This review aims to provide an overview of complications associated with surgical treatment for urinary incontinence and pelvic organ prolapse relating to synthetic mesh, as well as review the new International Urogynecologic Association (IUGA)/ International Continence Society (ICS) classification of complications for insertion of prosthesis or grafts in female pelvic floor surgery and the recent Food and Drug Administration (FDA) notifications.
A multitude of surgical procedures have been described and modified in hope of attaining a durable cure for stress urinary incontinence (SUI) and pelvic organ prolapse (POP). These surgeries were traditionally performed using the patient’s native tissues. In an effort to decrease morbidity, improve surgical outcomes and minimize the complexity of these operations, an increasing number of repairs using synthetic mesh and biomaterials from cadaveric or xenograft tissue have been employed. This review aims to provide an overview of complications associated with surgical treatment for urinary incontinence and pelvic organ prolapse relating to synthetic mesh, as well as review the new International Urogynecologic Association (IUGA)/ International Continence Society (ICS) classification of complications for insertion of prosthesis or grafts in female pelvic floor surgery and the recent Food and Drug Administration (FDA) notifications.
Mesh in SUISynthetic mesh has been used in the treatment of stress urinary incontinence with a wide variety of retropubic midurethral slings (MUS), transobturator MUS and single incision mini-slings. Success rates are estimated at 51 to 99% for retropubic and transobturator slings (1-3). Single incision mini-slings have demonstrated lower success rates so far. Success rates range from 31 to 91.9% (4, 5). Although extremely low rates of bowel injury, vascular injuries and death have been reported in the literature with the retropubic MUS, some surgeons prefer to use transobturator MUS to avoid these devastating complications and reduce the risk of bladder injury (3, 6, 7). Similarly, the minisling was devised as a less invasive procedure that could be performed safely in an office setting. Despite these technological advancements, placement of synthetic mesh for the treatment of stress urinary incontinence may result in both minor and serious complications. Lower urinary tract symptoms may be exacerbated with worsened or de novo urgency and urge incontinence in 11-28% (8-10). MUS placement focuses on tension-free positioning but ways of achieving a tension-free placement is not standardized and difficult to assess intraoperatively (11). Bladder outlet obstruction and/or voiding dysfunction can result from tension at time of sling placement but also from tissue contraction and fibrosis in response to secondary scarring. Mesh complications can also include vaginal extrusion with related symptoms of vaginal bleeding, vaginal discharge or pain with intercourse for the patient or their partner (hispareunia) (12). Erosion into the urinary tract most commonly involve the bladder and/or urethra presenting with urinary frequency, urgency, dysuria, recurrent urinary tract infections or calculi. Although persistent groin and medial thigh pain have been reported following transobturator MUS, transient pain is fortunately more common occurring in 5-31% (13-16). Pelvic pain and dyspareunia have been reported in up to 24% following MUS, and can be a most distressing and potentially irreversible complication to rectify (17, 18).
Mesh in POPMesh use for abdominal sacrocolpopexy dates back to 1962 (19) and is well established through long-term data (20, 21). On the other hand, transvaginal repairs with either self-fashioned prolene mesh or commercial mesh kits are very controversial. Mesh for anterior repair may improve anatomic outcomes but has not demonstrated a clear benefit regarding quality of life and patient satisfaction in a recent meta-analysis (21, 22). Efficacy of mesh repairs for vault repair and posterior repair has not been demonstrated, with low level evidence and short term studies (21, 22). Most frequently cited complications are vaginal extrusion and exposure ranging from 5.8 to 20% (22, 23) De novo dyspareunia and pelvic pain is also a significant concern reported in 1 to 69% (24). Pain seems to be related to the amount of implanted mesh and likely partially attributable to mesh contraction (23). Fistulae may involve the urinary tract and/ or colo-rectal tract requiring aggressive intervention. (See Figure 1.) Recurrent prolapse, infection, neuromuscular impairment, vaginal shrinkage, psychological problems and death have been reported complications associated with mesh for transvaginal POP repair (21).
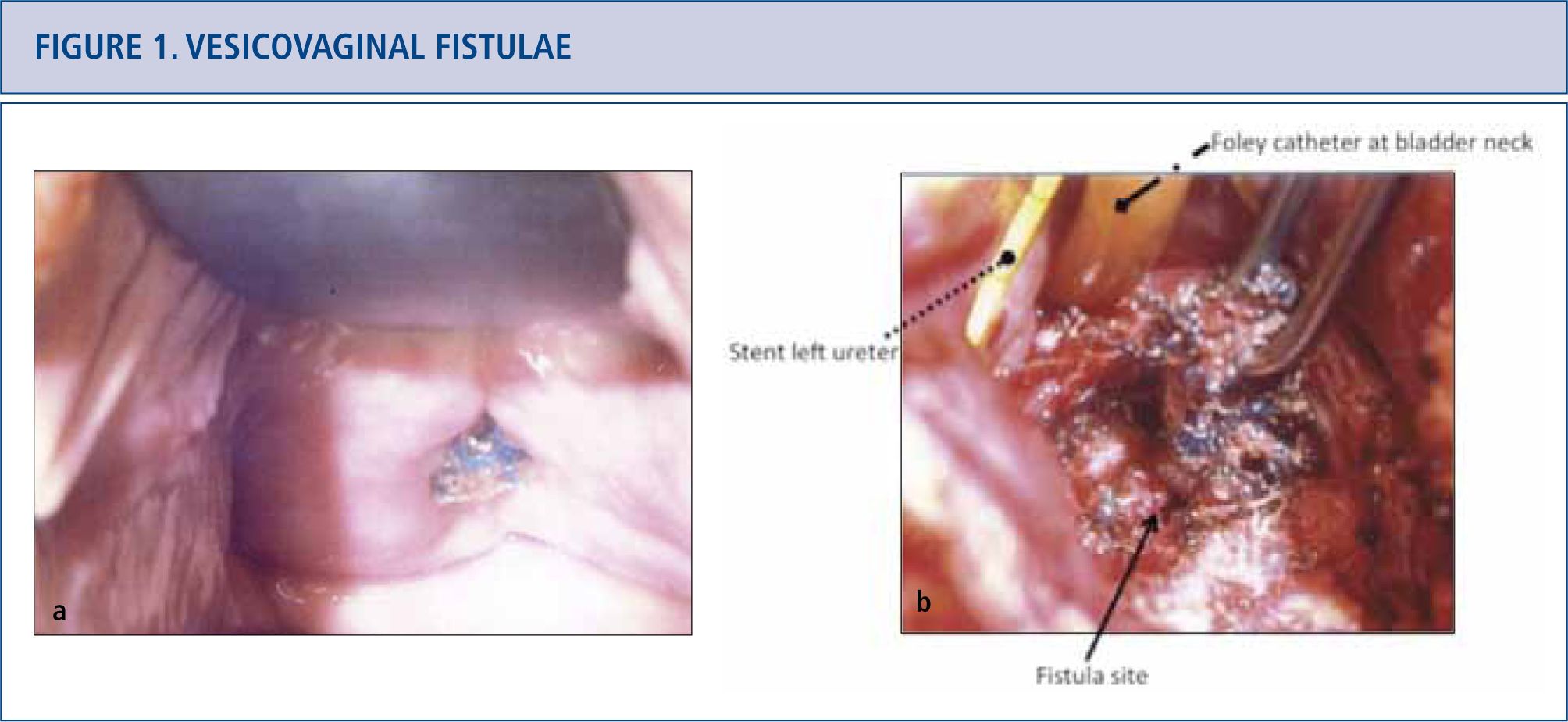
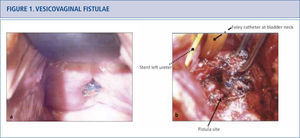
Vesicovaginal fistulae
a) Patient presented with anterior vaginal midline mesh erosion and an associated vesicovaginal fistula. Site of mesh erosion was located near the left ureteric orifice by cystoscopy. b) Surgical options for vesicovaginal fistula involving an exposed mesh include transabdominal or transvaginal repairs. Transabdominal repair of the vesicovaginal fistula with removal of mesh was performed. The left ureteric orfice was in very close proximity to the fistula and is depicted by the arrow, but was not reimplanted.
The FDA released a Public Health Notification in October 2008 in response to complications associated with urogynecologic use of surgical mesh (25). The FDA conducted a search of the adverse events in Manufacturer’s and User Device Experience (MAUDE) database, revealing 3,979 cases from January 2005 to December 2010 with a 5 fold increase in reports of adverse events in POP repairs from January 2008 to December 2010 (21). An “Update on the Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse” was issued by the FDA in July 2011. Unlike the 2008 notification, the 2011 FDA Safety Communication stated that complications “are NOT rare” and that “transvaginally placed mesh in POP repairs does NOT conclusively improve clinical outcomes over traditional non-mesh repairs” (21). The Safety Communication aimed to educate the public and health care providers with adverse events relating to these devices and provided recommendations for informed decision-making regarding transvaginal mesh (21). In September 2011, an advisory panel of experts assembled for an open public hearing and presentations by both industry and the FDA to address questions regarding mesh safety for urogynecological applications for POP and SUI (21). Regarding transvaginal placement of mesh, the advisory panel reached a number of consensus including the following:
- (i)
The safety, efficacy and benefit ratio is not well established in transvaginal mesh.
- (ii)
Improved premarket studies comparing mesh to non-mesh options need at least 1 year follow-up.
- (iii)
Transvaginal meshes should be reclassified to Class III.
- (iv)
Postmarket studies need to be ongoing.
- (v)
Mesh for abdominal sacrocolpopexy would not require reclassification (21). Patients are encouraged to ask their surgeons several pertinent questions before proceeding with mesh placement (21). (See Table 1.1.) The advisory panel felt that the safety and efficacy of retropubic and transobturator MUS is established, whereas single-incision mini slings require further investigation and should be used in study setting with long-term follow-up (21). More recently, Johnson & Johnson have withdrawn some of their mesh products from the global market (26). Although it is recommended that mesh and device complications are reported to the FDA through its MedWatch, the FDA Safety Information and Adverse Event Reporting program or respective national equivalent, surgeons and clinicians underreport adverse events as the reporting process can be time consuming and is completely voluntary (21). Many acknowledge the need for a comprehensive registry of mesh use and outcomes (27-29). Until such a national registry exists, recognition of device-associated complications will be further delayed until reported in the literature, thus exposing even more patients to these risks (28). Fortunately, a national registry of outcomes of mesh in incontinence and prolapse is underway in both Australia and the United Kingdom, initiated by their national urogynecological societies (30). The Urogynaecological Society of Australia (UGSA) database encourages its members to report their outcomes by offering the database at a low annual cost, giving CME credits for participating and arguing for the greater good since accurate surgical data will better support clinical and regulatory decisions (30). Companies marketing mesh products should be encouraged to employ code numbers and tracking systems to make identification and follow-up of mesh easier.
Table 1.1.Questions before-after surgery
Before surgery After surgery - 1
Are you planning to use mesh in my surgery?
- 2
Why do you think I am a good candidate for surgical mesh?
- 3
Why is surgical mesh being chosen for my repair?
- 4
What are the alternatives to transvaginal surgical mesh repair for POP, including non-surgical options?
- 5
What are the pros and cons of mesh in my particular case?
- 6
How likely is it that my repair could be successfully performed without surgical mesh?
- 7
Will my partner be able to feel surgical mesh during sexual intercourse?
- 8
What if the surgical mesh erodes through my vaginal wall?
- 9
If surgical mesh is to be used, how often have you implanted this particular product? What results have your other patients had with this product?
- 10
What can I expect to feel after surgery and for how long?
- 11
Which specific side effects should I report to you after surgery?
- 12
What if the mesh surgery doesn’t correct my problem?
- 13
If I develop a complication, will you treat it or will I be referred to a specialist experienced with surgical mesh complications?
- 14
If I have a complication related to the mesh, how likely is it that the surgical mesh could be removed and what could be the consequences?
- 15
If a surgical mesh is to be used, is there patient information that comes with the product, and can I have a copy?
- 1
Continue routine follow-up care.
- 2
Notify health care provider if complications or symptoms:
- -
Persistent vaginal bleeding or discharge
- -
Pelvic or groin pain
- -
Pain with sex
- -
- 3
Let health care provides know if they have surgical mesh, especially if planning to have another related surgery or other medical procedures
- 4
Talk to health care provider about any questions or concerns.
- 5
Ask the surgeon at her next check-up if she received mesh for POP surgery if she does not know if mesh was used.
Important questions patient should address with the surgeon preoperatively according to the FDA Safety Communication Update (July 12, 2011) are included in this table. A summary of basic aspects of care following mesh surgery is included for the patient.
Modified from: FDA, Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse. July 2011.
- 1
A classification system of complications related directly to the insertion of prosthesis in female pelvic floor surgery has been instituted by both the International Urogynecological Association (IUGA) and International Continence Society (ICS) in efforts to standardize terminology for more precise reporting of complications which should help facilitate the implementation of a reliable registry (28, 31). (See Table 2.1 for a list of the terminology.) The classification system coding is based on category of complication, time of clinical diagnosis and site of complication (31). Pain is subclassified into 5 grades ranging from a (asymptomatic/ no pain) to e (spontaneous pain) (31). Although a patient may suffer different complications at different times, all complications should be listed with the final category for a single complication reported at its maximal score (31). (See Table 2.2 for classification.)
Terminology involved for classification
| TERMS USED | DEFINITIONS |
|---|---|
| PROSTHESIS | A fabricated substitute to assist a damaged body part or to augment or stabilize a hypoplastic structure |
| a. MESH | A (prosthetic) network of fabric or structure |
| b. IMPLANT | A surgically inserted or embedded prosthesis |
| c. TAPE (SLING) | A flat strip of synthetic material |
| GRAFT | Any tissue or organ for transplantation. This term will refer to biological materials inserted |
| a. AUTOLOGOUS GRAFTS | From the woman’s own tissues (e.g. dura mater, rectus sheath or fascia lata) |
| b. ALLOGRAFTS | From post-mortem tissue banks |
| c. XENOGRAFTS | From other species (e.g. modified porcine dermis, porcine small intestine, bovine pericardium) |
| COMPLICATION | A morbid process or event that occurs during the course of a surgery that is not an essential part of that surgery |
| CONTRACTION | Shrinkage or reduction of size |
| PROMINENCE | Parts that protrude beyond the surface (e.g. due to wrinkling or folding with no epithelial separation) |
| SEPARATION | Physically disconnected (e.g. vaginal epithelium) |
| EXPOSURE | A condition of displaying, revealing, exhibiting or making accessible (e.g. vaginal mesh visualized through separated vaginal epithelium) |
| EXTRUSION | Passage gradually out of a body structure or tissue |
| COMPROMISE | Bring into danger |
| PERFORATION | Abnormal opening into a hollow organ or viscus |
| DEHISCENCE | A bursting open or gaping along natural or sutured line |
Terminology involved in the classification of complications related directly to insertion of prosthesis (meshes, implants, tapes) or grafts in female pelvic floor surgery. From: Haylen, B.T., Freeman, R.M., Swift, S.E. et al., IUGA/ICS Joint Terminology and Classification of Complications Related Directly to the Insertion of Prosthesis (Meshes, Implants, Tapes) or Grafts in Female Pelvic Floor Surgery. 2012.
IUGA/ICS classification of complications related with directly insertion of prosthesis
| General Description | A (Asymptomatic) | B (Symptomatic) | C (Infection) | D (Abscess) |
|---|---|---|---|---|
| 1. Vaginal: No epithelial separation Include prominence (e.g. due to wrinkling or folding), mesh fiber palpation or contraction (shrinkage) | 1A: Abnormal prosthesis or graft finding on clinical exam | 1B: Symptomatic e.g. unusual discomfort/ pain; dyspareunia (either partner); bleeding | 1C: Infection (suspected or actual) | 1D: Abscess |
| 2. Vaginal: Smaller <1 cm exposure | 2A: Asymptomatic | 2B: Symptomatic | 2C: Infection | 2D: Abscess |
| 3. Vaginal: Larger >1cm exposure, or any extrusion | 3A: Asymptomatic 1-3Aa if no prosthesis or graft related pain | 3B: Symptomatic 1-3B(b-e) if prosthesis or graft related pain | 3C: Infection 1-3C(b-e) if prosthesis or graft related pain | 3D: Abscess 1-3D(b-e) if prosthesis or graft related pain |
| 4. Urinary tract: Compromise or perforation including prosthesis (graft) perforation, fistula and calculus | 4A: Small intraoperative defect e.g. Bladder perforation | 4B: Other lower urinary tract complication or urinary retention | 4C: Ureteric or upper urinary tract complication | |
| 5. Rectal or Bowel: Compromise or perforation including prosthesis (graft) perforation and fistula | 5A: Small intraoperative defect (rectal or bowel) | 5B: Rectal injury or compromise | 5C: Small or large bowel injury or compromise | 5D: Abscess |
| 6. Skin and/or musculoskeletal: Complications including discharge, pain, lump or sinus tract formation | 6A: Asymptomatic, abnormal finding on clinical exam | 6B: Symptomatic eg. Discharge, pain or lump | 6C: Infection e.g. Sinus tract formation | 6D: Abscess |
| 7. Patient: Compromise including hematoma or systemic compromise | 7A: Bleeding complication including hematoma | 7B: Major degrees of resuscitation or intensive care* | 7C: Mortality* *(additional complication-no site applicable –S0) | |
| TIME (CLINICALLY DIAGNOSED) | |||
|---|---|---|---|
| T1: Intraoperative- 48 hours | T2: 48 hours – 2months | T3: 2-12 months | T4: Over 12 months |
| SITE | ||||
|---|---|---|---|---|
| S1: Vaginal: Area of suture line | S2: Vaginal: Away from area of suture line | S3: Trocar passage Exception: intra-abdominal (S5) | S4: Other skin or musculoskeletal site | S5: Intra-abdominal |
| GRADES OF PAIN: SUBCLASSIFICATION OF COMPLICATION CATEGORY |
|---|
| a: Asymptomatic or no pain b: Provoked pain only (during vaginal examination) c: Pain during sexual intercourse d: Pain during physical activities e: Spontaneous pain |
IUGA/ICS classification of complications related directly to insertion of prosthesis (meshes, implants, tapes) or grafts in female pelvic floor surgery.
From: Haylen, B.T., Freeman, R.M., Swift, S.E. et al., IUGA/ICS Joint Terminology and Classification of Complications Related Directly to the Insertion of Prosthesis (Meshes, Implants, Tapes) or Grafts in Female Pelvic Floor Surgery. 2012.
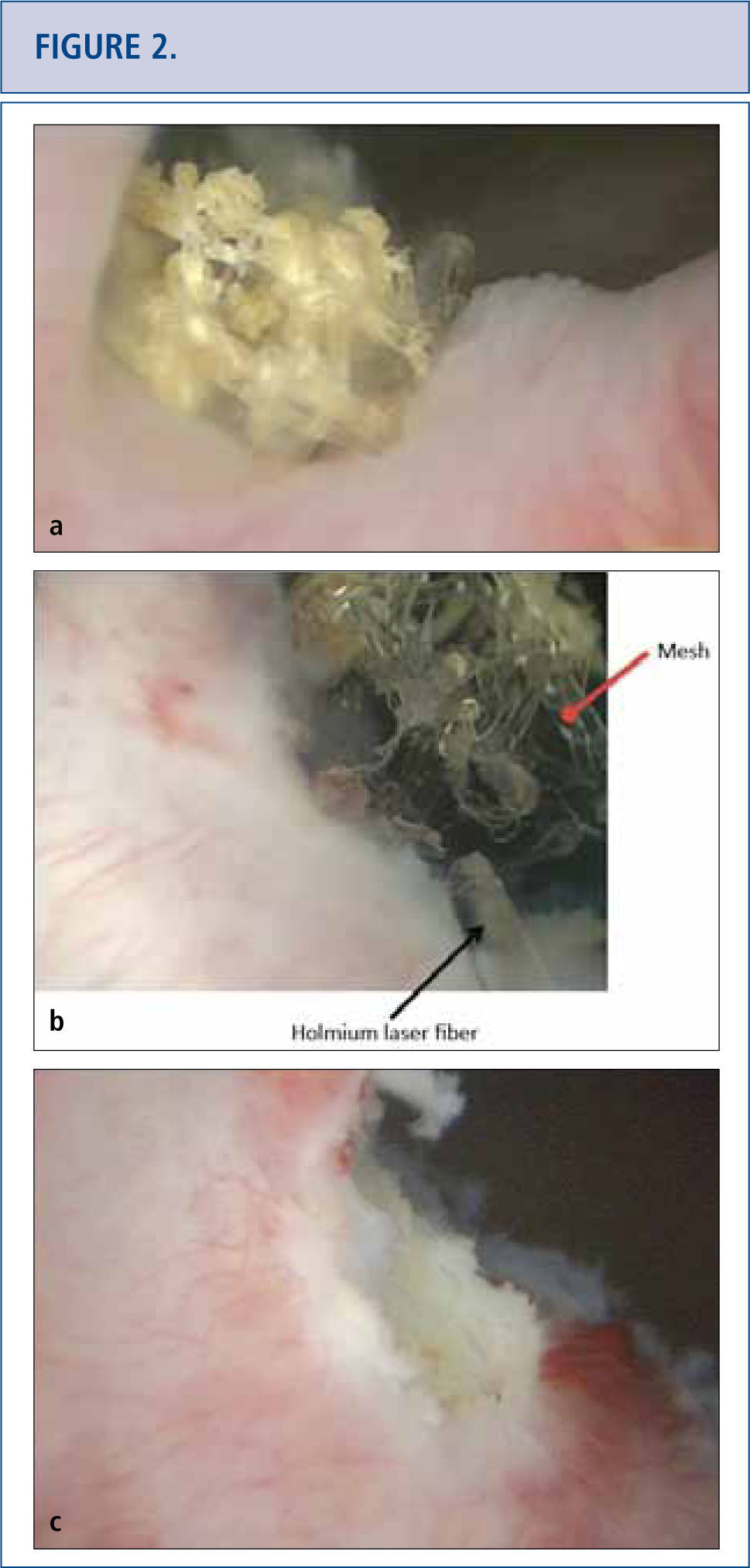
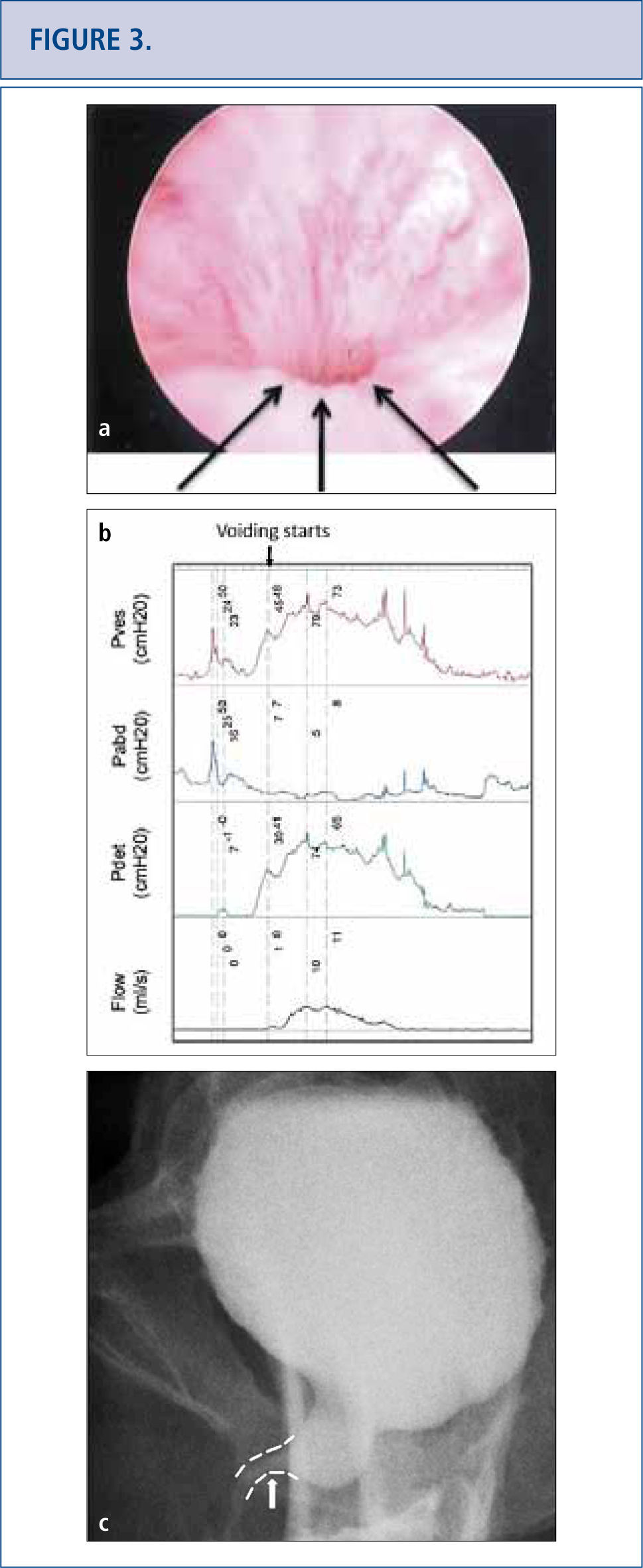
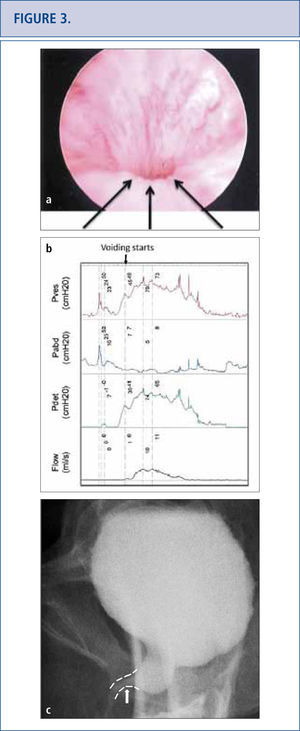
As the long-term consequences of mesh are still unknown, patients with mesh placed for SUI and POP should have long-term (>10 years) follow-up to monitor for complications or symptoms (32-34). Complications with mesh can occur several years later and the field is becoming increasingly litigious (34, 35). Patients with mesh who do not have complications should not undergo mesh explantation (32). A detailed history should screen for vaginal discharge, vaginal bleeding, pelvic or groin pain, dyspareunia, hispareunia, UTIs, urinary urgency, incomplete emptying, prolonged or slow urinary stream as well as bowel complaints. Onset of the symptoms, type of mesh used preferably based on an operative report, prior pelvic surgeries, investigations and treatments should be attained. A pelvic exam is necessary to assess for mesh exposure, prominence of scar tissue, recurrence of prolapse or SUI, and areas of tenderness or discomfort. In severe cases, patients unable to tolerate the exam may require an examination under anesthesia. Cystourethroscopy can be useful to identify mesh exposed in the lower urinary tract (Figure 2) and distortion of the urethral lumen (Figure a). For voiding complaints, urodynamic studies and voiding cystourethrogram (VCUG) have been useful. For bladder outlet obstruction following MUS placement, patients may demonstrate detrusor overactivity but more consistently will exhibit a prolonged or intermittent flow curve with an elevated detrusor pressure on urodynamic testing (Figure b). Another finding of bladder obstruction secondary to MUS on VCUG is urethral narrowing and kinking at the level of the MUS with proximal urethral dilatation (Figure 3c) (36). Present imaging strategies with MRI and ultrasound are generally of limited use for pre-surgical planning, but sometimes identify the mesh.
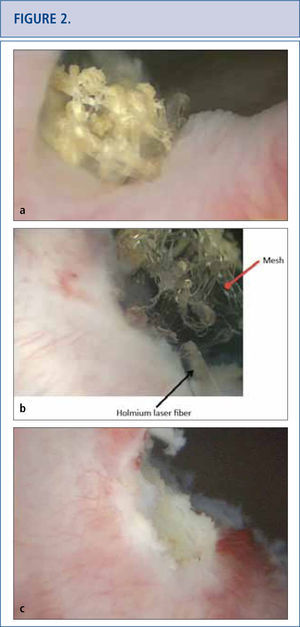
a) Cystoscopic view of mesh extended at the right side of the bladder neck, covered with calcifications 5 years after placement of a retropubic midurethral sling. b) Holmium laser (365 micron fiber) was used to eliminate as many mesh fragments as possible. c) Cystoscopic view of completed laser resection of the bladder neck mesh revealing no residual tape.
Persistent lower urinary tract symptoms (frequency, urgency and mixed urinary incontinence), recurrent UTIS and incomplete emptying in a 50 year old woman who underwent a “loosening of her tape” at 3 months post-op. Cystoscopy revealed no exposed tape to explain her UTIs, but a very narrow lumen with elevation and flattening of urethral floor depicted by the arrow in Figure 3a. Urodynamics (3b) and voiding cystogram confirmed obstruction and its site (arrow on 3c). Tape loosening or incision does not always release an obstruction completely and persistent symptomatology should raise the concern for residual obstruction.
Vaginal extrusions and exposure may be managed conservatively if exposure is < 1cm and not associated with any complicating factors (23, 37). Local estrogen therapy is often employed but the literature reflects mixed results (23, 38). If vaginal extrusion/exposure is larger or fails to heal satisfactorily with conservative measures, mesh excision should be considered (23, 29, 37, 38). Often a limited excision of mesh is attempted under local anesthesia in cases of small persistent areas of vaginal mesh exposure (29, 38). Management of mesh involving the urinary tract has been reported with excision via either the vaginal or abdominal approaches, endoscopically with ablation with holmium laser or transurethral resection with electrocautery (39, 40). Combined laparoscopic and endoscopic procedures have also been described (41).
For urinary retention following placement of a suburethral tape that persists for > 1 week, loosening the sling or sling incision is recommended. Despite a prior sling incision at another institution, we caution the reader about some patients who continue to have obstructive symptoms and clinical evidence of obstruction on urodynamics and VCUG, and may ultimately require excision of the tape and/or urethrolysis. It is likely that the longer the obstruction goes untreated, prolonged compression and ischemia of the midurethra can result in permanent scarring of the urethral lumen and consequential voiding dysfunction and bladder remodeling (42). Behavioral therapy and anticholinergics have been reported for de novo detrusor overactivity following sling placement. Urgency symptoms frequently occur as a result of BOO; and thus BOO be excluded for any de novo symptoms after a sling procedure (43-45). In this case, tape excision to relieve the obstruction would be necessary.
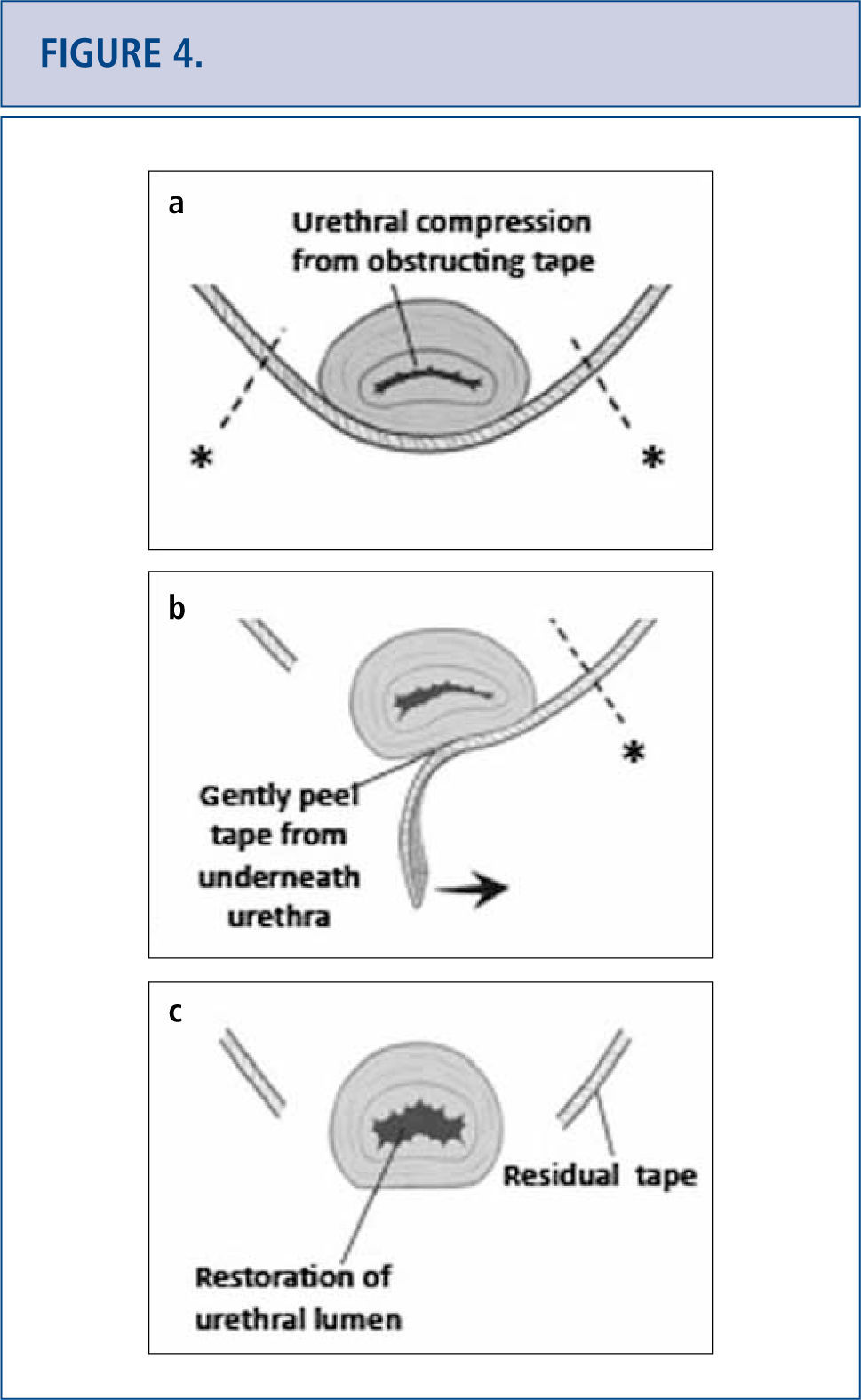
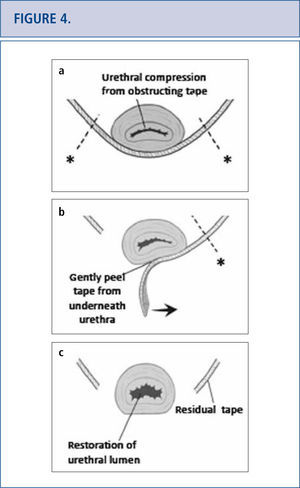
In some patients, either complete or partial removal of the mesh is the only effective treatment modality. Mesh removal can be performed transvaginally or in a combined abdominal-vaginal approach. Mesh removal is challenging as visualization is often limited and extent of tissue damage from the mesh is often unknown. Success of mesh removal often depends on surgical experience in dealing with these complications. As a result, many patients travel great distances to tertiary referral centers to deal with their mesh complication as a last resort (42). Tape excision technique is depicted in Figure 4 (46). Specific complications following tape removal include recurrent incontinence, urethral stricture, persistent pain, bladder neck injury, vesicovaginal fistula and need for repeat surgery. Complications following removal of transvaginal mesh are related to the affected compartment. For apical and anterior meshes, bladder and ureteric injury are of particular concern. Following mesh removal, we routine perform cystoscopy with indigo carmine to exclude ureteric injury. For mesh complications involving the posterior compartment, bowel injury and need for colostomy have been reported (23). Other complications associated with mesh excision include large vaginal defects, possibly requiring skin grafting, residual pain which can be unremitting and life altering, and/or need for repeat surgery.
(Images modified from Dillon B, Gurbuz C, Zimmern P. Long term results after complication of “prophylactic” suburethral tape placement. Can J Urol. 2012; 19:6424-30.) a) MUS placed underneath the urethra should be tension free but can result in urethral kinking and distortion. It is preferable to incise the tape on the side of the urethra (marked by *) to reduce risk of urethral injury. b) Tape is carefully peeled away from underneath the urethra. c) After midurethral tape excision, urethroscopy helps confirm no urethral injury and documents restoration of a normal urethral lumen.
Management of mesh complications in POP and SUI is a rapidly growing field for surgeons, therapists and lawyers. These complications emphasize the need for more deliberate and careful consideration by both the patient and the surgeon prior to surgery. The literature reporting mesh complications is mostly retrospective. As surgeons, we are unable to predict who will suffer an adverse event. It is unclear whether the contributing factors of these devastating complications result from poor surgical technique, deficient training, infection, patient factors or an inherent defect of the synthetic material (27). Marketing strategy rather than evidence-based data resulted in rapid adoption of mesh for POP (42, 47). In retrospect, surgical expertise with specialized training in proper patient selection, mesh insertion and management of associated complications is now advocated (21, 28, 32). Tightening FDA approval with more rigorous safety and efficacy testing for the licensing of new surgical devices will be necessary to improve patient safety and trust (21, 32, 38, 47). There are still many unanswered questions in understanding vaginal tissue, its aging process and how exactly mesh placement affects the vaginal wall healing and inflammatory responses (42). We also need to better understand mesh properties and biomechanics to ultimately create a more biologically compatible material to avoid potentially devastating and permanent complications (27). With increased vigilance, understanding and expertise in the field, it will be possible to achieve the best outcomes for our patients.
The authors have no interest conflicts with this article.