not satísfied. Patient's sports activity level is documented using a Valderrabano score: grade 0, none; grade 1, moderate; grade 2, normal; grade 3, high; and grade 4, elite. Patients’ gait is observed clinically and then analyzed using pedobarography.
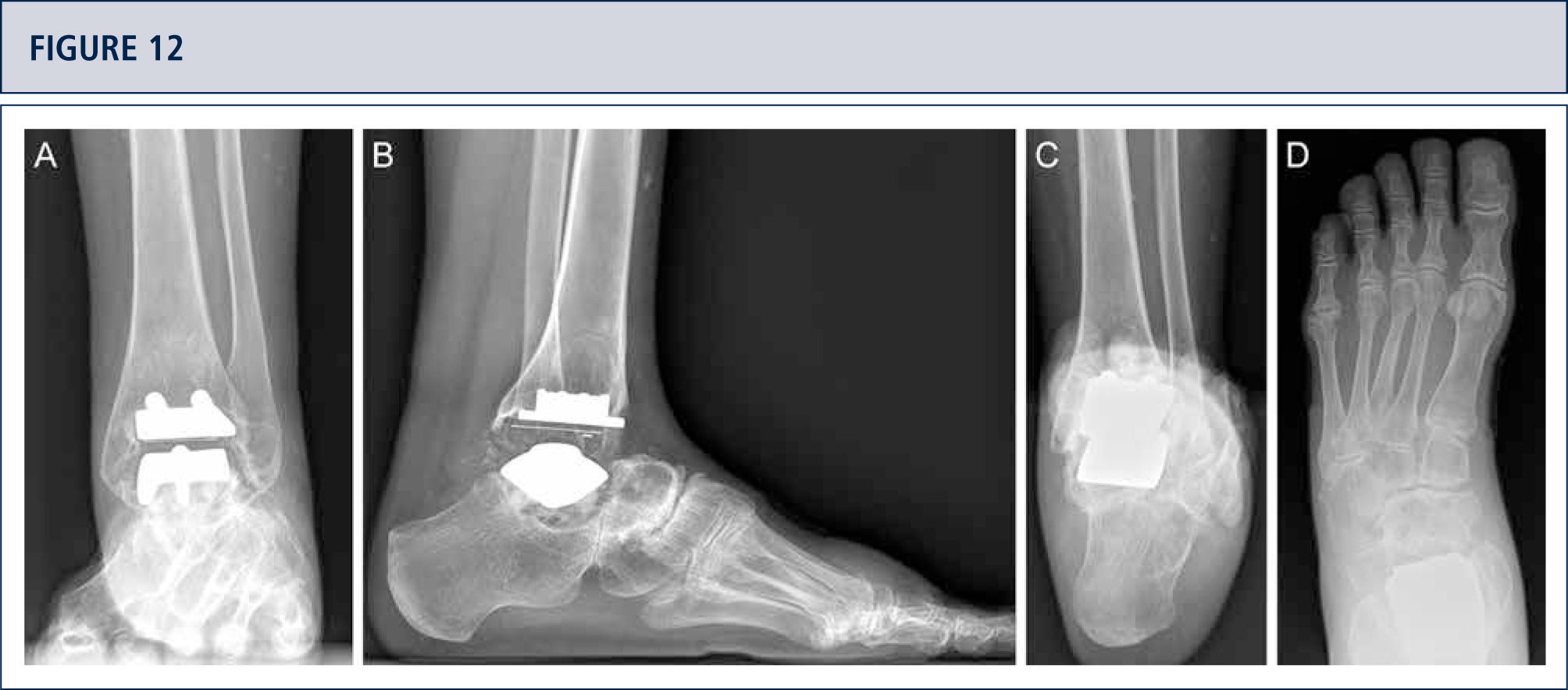
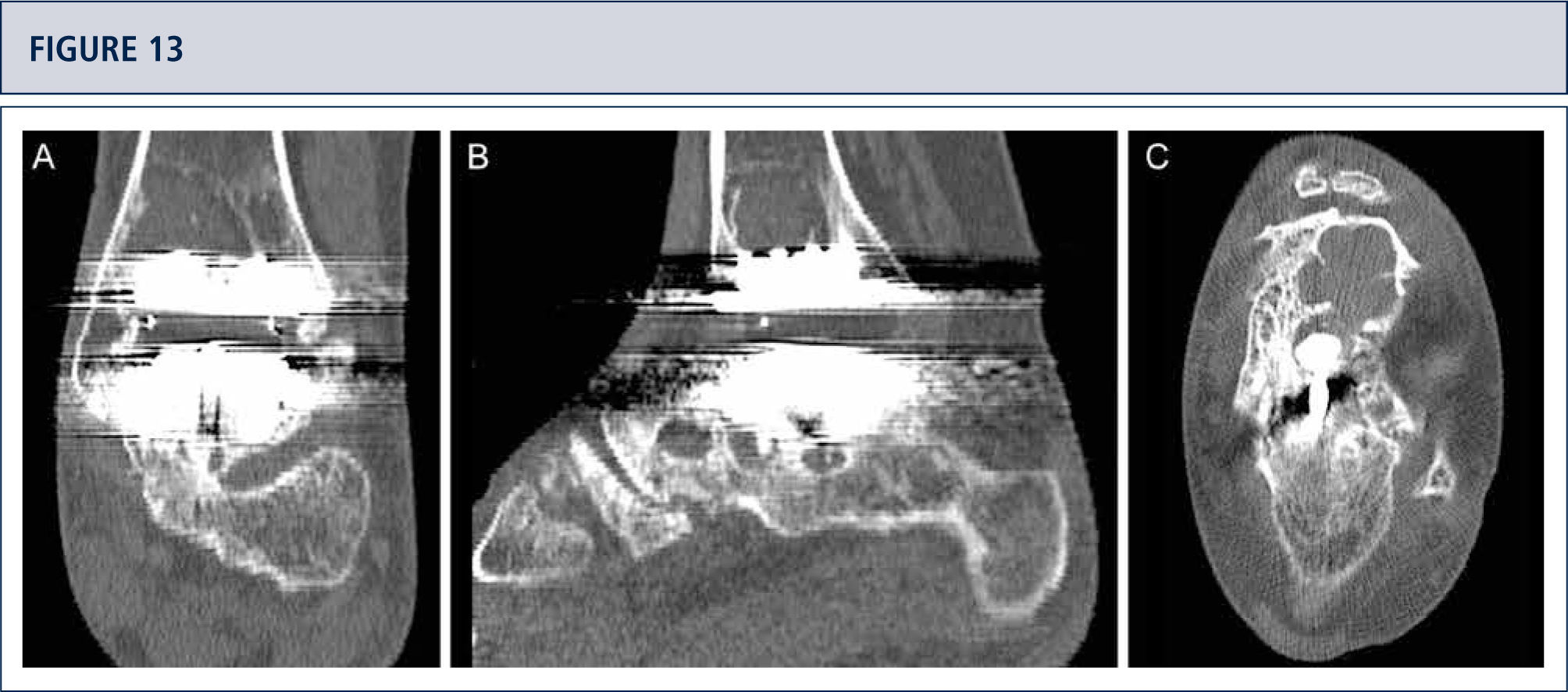
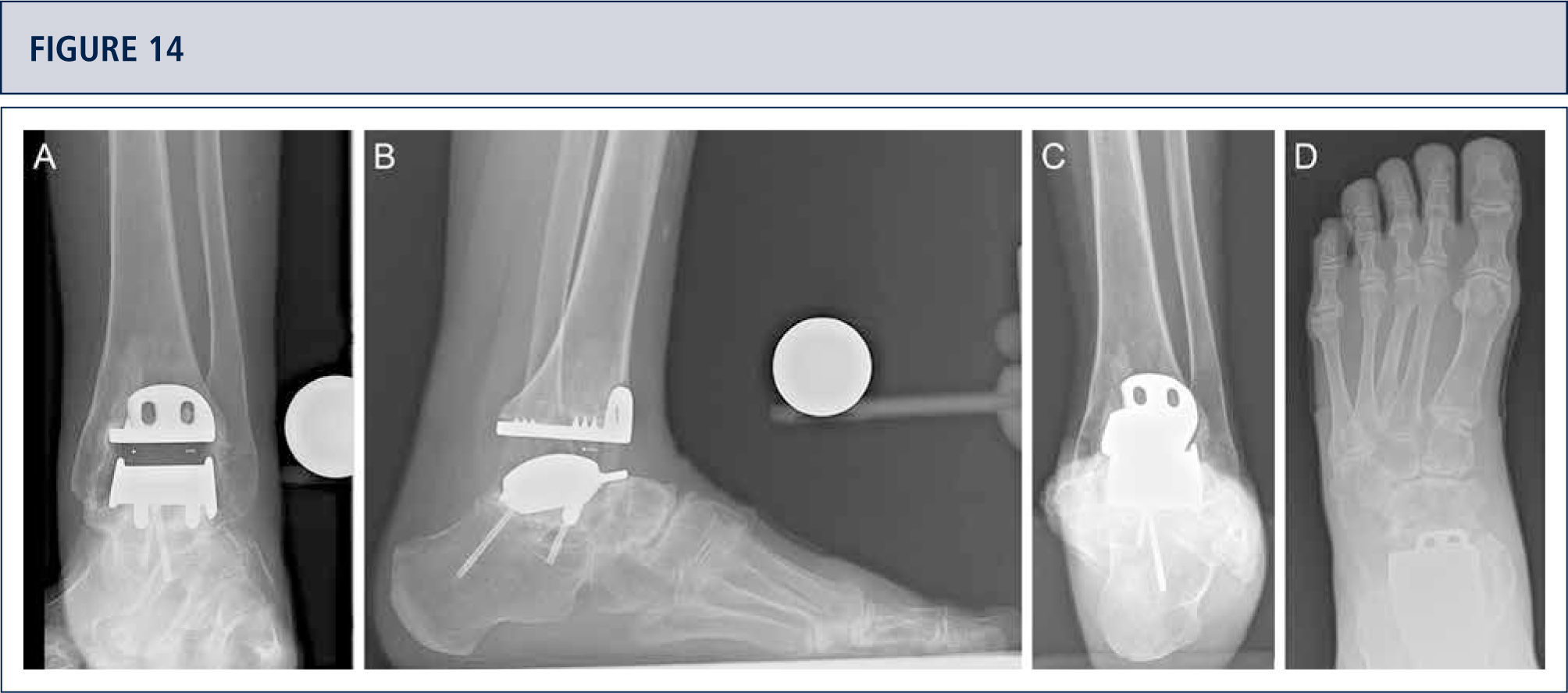
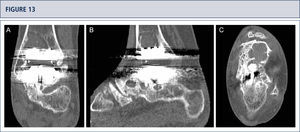
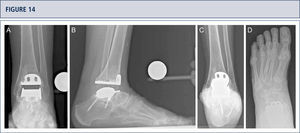
Radiographic Follow-upRadiographic assessment is performed using weight-bearing radiographs with fluoroscopy to standardize the radiographic technique. The postoperative hindfoot alignment is assessed using a Saltzman view. Following angular values are used for standardized assessment of prosthesis components: α-, (β-, and γ-angles. α-and β-angles are used for assessment of the tibial component and measured between the longitudinal axis of the tibia and the articular surface of the tibial component in the anteroposterior and lateral views, respectively. All radiographs are analyzed regarding the localization and degree of heterotopic ossifications. Heterotopic ossifications are described according to the modified Brooker's classification: 0: no heterotopic ossifications; I: islands of bone within the soft tissues about the ankle: II and III: bone spurs from the tibial or talus, reducing the posterior joint space by <50% or ≥50%, respectively; IV: bridging bone continuous between the tibia and the talus. Change in position of the tibial component's fíat base by more than 2° relative to the longitudinal axis of the tibia and/or progressive radiolucency greater than 2mm on the anteroposterior or lateral radiographs is defined as loosening of the tibial component. Subsidence of the talar component by more than 5mm or a position change of greater than 5° relative to a line drawn from the top of the talonavicular joint to the tuberosity of the calcaneus is defined as loosening of the talar component. Because of prosthesis design it is difficult to assess the position changes of the talar component. Therefore, in cases with suspicion of loosening or subsidence a CT scan or SPECT-CT should be performed. Meticulous working-up is in particular important for planning od revision TAR (figure 12–14).
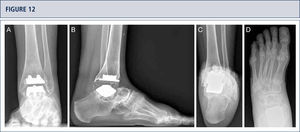
39-year old woman with rheumatoid arthritis presenting with a painful ankle 9 years after TAR (S.T.A.R ankle). A) AP-view of the ankle showing some trabecular formation over the pegs following stress shielding and cyst formation; B); lateral view of the foot showing subsidence of talar component and extensive cyst formation at tibial and talar side; C), Saltzman alignment view showing a slightly varus malalignment; D) AP-view of the foot showing a supination and adductus deformity.
CT scan confirms excessive cyst formation (same patient as figure 12). A) coronal plane; B) sagittal plane, and Q horizontal plane.
3 years after revision TAR with the HINTEGRA ankle and cyst fllling with allograft (Tutoplast), the implants are stable and the ankle is well balanced. The patient is highiy satisfied. A) AP-view of the ankle showing complete incorporation of allograft and stable implants; B); lateral view of the foot showing complete incorporation of allograft and stable implants; C), Saltzman alignment view showing a minimal varus malalignment left; D) AP-view of the foot showing a minimal supination and adductus deformity left.
A most recent meta-analysis on 58 papers (7942 TARs) calculated an overall survivorship of 89% at 10 years with an annual failure rate of 1.2% (95% confidence interval (CI) 0.7 to 1.6) (28). The mean AOFAS score changed from 40 (95% CI 36 to 43) pre-operatively to 80 (95% CI 76 to 84) at a mean follow-up of 8.2 years (7 to 10) (p<0.01). Radiolucencies were identified in up to 23% of TARs after a mean of 4.4 years (2.3 to 9.6). The mean total range of movement improved from 23° (95% CI 19 to 26) to 34° (95% CI 26 to 41) (p=0.01). The study demonstrated that TAR has a positive impact on patients’ lives, with benefits lasting 10 years, as judged by improvement in pain and function, as well as improved gait and increased range of movement. However, the quality of evidence was weak and fraught with biases and high quality randomized controlled trials are required to compare TAR with other forms of treatment such as fusion.
Recently we reported on survivorship of 722 TAR's (741 patients) performed with the HINTEGRA ankle (12). A logistic multiple regression model was used to identify independent risk factors for prosthesis failure in 684 patients (722 ankles). The mean time to final follow-up (and standard deviation) was 6.3±2.9 years. The overall survival rates were 94% and 84% after 5 and 10 years, respectively. Sixty-one ankles had a revision TAR (27 both components, 13 the tibial component only, and 14 the talar component only) or were converted to a fusion (7 ankles). There were no polyethylene failures. There were no amputations. The generation category of the prosthesis, the cause of ankle osteoarthritis, and the age of the patient were identified as independent risk factors for prosthesis failure.
ConclusionsAs TAR continues to evolve as a viable treatment option for end-stage ankle OA, the adverse clinical and biomechanical consequences of ankle arthrodesis are far more apparent. Proper patient selection is a critical aspect of promoting successful results. Acceptable results have been reported in older, low-demand patients who have osteoarthritis or rheumatoid arthritis. A significant percentage of patients with end-stage ankle OA, however, are younger patients with post-traumatic osteoarthritis. The use of TAR in younger, more physically active patients, and in those with significant deformity in the ankle or hindfoot remains a question to debate. More studies must be completed and further developments must be made to maximize the longevity and functional results of TAR in future designs and applications.
Along with improved implants that are typically more respectful of anatomic concerns, proper positioning of the implants (particularly of the talar component with respect to the center of rotation of the talus), accurate balancing of the soft tissues, and appropriate correction of malalignment are far more important for the success of TAR than previously believed. Careful clinical investigation and reliable diagnostic tools should thus be used to identify all of the associated problems so that they can be properly addressed during TAR.
The authors declare no conflict of interests with this article.