While biometry and Doppler have proved useful in the management of fetal growth restriction, the same battery has been of less help in diabetic pregnancies. It is not surprising since the underlying pathophysiology is fundamentally different. Recent studies of the fetal liver, a key metabolic organ, have shown that its venous circulation reflects the impact of maternal hyperglycemia. Umbilical return from the placenta is disproportionately distributed to the fetal liver (more than in normally growing fetuses, and more than in non-diabetic macrosomia). However, what is set as a pattern at midtrimester is not followed up in the 3rd trimester when high fetal growth continues but no longer correspondingly supported by the umbilical flow to the liver (mLmin−1kg−1 is low). Thus, the status at 3rd trimester is as follows: umbilical flow does not match fetal growth, but the fetal liver still takes a major proportion of the placental return leaving less for the ductus venosus (DV). A distended DV does not help; rather it indicates reduced residual compensatory mechanisms to face hypoxic challenges.
The new insights suggest taking into consideration the fetal liver when assessing risks in diabetic pregnancies at 3rd trimester. Measuring umbilical venous flow and its distribution requires high level of skills and accurate techniques, but in the left portal branch (between the DV inlet and the junction with the portal main stem), the blood velocity is regularly accessible and it reflects the skewed umbilical flow to the liver, and its consequences, in a graded manner.
Si bien la biometría y el Doppler han demostrado ser útiles en el manejo de la restricción del crecimiento fetal, dichos exámenes han sido de menor ayuda en los embarazos en pacientes diabéticas. Esto no sorprende dado que la fisiopatología subyacente es fundamentalmente diferente. Estudios recientes del hígado fetal, un órgano metabólico clave, han demostrado que su circulación venosa refleja el impacto de la hiperglucemia materna. El retorno umbilical desde la placenta se distribuye de manera desproporcionada al hígado fetal (más que en los fetos de crecimiento normal y más que en la macrosomía no diabética). Sin embargo, lo que se establece como un patrón en el segundo trimestre no persiste en el tercer trimestre cuando al continuar el alto crecimiento no es apoyado proporcionalmente por el flujo umbilical al hígado (mLmin−1kg−1 es bajo). Por lo tanto, el estado en el tercer trimestre es el siguiente: el flujo umbilical no coincide con el crecimiento fetal, pero el hígado fetal sigue tomando una proporción importante del retorno placentario, dejando menos para el ductus venosos (DV). Un DV distendido no ayuda; más bien indica mecanismos compensatorios residuales reducidos para enfrentar desafíos hipóxicos.
Los nuevos conocimientos sugieren tener en cuenta el hígado fetal al evaluar los riesgos en los embarazos diabéticos en el tercer trimestre. Medir el flujo venoso umbilical y su distribución requiere de un alto nivel de habilidad y técnicas precisas, pero en la rama portal izquierda (entre la entrada del DV y la unión con la vena porta), la velocidad de la sangre es habitualmente accesible y refleja el flujo umbilical sesgado al hígado, y sus consecuencias, de manera medible.
In 1922, FG Banting and CH Best revolutionized diabetes mellitus (DM) understanding and treatment when they extracted what they called insulin from the pancreas and could show the effect of reducing blood glucose in normal animals1. The following 100 years of continued research, innovation, and clinical implementations have brought improved treatment and health to millions of patients, but the disease continues to rise globally2,3.
In pregnancy, tight glycemic control has been a cornerstone of care that has reduced the incidence of congenital malformations and perinatal morbidity4–6. The latest improvement was achieved through a glucose monitoring system that led to reduced glucose excursions7. However, still today, DM in pregnancy has an increased perinatal mortality and morbidity compared with the background population4,5 and researchers strive to understand why and how they can better identify and manage those at risk of stillbirth and perinatal morbidity. In the following article we are prioritizing human research when available, with a pathophysiological and clinical perspective.
Physiological backgroundMaternal glucose is transmitted through the placenta to the fetus, and an oral glucose load increases fetal glucose and umbilical flow to the fetal liver, affecting fetal abdominal circumference, which reflects fetal liver size8. Fetal glucose stimulates insulin secretion and glucose uptake in the fetal liver where roughly 80% of the insulin is received9. Insulin also increases glucose uptake in peripheral tissues such as striate muscles and heart. High glucose levels also promote fetal fat accretion and development of macrosomia. Such a drive includes an increased oxidative activity and oxygen consumption, further conditioned by degrees of maternal adiposity and gestational weight gain which has an independent driving effect10.
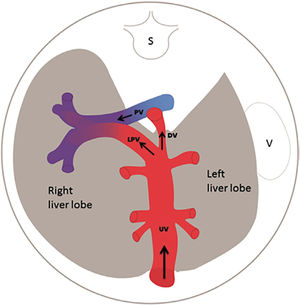
The fetal liver has a key role in metabolic adaptation and growth. Under normal conditions it receives 70–80% of the umbilical venous return from the placenta11–13, the rest being fed into the ductus venosus forming the nutritious central pathway via sinistra (Figure 1) ensuring cardiac and cerebral oxygenation, particularly under hypoxic challenges14–17. Fetal liver growth depends on the volume of umbilical blood perfusing its tissue inducing hepatocyte proliferation18,19. The liver also produces IGF-1 and IGF-2 directed at differential somatic growth19. Although degrees of maternal adiposity condition fetal fat accretion, the fetal liver per se is responsible for a basic fetal fat accumulation determined by the amount of umbilical liver perfusion. It ensures some fetal fat formation even in a slim mother20. A certain degree of fetal hepatic auto-regulation seems to operate by increasing its share of umbilical blood when a mother's skinfold is small, or she eats an unbalanced diet21.
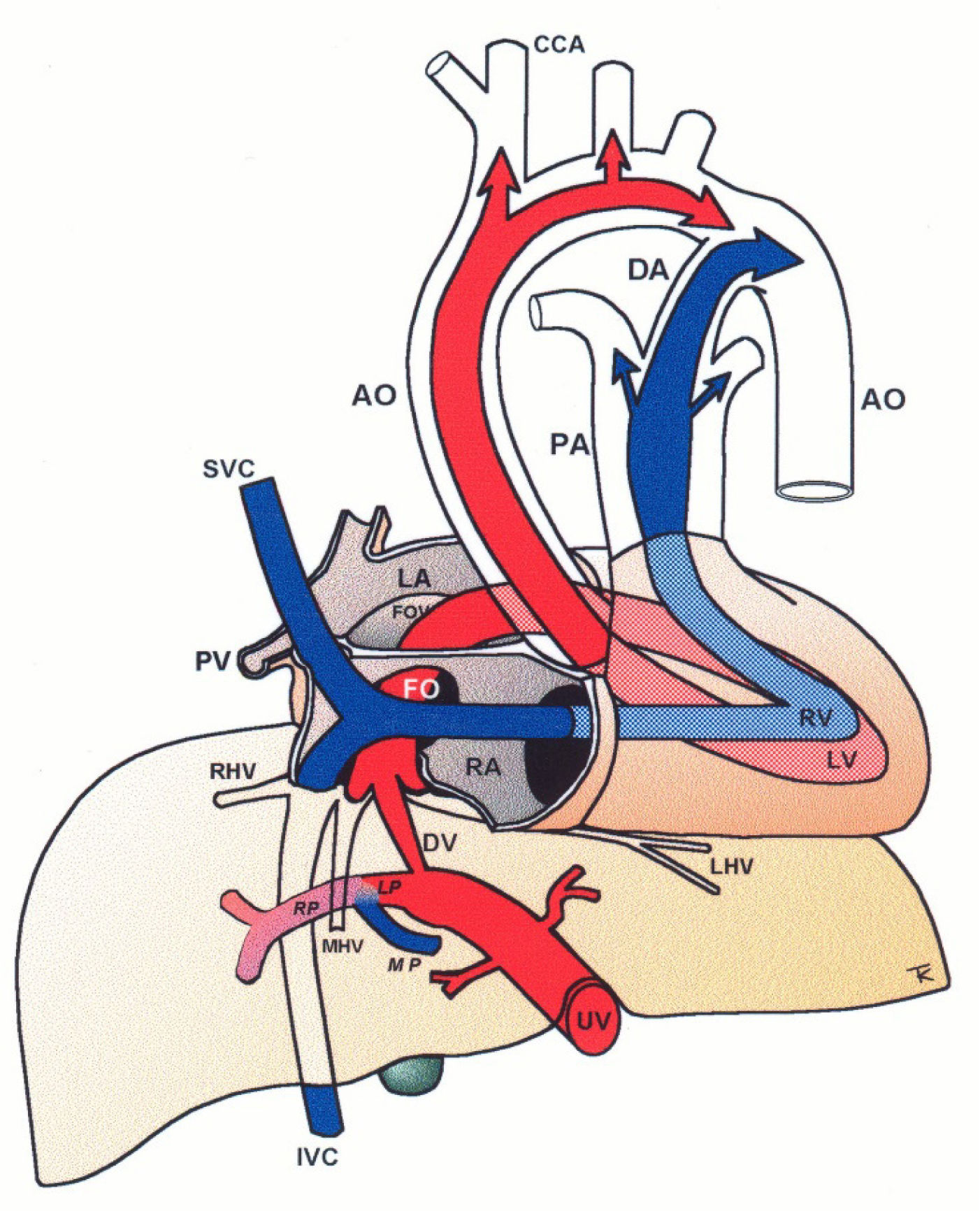
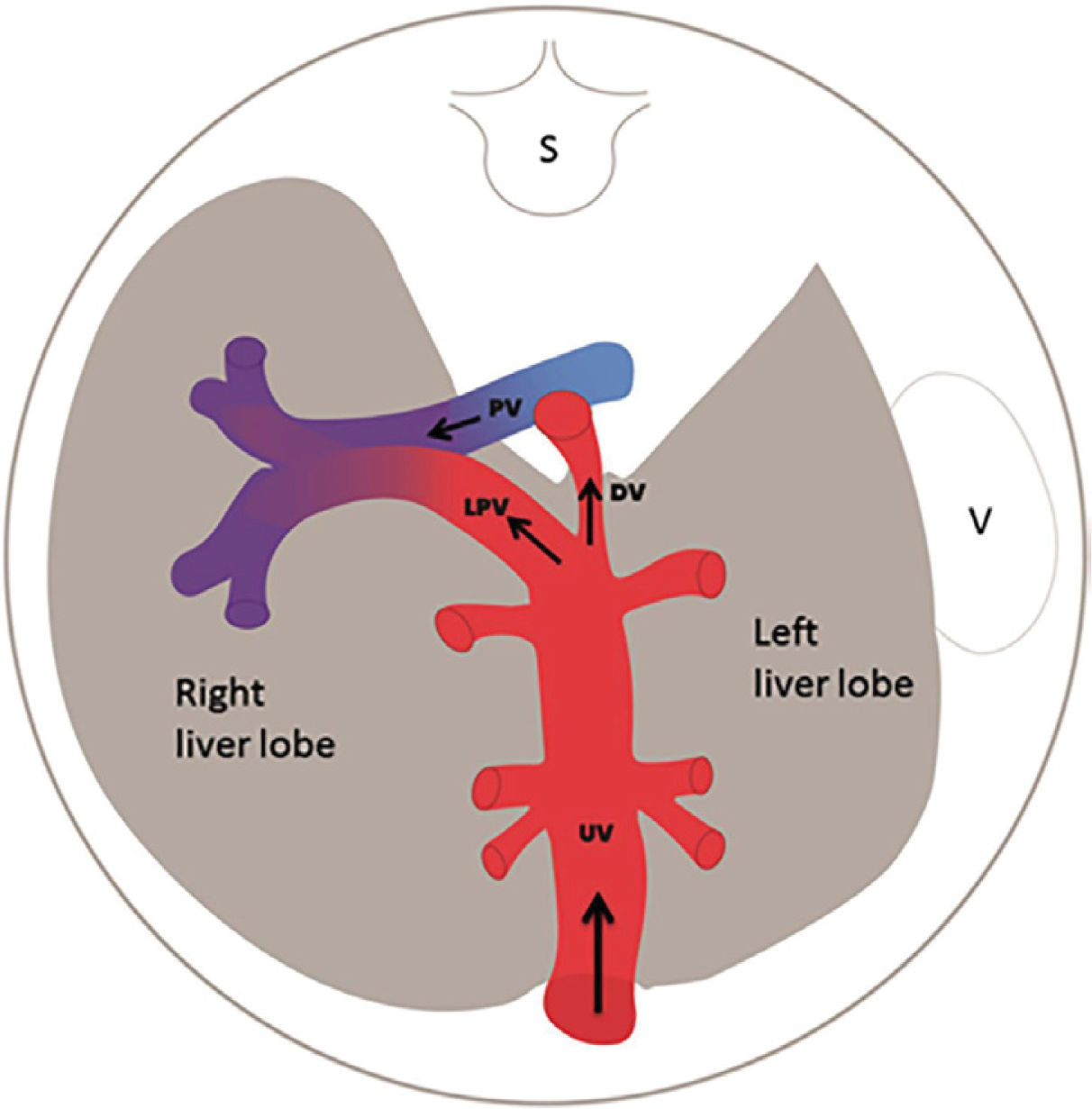
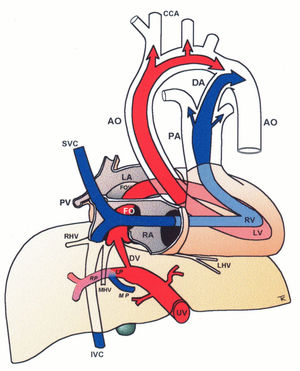
Central circulatory pathways of the fetus
Via sinistra (red) directs well-oxygenated umbilical blood from the umbilical vein (UV), through the ductus venosus (DV) across the proximal inferior vena cava (IVC) to pass through the foramen ovale (FO) above the foramen ovale valve (FOV) to reach the left atrium (LA) and the left ventricle (LV) to be pumped into the aorta (AO) feeding the coronary arteries, head and arms before reaching the descending AO. Via dextra directs low-oxygenated blood (blue) from the superior vena cava (SVC) and inferior vena cava (IVC) through the right atrium (RA) to the right ventricle (RV) where it is pumped into the pulmonary artery (PA) and ductus arteriosus (DA) to reach the descending AO. CCA, common carotic arteries; LHV, left hepatic vein; LP, left portal branch; MHV, medial hepatic vein; MP, main portal vein; PV, pulmonary vein; RHV, right hepatic vein; RP, right portal branch (modified with permission11).
The fetal brain is the highest energy consumer and the heart is equally dependent on a continuous supply of oxygen and energy substrate. The via sinistra with the ductus venosus conveys fresh blood with the highest oxygen saturation (typically 85%) constituting, under all circumstances, the essential core of feeding for these prioritized organs14. All other blood supply represents blood that has recycled in the fetal body before returning for the second or third extraction of oxygen22. The via sinistra, however, competes directly with the perfusion of the liver.
When the placenta is compromised, the growth-restricted fetus spares the heart and brain by maintaining the ductus venosus blood flow at the expense of liver growth, general somatic growth, and fat accretion17,23,24. These fetuses have an impaired umbilical circulation with less placental blood reaching the abdominal umbilical vein, but the ductus venosus shunting maintains its flow by enlargement. The bottom line is that the fetal liver receives less, and the distribution liver/ductus venosus shifts from, e.g.,75/25 (%) to 50/50, or worse depending on the severity of the placental dysfunction (Figure 2).
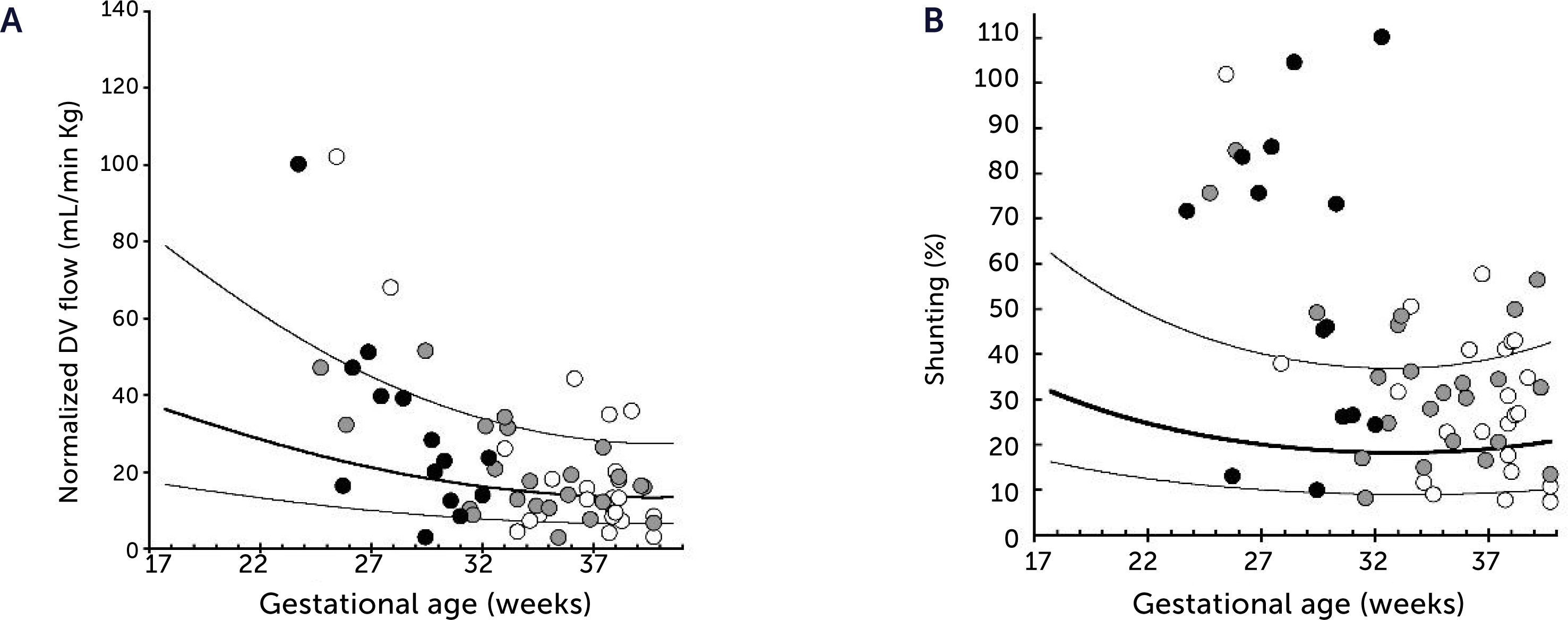
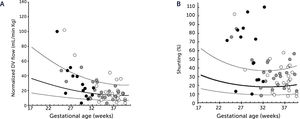
Ductus venosus flow pr kg and degree of shunting in severe fetal growth restriction (FGR)
A) Ductus venosus (DV) blood flow normalized for estimated fetal weight in 60 growth-restricted fetuses (≤2.5 centile) was not different from background population (10th, 50th, and 90th centile) (p=0.8). B) However, since the umbilical flow was low in these fetuses, the degree of DV shunting was increased (p<0.0001) leaving correspondingly less for the liver perfusion. White circle, normal umbilical artery PI; grey circle, umbilical artery PI >97.5 centile; black circle, umbilical artery absent or reversed diastolic flow17.
Women with DM may at times experience pregnancies with fetal growth-restriction as pregestational DM (PGDM), in particular, also carries a risk of disruptive pathophysiology. In such cases, the hyperglycemic condition is expected to modify and skew the known adaptive responses of fetal growth-restriction making the clinical interpretation of findings a challenge.
Normal macrosomic fetal growth and its underlying circulationBefore jumping at the question of how the underlying circulation in fetal macrosomia develops in diabetic pregnancies, we thought it prudent to understand how the circulation is organized in normal macrosomia, i.e., birthweight >80th centile in pregnancies without DM. Interestingly, the macrosomic fetus has the same circulatory pattern as the rest of the background population, but scaled up to fit their size25,26. This means that the umbilical flow supported macrosomic growth throughout pregnancy and into the last days of pregnancy without blunting (Figures 3A and 4A), that the fetal liver perfusion was correspondingly scaled up (Figure 5), and that the ductus venosus blood flow was maintained proportionate to fetal size (Figures 3B and 4B). It is interesting at this point to comment the results of a national birth-registry from a high-income country showing that birthweights at 80–85 centiles are associated with the lowest perinatal mortality and morbidity27. This presumably implies the availability of adequate obstetric services.
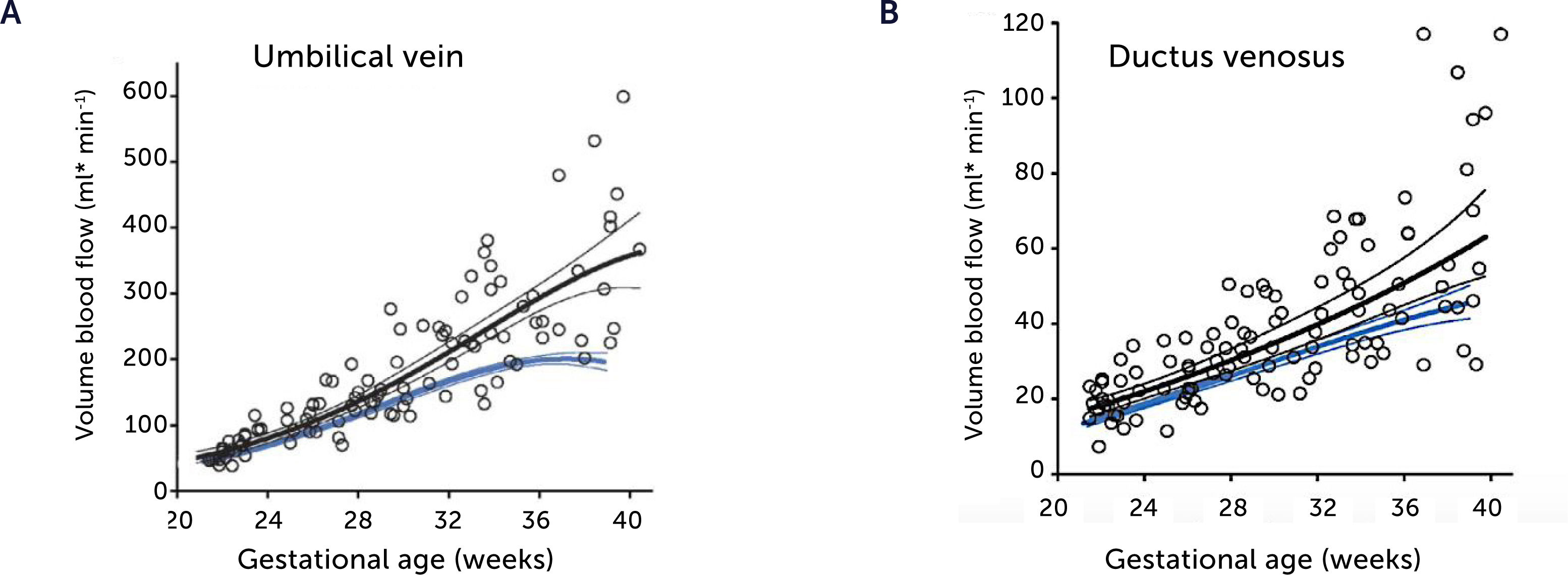
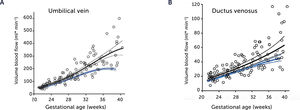
Blood flow in the umbilical vein and ductus venosus of macrosomic in non-diabetic mothers
Blood flow in the umbilical vein (A) and ductus venosus (B) of macrosomic fetuses in non-diabetic mothers (black) is upscaled compared with background population (blue), and less blunted towards term. Lines represents mean and 95% CI of the mean25.
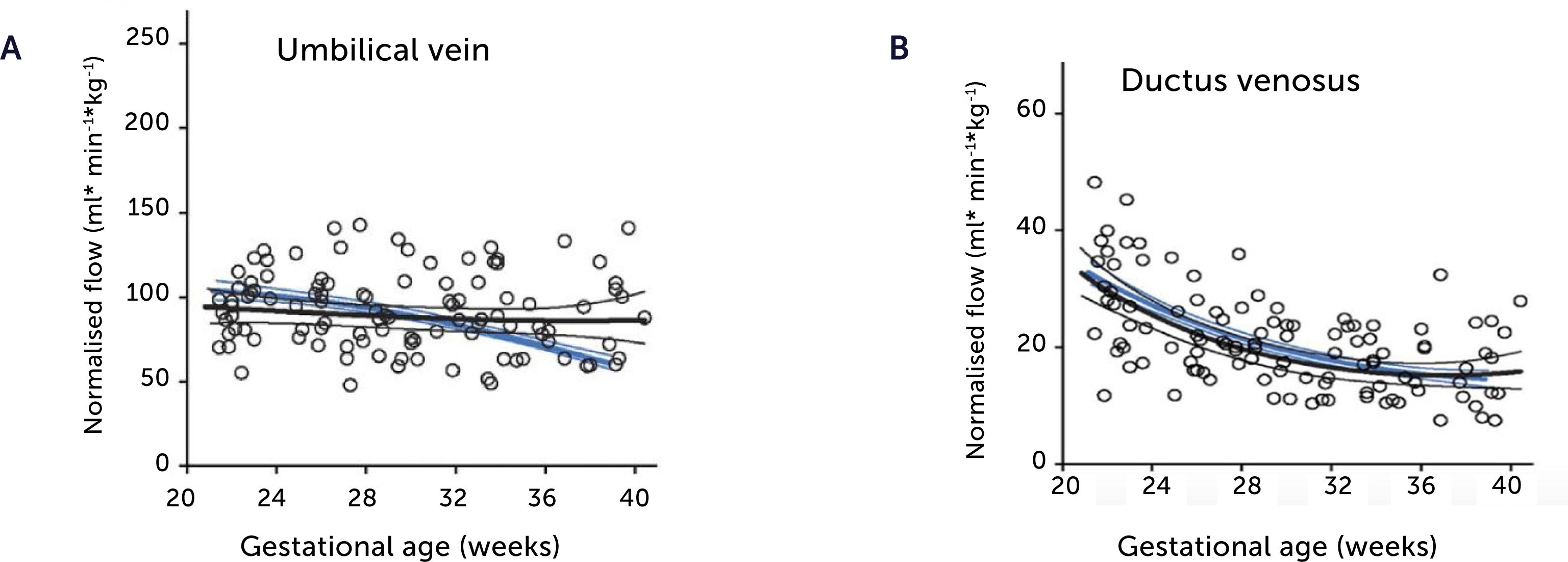
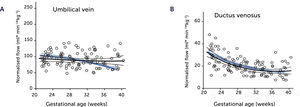
Blood flow normalized for estimated fetal weight in the umbilical vein and ductus venosus of macrosomic in non-diabetic mothers
Blood flow normalized for estimated fetal weight in the umbilical vein (A) and ductus venosus (B) of macrosomic fetuses in non-diabetic mothers (black) confirms a proportionate blood flow support of growth until birth, compared with low-risk background population (blue). Lines represent mean and 95% CI of the mean25.
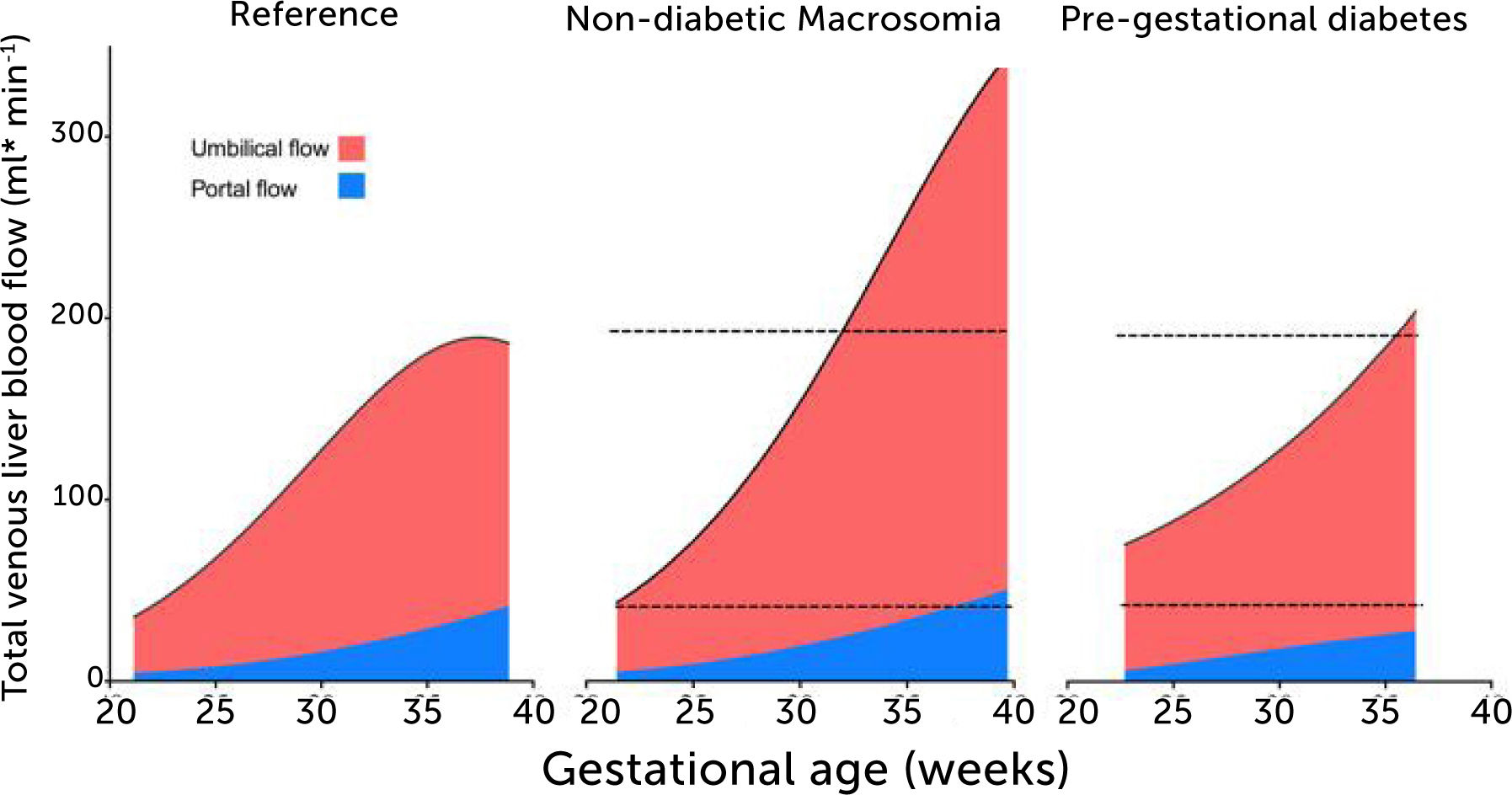
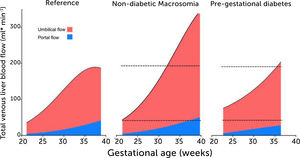
Total venous blood flow to the fetal liver in low-risk pregnancies, fetal macrosomia in non-diabetic pregnancies, and fetuses in mothers with prepregnant diabetes mellitus
Total venous blood flow to the fetal liver in low-risk pregnancies (left), fetal macrosomia in non-diabetic pregnancies (center), and fetuses in mothers with prepregnant diabetes mellitus (right) where 39% had macrosomia. Red color signifies umbilical blood, and blue color splanchnic blood from the portal stem. Data collation from four studies25,28,33,43.
After discussing liver circulation in fetal macrosomia in non-diabetic mothers, we turn to DM and focus primarily on PGDM as it is a more sinister condition than gestational DM, which encompasses pregnancies with various degrees of hyperglycemia and where the cut-off with regards to normal pregnancies has been shifting with time and society.
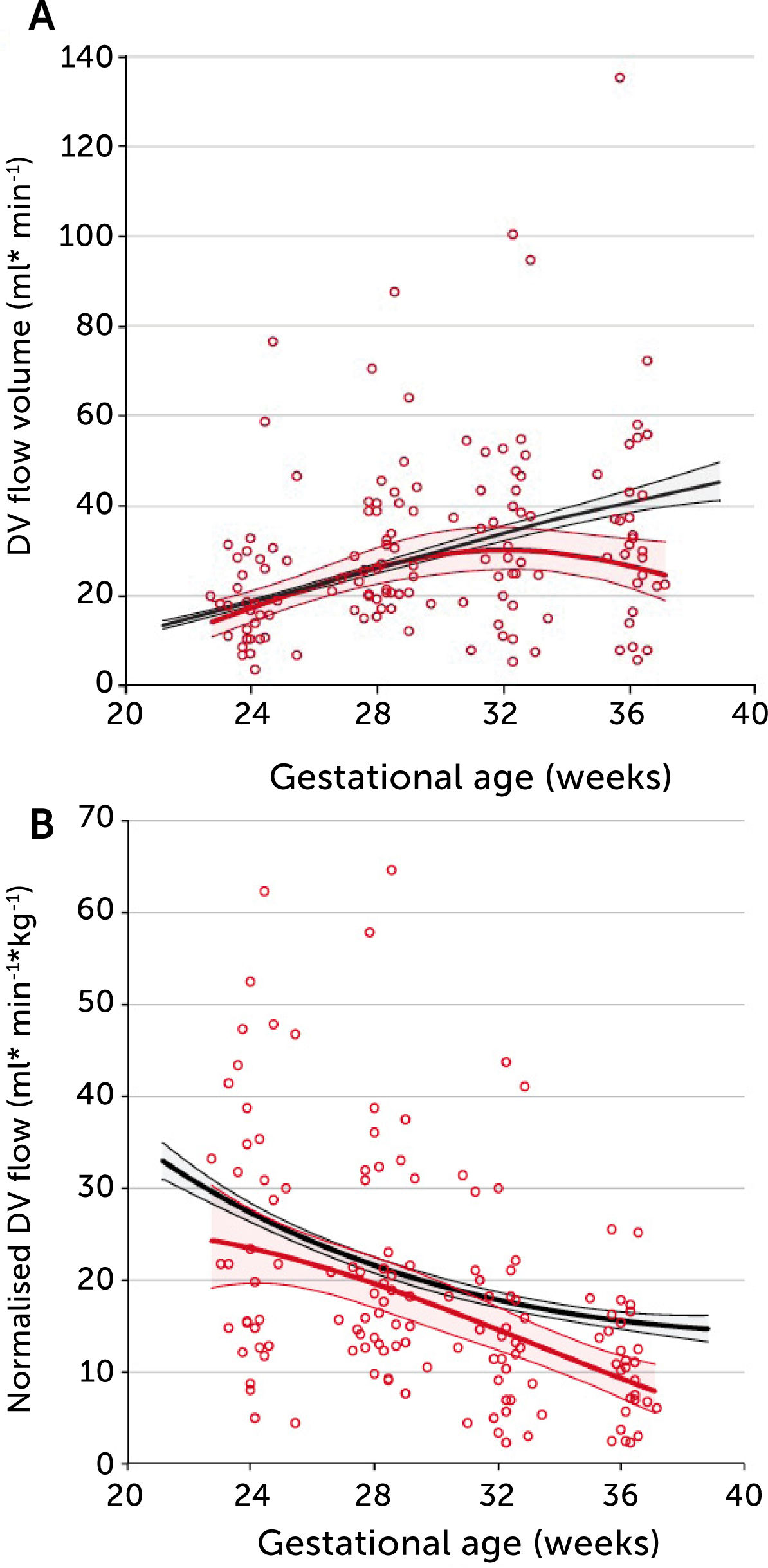
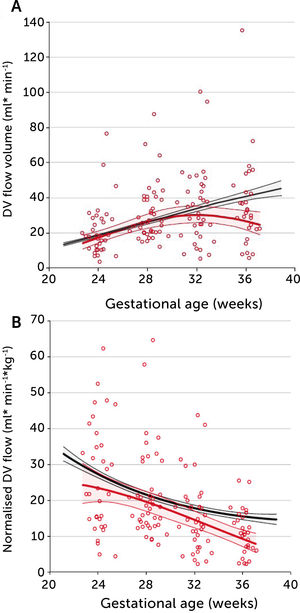
In PGDM, umbilical flow at mid gestation is high with a large volume directed to the fetal liver (Figure 5)28. Together with high fetal glucose concentration, it sets the standard and promises high growth rates. In fact, 39% of these fetuses are born with macrosomia (>80 centile). The umbilical flow, however, does not grow proportionately, resulting in a significantly lower umbilical flow when it is normalized for weight. In other words, the blood volume distributed to the fetal liver does not match fetal growth and is inferior to the circulatory support non-diabetic macrosomic fetuses would have (Figure 5). Apparently, the distributional pattern was set at mid gestation, but the following development failed to follow up as less placental blood reached the intraabdominal umbilical vein possibly due to a diabetic dysfunctional placenta. On the other hand, the vascular regulation in the liver venules is several times more sensitive than in the ductus venosus29 and ensures a continued high fraction of umbilical blood to the liver, at the expense of the ductus venosus. The ductus venosus on its side is significantly distended, has a low blood velocity and a blunted flow development during 3rd trimester (Figure 6)30. It is likely that this state of distribution constitutes a restrictive capacity to respond to hypoxic challenge, and therefore a correspondingly increased risk for serious events.
A) Longitudinal observations of ductus venosus blood flow showed a blunted development during 3rd trimester in pregestational diabetes mellitus (red) compared with low-risk background population (black). B) The corresponding ductus venosus flow normalized for estimated fetal weight is less than in the low-risk control group. Lines signify mean and 95% CI of the mean30.
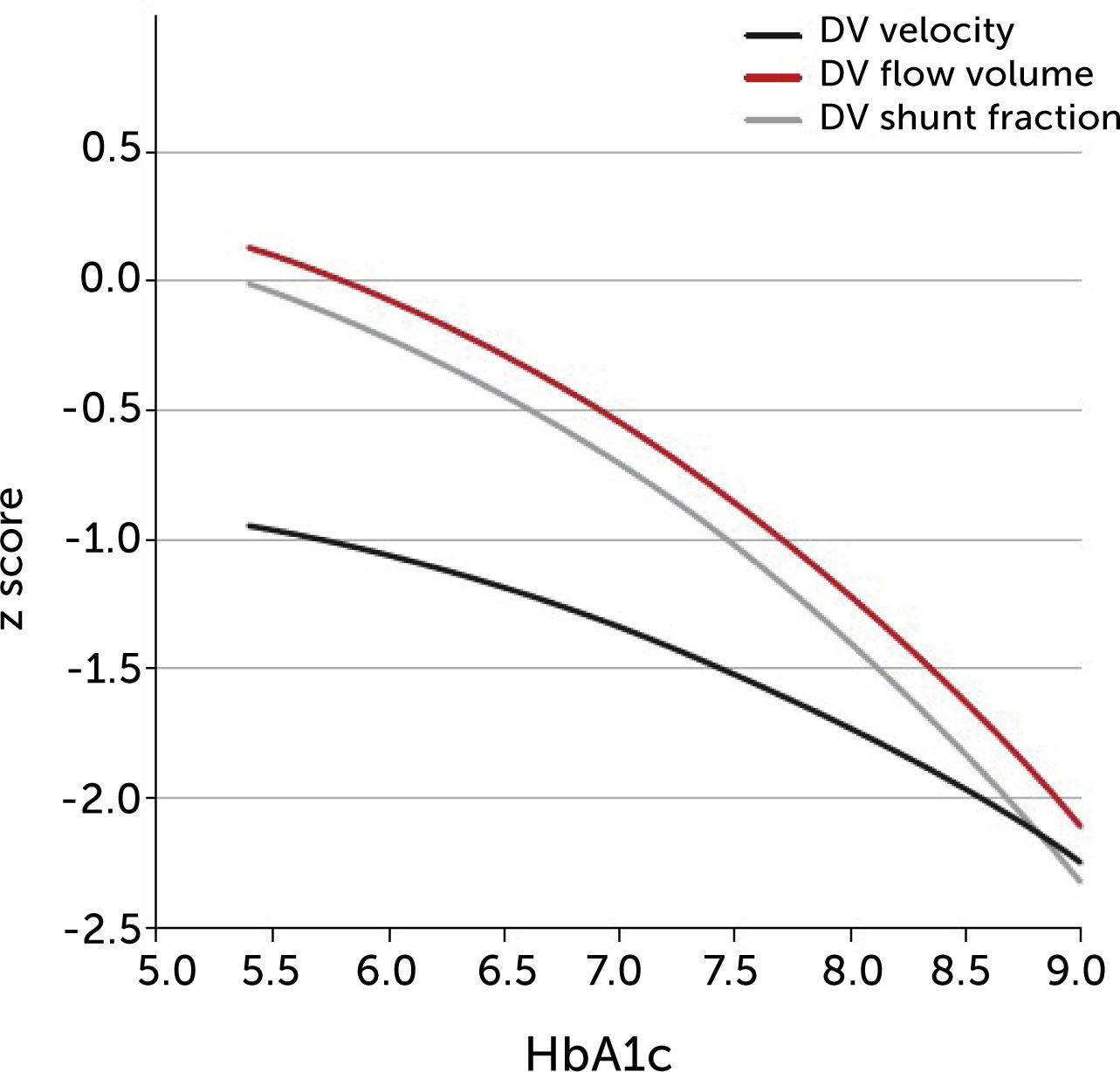
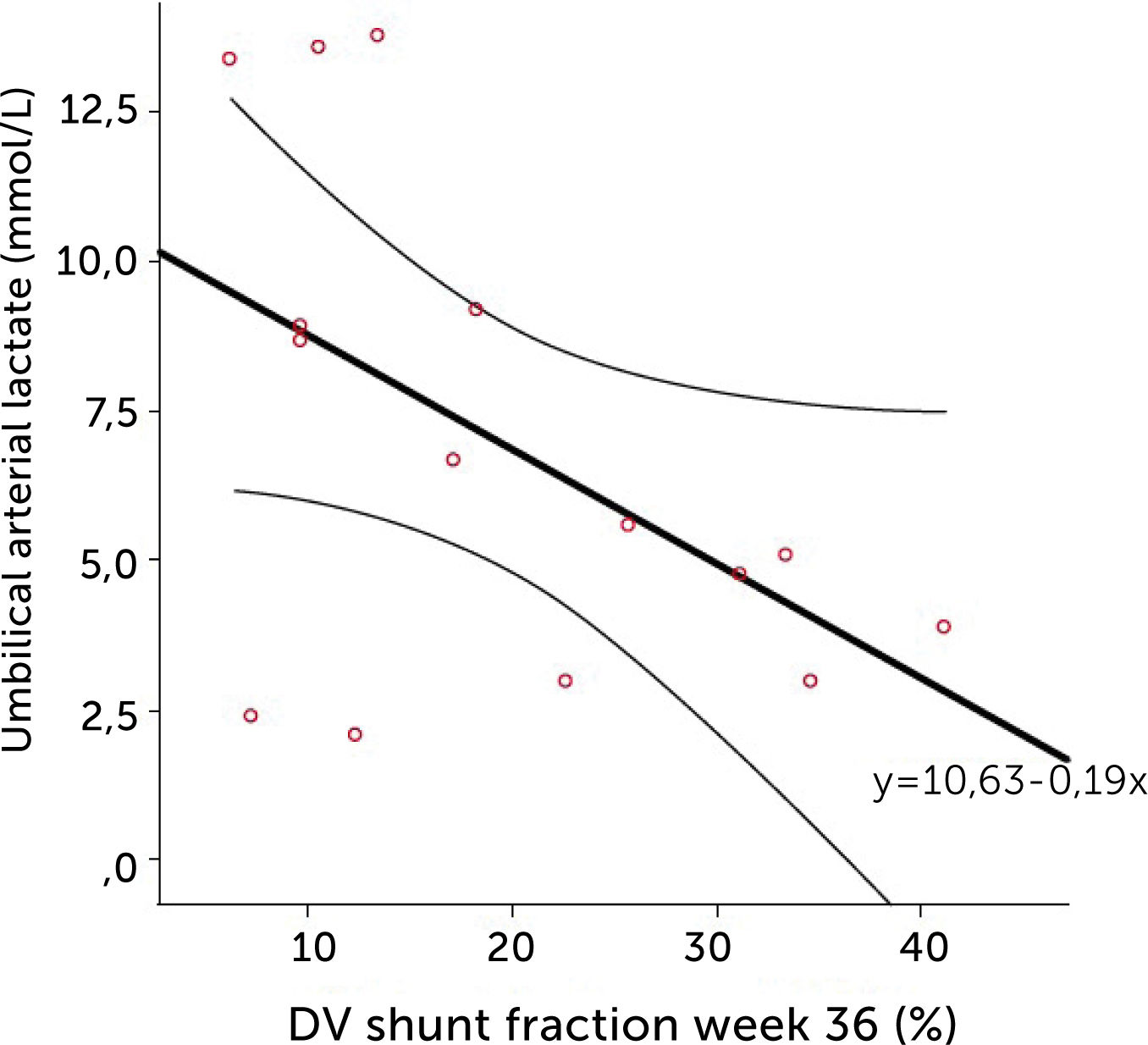
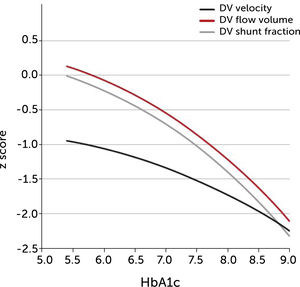
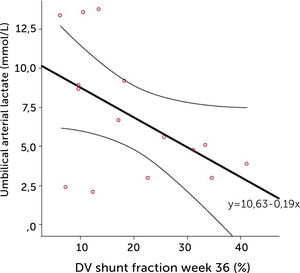
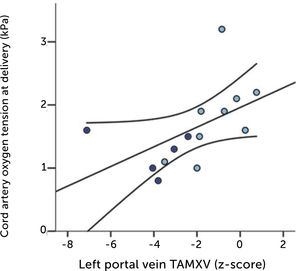
The degree of these circulatory changes (ductus venosus low velocity, low volume flow and low fraction shunted) are directly linked to degree of glycemic control (HbA1c) (Figure 7). At birth, arterial lactate in the cord is linearly related to the degree of shunting through the ductus venosus at 36 weeks of gestation; i.e., low shunting is associated with high lactate levels supporting the notion that these fetuses are at increased risk (Figure 8).
The degree of ductus venosus shunting impairment in pregnancies with pregestational diabetes mellitus is related to degree of glycemic control (HbA1c)
I.e., both shunted fraction of umbilical blood (grey), blood volume shunted (red), and time-averaged maximum blood velocity in the ductus venosus (green), which represents the driving umbilical pressure for perfusing both ductus and liver, are affected in a graded manner30.
The relation of cord arterial lactate at birth to degree of ductus venosus shunting (%) at 36 weeks of gestation in pregnancies with pregestational diabetes mellitus Regression line with 95% CI is shown30.
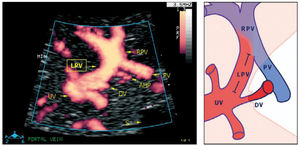
The left portal branch deserves a particular focus as it serves as a watershed area between the umbilical and splanchnic venous supply to the fetal liver (Figure 9)31–33. Thus, it has the potential of gauging an imbalance associated with fetal growth-restriction23, DM28, twin-twin transfusion syndrome, fetal liver diseases or anomalies of the abdominal venous system34,35.
The left portal vein (PV) directs umbilical flow beyond the ductus venosus (DV) towards the right side to the junction with the portal stem, or the main portal vein (PV), where umbilical blood blends in with low-oxygenated splanchnic blood and enters the right portal branch and liver lobe. LPV constitutes a watershed area where blood may even reverse to feed splanchnic blood into the umbilical vein and ductus venosus when perfusion pressure in the umbilical vein is low. After birth when the umbilical vein obliterates, the velocity is permanently reversed, and the portal stem circulates both right and left liver lobes. S, spine; UV, intra-abdominal umbilical vein; V, stomach33.
The intra-abdominal umbilical vein, half embedded in the caudal surface of the fetal liver, feeds the portal branches of the left liver lobe as it heads towards the ductus venosus inlet. Here the umbilical venous flow turns right following the left portal branch to join with the main portal stem and the right portal branch (Figure 10).
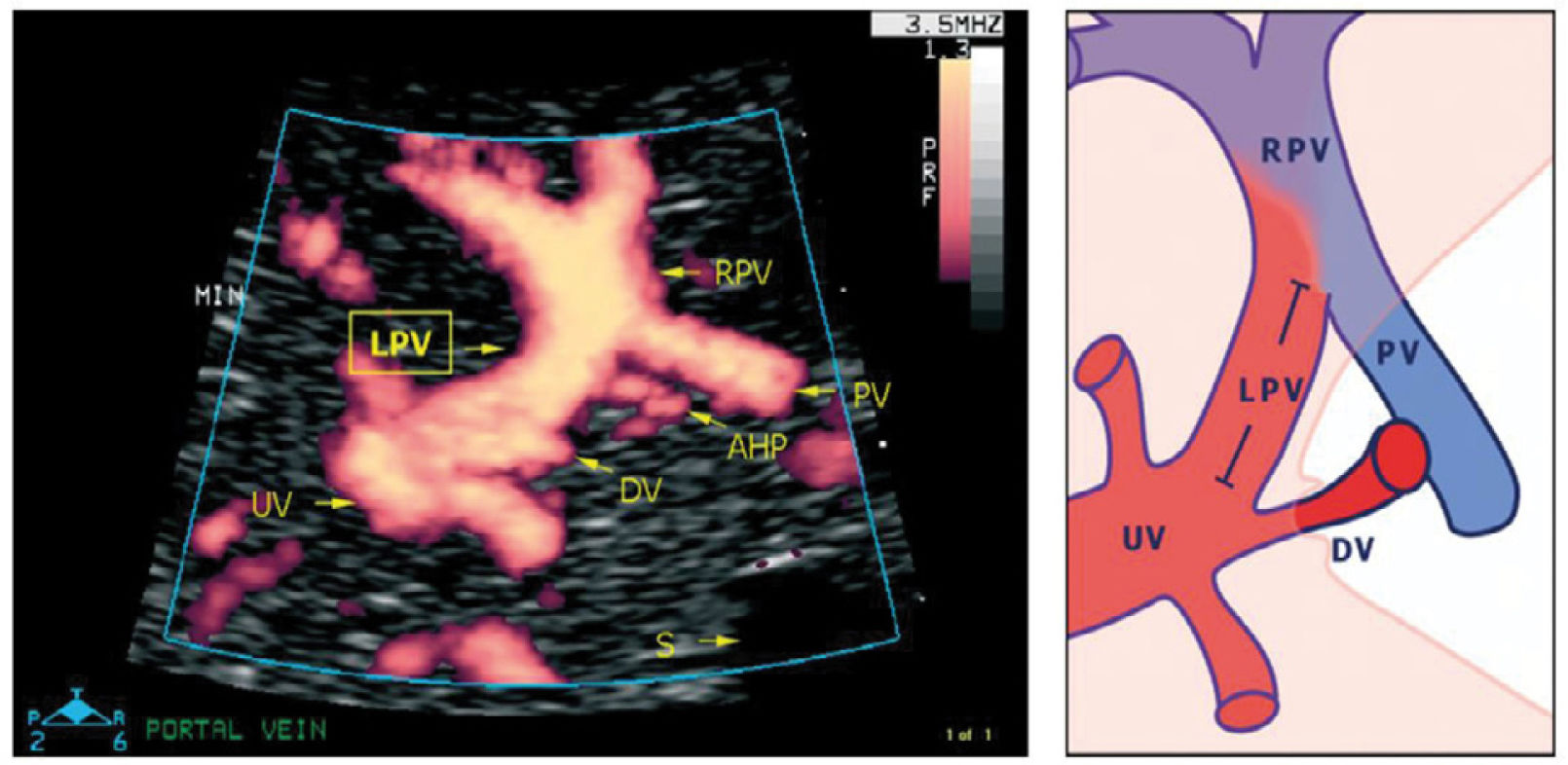
Details of the left portal vein connections visualized using power Doppler sonography
A magnified sketch (right panel) clarifies the details. The site for Doppler recording of the LPV would be the section between the istmus of the ductus venosus (DV) and the junction with the portal vein (PV) and the right portal vein (RPV). The insonation should be aligned and the sample volume reduced to 2-3mm to minimize interference. AHP, arteria hepatica; S, stomach; UV, umbilical vein33.
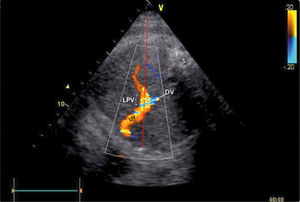
There has been some discussion of the nomenclature of this venous section, i.e., whether to name according to prenatal developmental origin, postnatal anatomic naming traditions or current clinical needs of simplicity36. The section between the ductus venosus inlet and the junction with the portal stem is, for simplicity reasons, called the ‘left portal vein’ (Figure 9), also known as the transverse sinus, or portal sinus, particularly when including the somewhat wider portion in front of the ductus venosus inlet. It is readily accessible in a modified transverse scan of the fetal abdomen tilted slightly caudally (Figure 11). The Doppler recording achieves a complete alignment when interrogating at 30–60° to the sagittal plane. The sample volume should be reduced to 2–3mm to avoid interference from the umbilical vein, ductus venosus, portal stem or right portal branch.
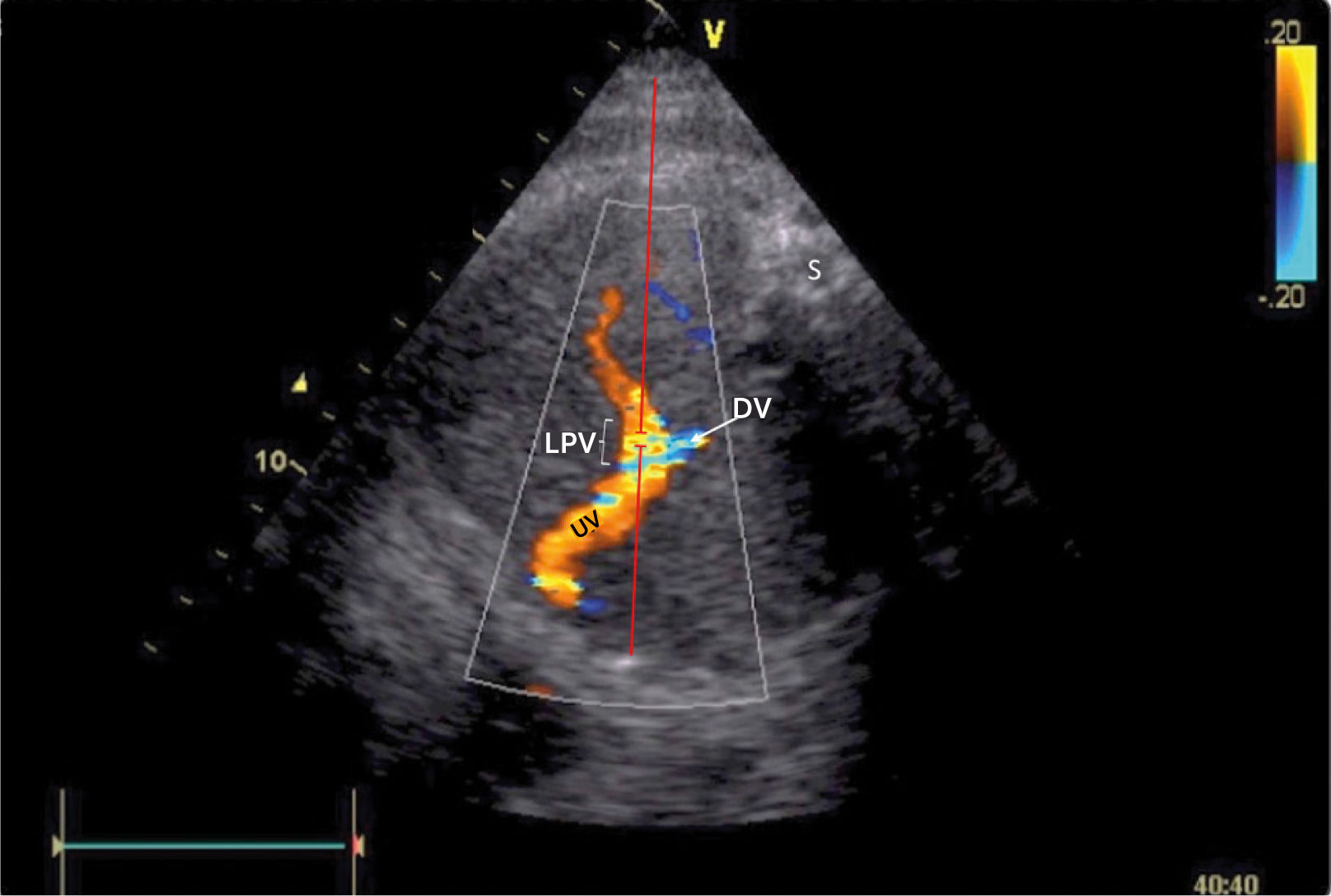
Doppler insonation of the left portal vein (LPV)
Doppler insonation (red line) of the left portal vein (LPV) in an almost transverse scan at the level of the inlet of the ductus venosus (DV) aligning the Doppler beam with the LPV from the posterior right side of the fetus. Color Doppler optimized for velocities of 10-25 cm/s confirms identification and connection to the umbilical vein (UV). The opposite direction is also favorable, should the fetus rotate 180̊. Spine (S).
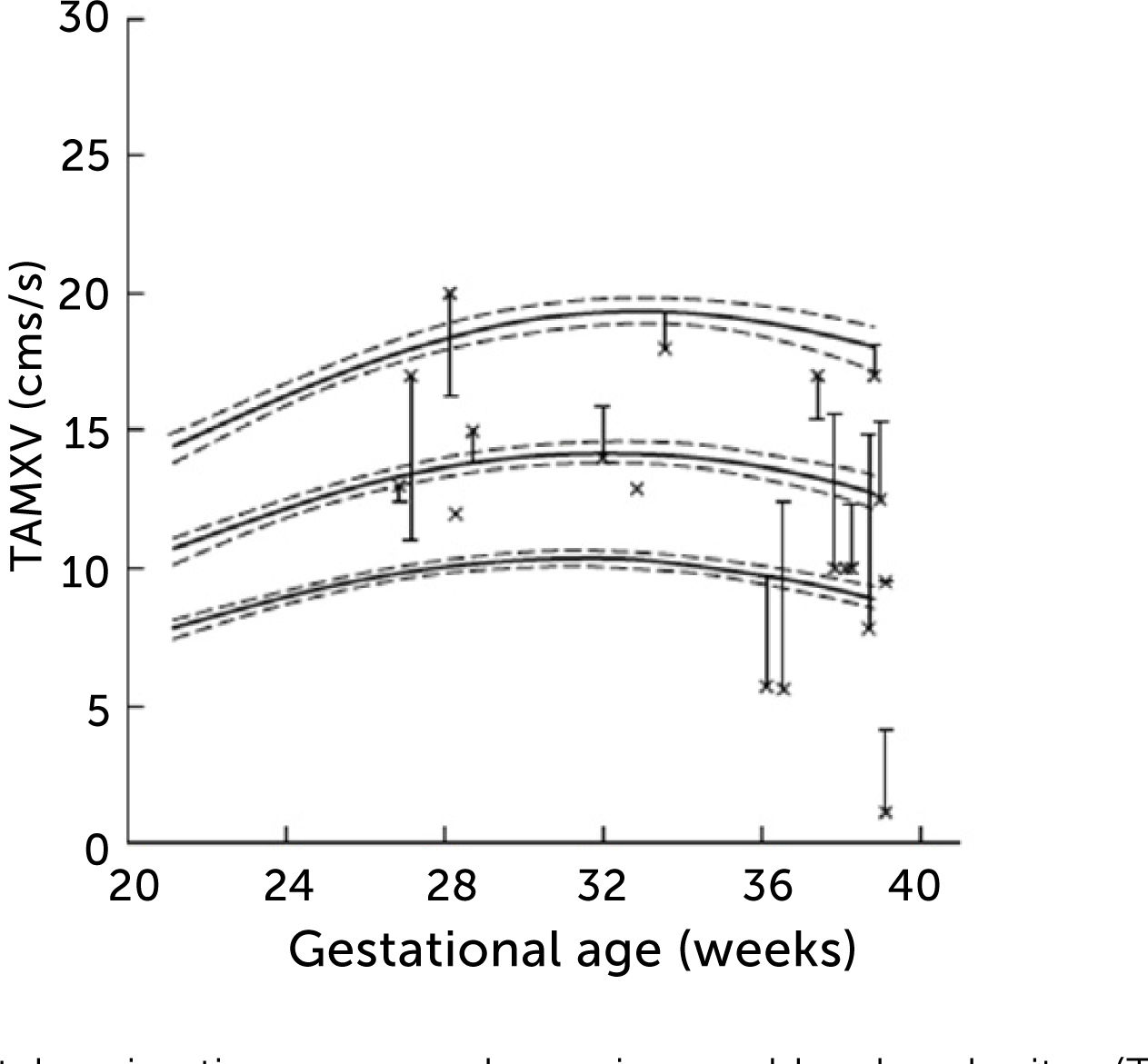
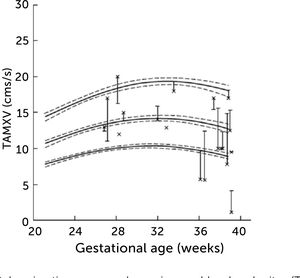
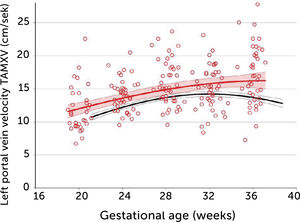
In normal pregnancies the time-averaged maximum velocity ranges between 7–21cm/s corresponding to the 2.5–97.5 centile prediction span depending on gestational age (Table 1)33. During the last days (weeks) before birth the velocity may reduce to near zero or turn negative (i.e., reversed) particularly under the influence of fetal breathing movements (Figure 12)33. Thus, the effect of breathing is to push some of the well-oxygenated umbilical blood to enter the ductus venosus (and via sinistra) rather than the right liver lobe at a time when pCO2 is elevated and pO2 is reduced. Additionally, during the last days of pregnancy, it looks like the portal circulation initiates a transition to postnatal pattern as splanchnic blood increasingly flows through the portal stem to feed the right liver lobe13.
Reference ranges for time-averaged maximum blood velocity in the fetal left portal vein.
| Gestation | Centile | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| [weeks] | 2.5 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97.5 |
| 21 | 7.3 | 7.8 | 8.3 | 9.4 | 10.6 | 12.0 | 13.4 | 14.3 | 15.2 |
| 22 | 7.7 | 8.2 | 8.8 | 9.9 | 11.2 | 12.7 | 14.1 | 15.1 | 15.9 |
| 23 | 8.1 | 8.6 | 9.2 | 10.3 | 11.7 | 13.2 | 14.8 | 15.7 | 16.6 |
| 24 | 8.5 | 9.0 | 9.6 | 10.8 | 12.2 | 13.8 | 15.4 | 16.4 | 17.3 |
| 25 | 8.8 | 9.3 | 10.0 | 11.2 | 12.6 | 14.3 | 15.9 | 17.0 | 17.9 |
| 26 | 9.1 | 9.6 | 10.3 | 11.5 | 13.0 | 14.7 | 16.4 | 17.5 | 18.5 |
| 27 | 9.3 | 9.9 | 10.6 | 11.8 | 13.4 | 15.1 | 16.9 | 18.0 | 19.0 |
| 28 | 9.5 | 10.1 | 10.8 | 12.1 | 13.7 | 15.5 | 17.3 | 18.4 | 19.5 |
| 29 | 9.6 | 10.2 | 10.9 | 12.3 | 13.9 | 15.7 | 17.6 | 18.7 | 19.8 |
| 30 | 9.7 | 10.3 | 11.1 | 12.4 | 14.1 | 15.9 | 17.8 | 19.0 | 20.1 |
| 31 | 9.7 | 10.3 | 11.1 | 12.5 | 14.2 | 16.1 | 18.0 | 19.2 | 20.3 |
| 32 | 9.7 | 10.3 | 11.1 | 12.5 | 14.2 | 16.1 | 18.1 | 19.3 | 20.5 |
| 33 | 9.6 | 10.3 | 11.0 | 12.4 | 14.2 | 16.1 | 18.1 | 19.3 | 20.5 |
| 34 | 9.5 | 10.2 | 10.9 | 12.3 | 14.1 | 16.0 | 18.0 | 19.3 | 20.5 |
| 35 | 9.4 | 10.0 | 10.8 | 12.2 | 13.9 | 15.9 | 17.9 | 19.2 | 20.3 |
| 36 | 9.1 | 9.8 | 10.5 | 11.9 | 13.7 | 15.7 | 17.7 | 19.0 | 20.1 |
| 37 | 8.9 | 9.5 | 10.3 | 11.7 | 13.4 | 15.4 | 17.4 | 18.7 | 19.9 |
| 38 | 8.6 | 9.2 | 9.9 | 11.3 | 13.1 | 15.0 | 17.0 | 18.3 | 19.5 |
| 39 | 8.2 | 8.8 | 9.6 | 11.0 | 12.7 | 14.6 | 16.6 | 17.9 | 19.1 |
Reference ranges for time-averaged maximum blood velocity (cms−1) in the fetal left portal vein; based on 554 longitudinal observations in 160 low-risk pregnancies33.
Left portal vein time-averaged maximum blood velocity (TAMXV)
Left portal vein time-averaged maximum blood velocity (TAMXV) development during the second half of pregnancy with 5th, 50th, and 95th centile with their 95% CIs. Individual observations of effect of fetal breathing movement are plotted for quiescence (-) and connected with a line to breathing movements (x)33.
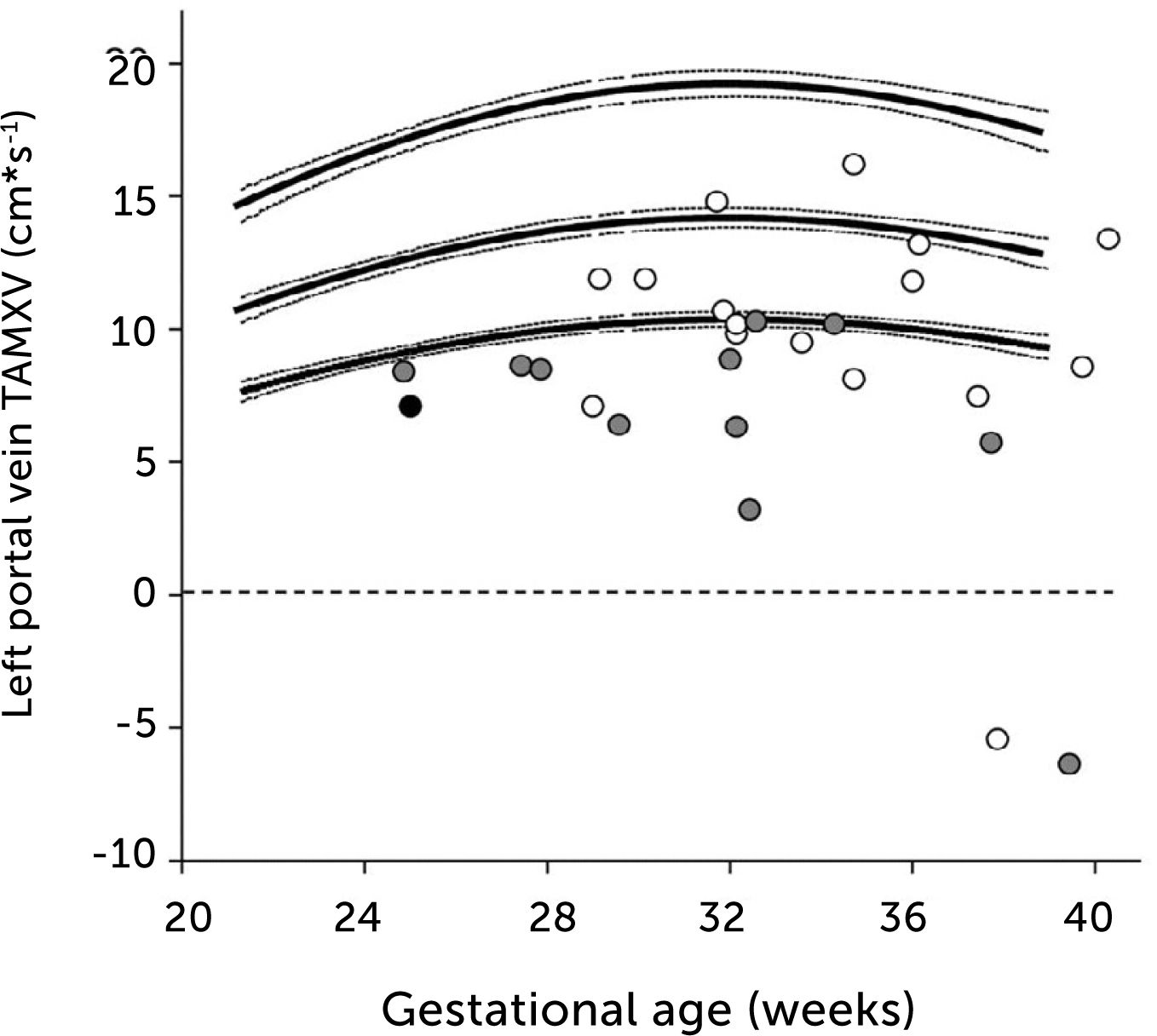
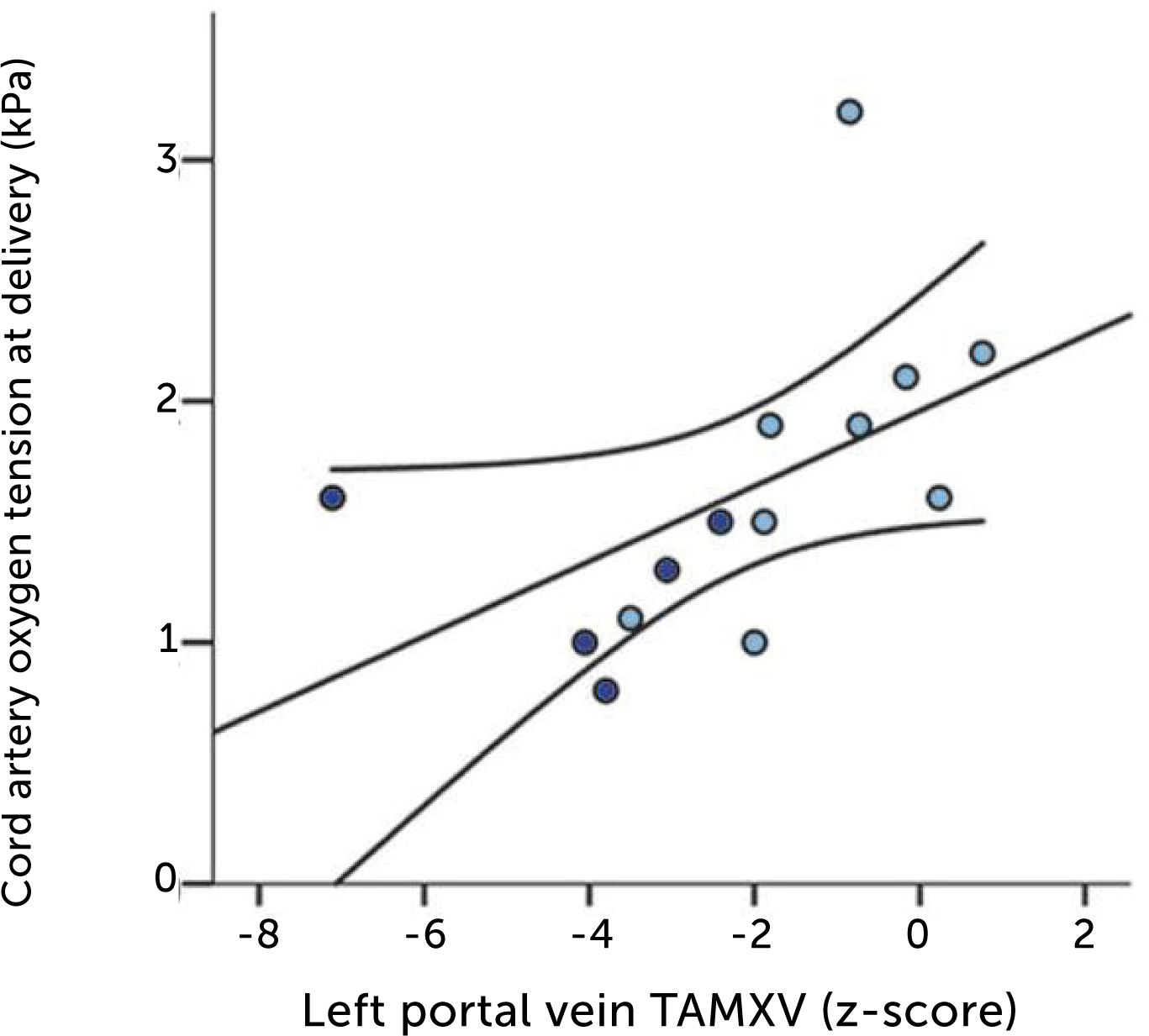
In fetal growth-restriction, however, the velocities are commonly reduced, and may even be reversed in the early weeks of the 3rd trimester when the condition is severe (Figure 13)23,37. The degree of low blood velocity in the left hepatic vein of growth-restricted fetuses tends to be linked to low arterial pO2 at birth (Figure 14)23.
Time-averaged maximum blood velocity
Time-averaged maximum blood velocity in growth-restricted. The effect is more pronounced in those with elevated PI (grey) or absent/reversed end-diastolic velocity (black) in the umbilical artery23.
Low blood velocity in the left portal vein of growth-restricted fetuses tends to be associated with low arterial pO2 at birth. Regression line with 95% CI. Light blue, normal umbilical artery PI; dark blue, umbilical artery PI >97.5 centile23.
In fetuses with macrosomia in non-diabetic mothers, blood flow in the left portal vein is increased when compared with background population, but is proportionate to fetal growth25.
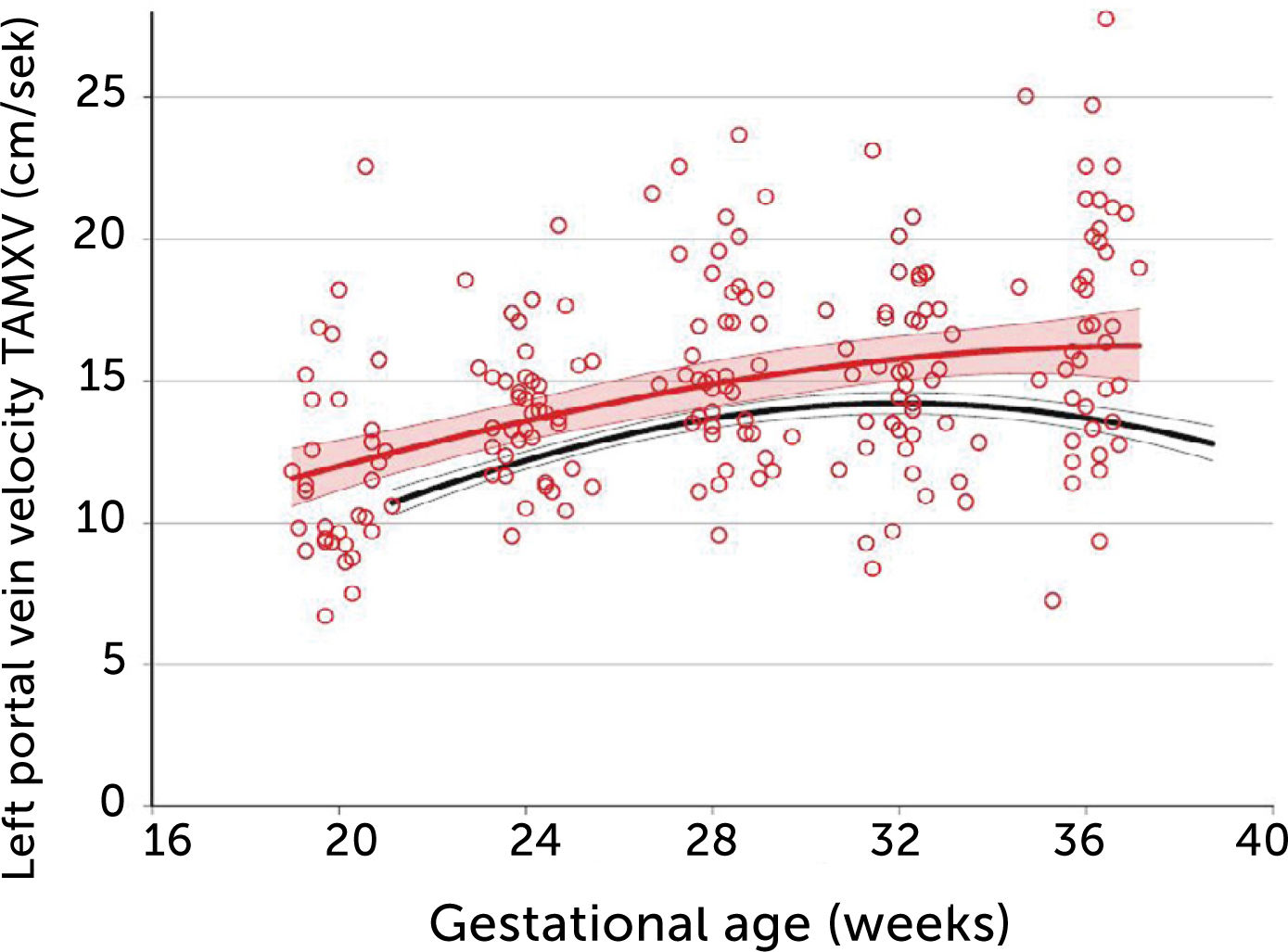
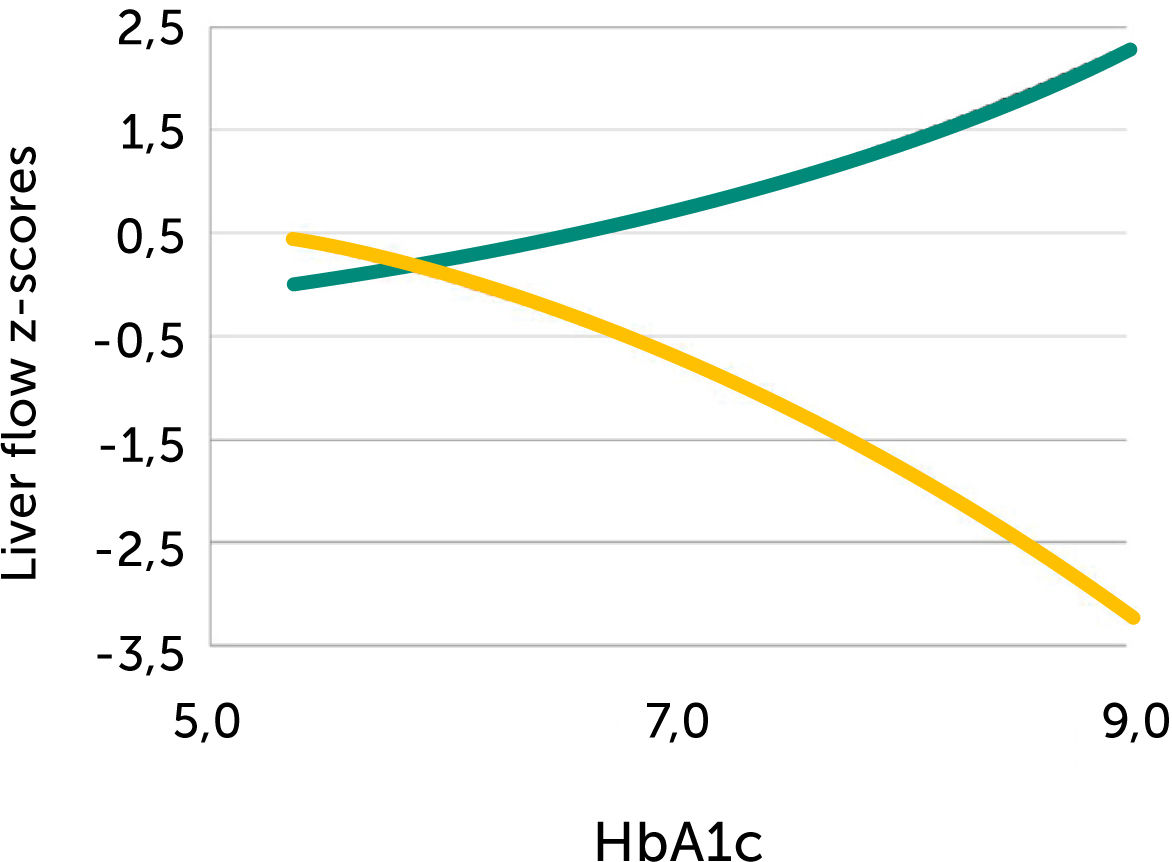
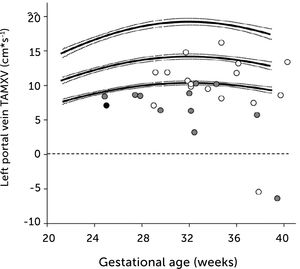
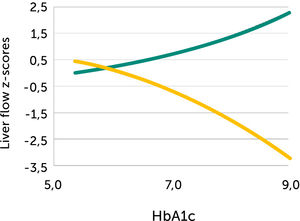
In pregnancies with PGDM, however, the umbilical flow and the amount directed to the fetal liver does not grow to match fetal growth although the fetal liver had been set to maximize umbilical venous intake at mid-gestation28,30. This is reflected in the generally high blood velocity in the left portal vein (Figure 15). It is also reflected in the increasing deficit in ductus venosus flow as the 3rd trimester develops30. This pattern is also related to the degree of maternal hyperglycemia assessed by HbA1c (Figure 16), and to the arterial concentration of lactate at birth30. This accumulated evidence of graded relation to maternal HbA1c, neonatal arterial lactate at birth and degree of ductus venosus shunting, makes the increased blood velocity in the left portal vein a candidate for fetal diagnosis and monitoring in DM pregnancies.
- •
Considerations and limitations
Time-averaged maximum blood velocity in the fetal left portal vein in pregestational diabetes mellitus
Time-averaged maximum blood velocity (TAMXV) in the fetal left portal vein is significantly increased in pregnancies with pregestational diabetes mellitus (red) compared with a low-risk reference group (black). Lines, mean with 95%CI for the mean28.
Fetal left portal vein blood velocity relation to glycemic control (HA1c)
Fetal left portal vein blood velocity z-score (green) increases with HbA1c while main portal vein fraction (z-score) to the liver reduces (yellow) with HbA1c in pregnancies with pregestational diabetes mellitus28.
It is obvious that for the future we will also use umbilical and middle cerebral artery pulsatility index (PI), and the ductus venosus PI to assess DM pregnancies, as they sometimes are complicated with placental dysfunction and growth impairment. The set of Doppler examinations that we have discussed in this review, should be interesting to add as they go after the pattern of metabolic effects that DM exerts on the fetal circulation. However, measurement of blood volume flow in the umbilical vein, ductus venosus, left portal vein and main portal stem requires painstaking techniques for Doppler interrogation and 2D-imaging, as well as consistency in repeating diameter measurement, particularly in the ductus venosus38,39. So far, these techniques have afforded valuable insights, but are less suitable for individual patient assessment in busy clinical settings staffed with sonologists of varying experience working with equipment of varying quality. In the literature, it is sometime possible to observe how results vary between research groups although the various groups produce knowledgeable results due to their internal consistency24,40–42. This is particularly true for the measurement of velocity and diameter of the ductus venosus. Thus, it will take time before these volume flow measurement techniques gain validation in clinical assessments. The umbilical vein flow assessment will probably become the most useful for the individual patient. It is commonly visualized in favorable positions both for Doppler measurement and for the needed repeat diameter measurement. Secondly, this vein has the largest bore in the fetus, thus being more robust against random error compared with the slim ductus venosus39.
Measuring blood velocity in the left portal vein, however, to gauge liver flow without measuring diameters could also be a candidate for clinical use. It is accessible in a transverse scan of the fetal abdomen and not very time-consuming (Figure 11). And, it is linked to status of glycemic control and fetal outcome.
Physicians should use all such insights as far as the present evidence takes her or him, as good doctors always have done.
However, the present evidence has limitations. The findings here stem from studies in PGDM where the physiopathology is expected to be more pronounced than in gestational DM and cannot easily be generalized. Secondly, prospective studies examining the predictivity of such measurements need to be carried out in order to refine the use and interpretation of the left portal vein Doppler measurement or any other of these measurement (e.g., umbilical flow).
ConclusionsPGDM in pregnancy does impact the fetal distributional circulation of the umbilical vein causing an augmented share to the fetal liver, but still not sufficient to match fetal growth in the 3rd trimester. Correspondingly, the ductus venosus shunting is blunted in the same period reducing the capacity to buffer off hypoxic events. Blood velocity in the left portal vein is commonly accessible for Doppler recording and reflects the augmented priority of liver perfusion. It is tempting to include this information in clinical management particularly since it has a graded relation to maternal glycemic control and to arterial lactate in the cord at birth. A refined use of this insight can be expected after prospective studies have been carried out to test its predictivity.
Conflict of InterestThe author reports no conflict of interest.
I am indebted to Svein Magne Skulstad, Guttorm Haugen, Jörg Kessler, Cathrine Ebbing, and Agnethe Lund, who have engaged deeply in our studies, and, in the present context, been instrumental in perpetuating consistency in techniques and methods through the years.