To describe the incidence, clinical characteristics and laboratory findings of meningitis and cephalic disorder from 2012 to 2014 at the Mexican Social Security Institute (MSSI).
Material and methodsDescriptive analysis of the epidemiological surveillance system for meningococcal disease. A univariate analysis was performed to measure the main trends and dispersion. The Wilson test was used to calculate 95% confidence intervals for proportions and a Kaplan–Meier analysis for survival.
Results113 probable cases of meningococcal disease. Cases with a positive result for meningococcus had an overall crude incidence rate of 0.02 cases per 100,000 social security beneficiaries. The disease is predominant in winter. The clinical symptoms in adults differ from those in children. In all, 20 deaths were reported, with an overall mortality rate of 17.7% and a rate of 20% for meningitis cases. The estimated mean survival time was 61.6 days (95% CI, 45.08, 75.18). The probability of surviving meningitis 2 days after the onset of symptoms was 0.944; after 20 days, it was 0.758 and after 87 days it was 0.427. Survival models were used, stratified by sex and age, with no statistically significant differences.
ConclusionsEpidemiological surveillance needs to be strengthened in terms of coverage between districts and timely reporting, with health personnel involved.
Describir la incidencia, características clínicas y hallazgos por laboratorio del síndrome de meningitis y encefálico de 2012 a 2014 en el IMSS.
Material y métodosAnálisis descriptivo del sistema de vigilancia epidemiológica de enfermedad meningococica. Se realizó análisis univariado con medidas de tendencia central y de dispersión. Se realizó prueba de Wilson para el cálculo de intervalos de confianza al 95% para proporciones y un análisis de sobrevida de Kaplan Meier.
Resultados113 casos probables de enfermedad meningococcica. Los casos con resultado positivo a meningococo, presentaron una tasa de incidencia cruda general de 0.02 casos por 100,000 derechohabientes. El comportamiento de la enfermedad predominó en invierno. El cuadro clínico entre adultos y niños fue diferente. Se reportaron 20 defunciones con una tasa de letalidad general 17.7% y 20% para casos por meningococo. El tiempo medio estimado de sobrevida fue de 61.6 días (IC95% 45.08, 75.18). La probabilidad de sobrevivir a la meningitis a los dos días fue de 0.944, a los 20 días de iniciado el cuadro clínico fue de 0.758, y a 87 los días fue de 0.427. Se realzaron modelos de supervivencia estratificados por sexo y edad, sin encontrar significancia estadística.
ConclusionesEs necesario reforzar la vigilancia epidemiológica en cobertura entre las Delegaciones y la oportunidad en la notificación, con involucramiento de todo el personal de salud.
Despite advances in diagnostic techniques and chemotherapy, bacterial meningitis has been a neurological emergency with a high mortality rate.1–3 The World Health Organization (WHO) considers bacterial meningitis to be a serious threat to health, with an estimated 171,000 deaths per year worldwide. Major outbreaks of this condition have been documented in the “meningitis belt” of sub-Saharan Africa.3
Optimal treatment depends on immediate treatment with the most appropriate antibiotic,4,5 but the data are limited regarding the epidemiological surveillance of meningitis. In this regard, epidemiological surveillance of meningitis is critical, owing to the wide range of aetiological agents. Outbreaks of meningitis have been identified in schools,6 prisons,7 and the community.8–10 In our country, meningitis is reported weekly through the Epidemiological Surveillance Single Information System (SUIVE)11,12; if meningococcal meningitis, invasive pneumococcal disease, invasive infection caused by Haemophilus influenzae, tuberculosis, human rabies, vector-borne disease or free-living amoebae infection are suspected, reporting is done immediately, and each disease must have a special surveillance system.13–15
In 2010, an outbreak of meningococcal meningitis in Mexico City and the metropolitan area was studied16; the outbreak started at the Social Rehabilitation Centre – North.7 From 2010 onward, special epidemiological surveillance of meningococcal meningitis has been implemented, and an epidemiological study was designed with a form for reporting cases of this disease. This study was endorsed by the establishments that make up the health sector within the National Epidemiological Surveillance Committee. It was modified in 2014.
The MSSI carries out meningitis reporting according to each of these specific guidelines for epidemiological surveillance; in this way probable and confirmed cases of meningococcal disease are studied and reported and, based on this, other aetiologies have been identified. In this document, the incidence, clinical characteristics and laboratory findings from cerebrospinal fluid (CSF) samples are described and analysed, over a period from 2012 to 2014, at the MSSI.
Materials and methodsA descriptive analysis was performed using the variables in the epidemiological studies of reported and studied cases of probable meningococcal disease, from 2012 to 2014, in social security beneficiaries with the MSSI.
A case was considered to be probable if the patient had a fever and two or more of the following signs or symptoms: (a) meningeal disorders: bulging fontanelle (<1 year), stiff neck, Kernig's sign, Brudzinski's sign, lumbar pain or photophobia; (b) cephalic disorders: irritability, disorientation, confusion, sleepiness, drowsiness, stupor, coma, apathy, aggressiveness, headache, slurred speech, cranial nerve palsies or convulsions; (c) suggestive CSF results: increased pressure, turbidity, elevated cellularity, low CSF glucose level, elevated protein, polymorphonuclear pleocytosis. In infants simply a fever/hypothermia with refusal to feed and irritability/lethargy were suggestive. A confirmed case was any probable case in which the presence of Neisseria meningitidis was identified according to the confirmation criteria established at the laboratory; or in the absence of a diagnostic test, association with a confirmed case.16
A descriptive analysis of demographic variables, level of medical care, clinical manifestations, laboratory parameters, treatments and outcome was performed. Incidence rates were calculated per 100,000 social security beneficiaries who saw their GP; in general, the population mean during the period was considered halfway through each year. General and specific crude incidence rates were calculated by age group and by state. The descriptive analysis, with a univariate determination of rates, ratios and proportions, was done in Excel. The Wilson test was used to calculate 95% confidence intervals for proportions in Open-Epi. In addition, a Kaplan–Meier analysis for survival was used. Follow-up time was calculated in days, with subtraction between the date of hospital discharge and the date of the onset of symptoms (patients with none of these data were excluded); this was performed in Stata.
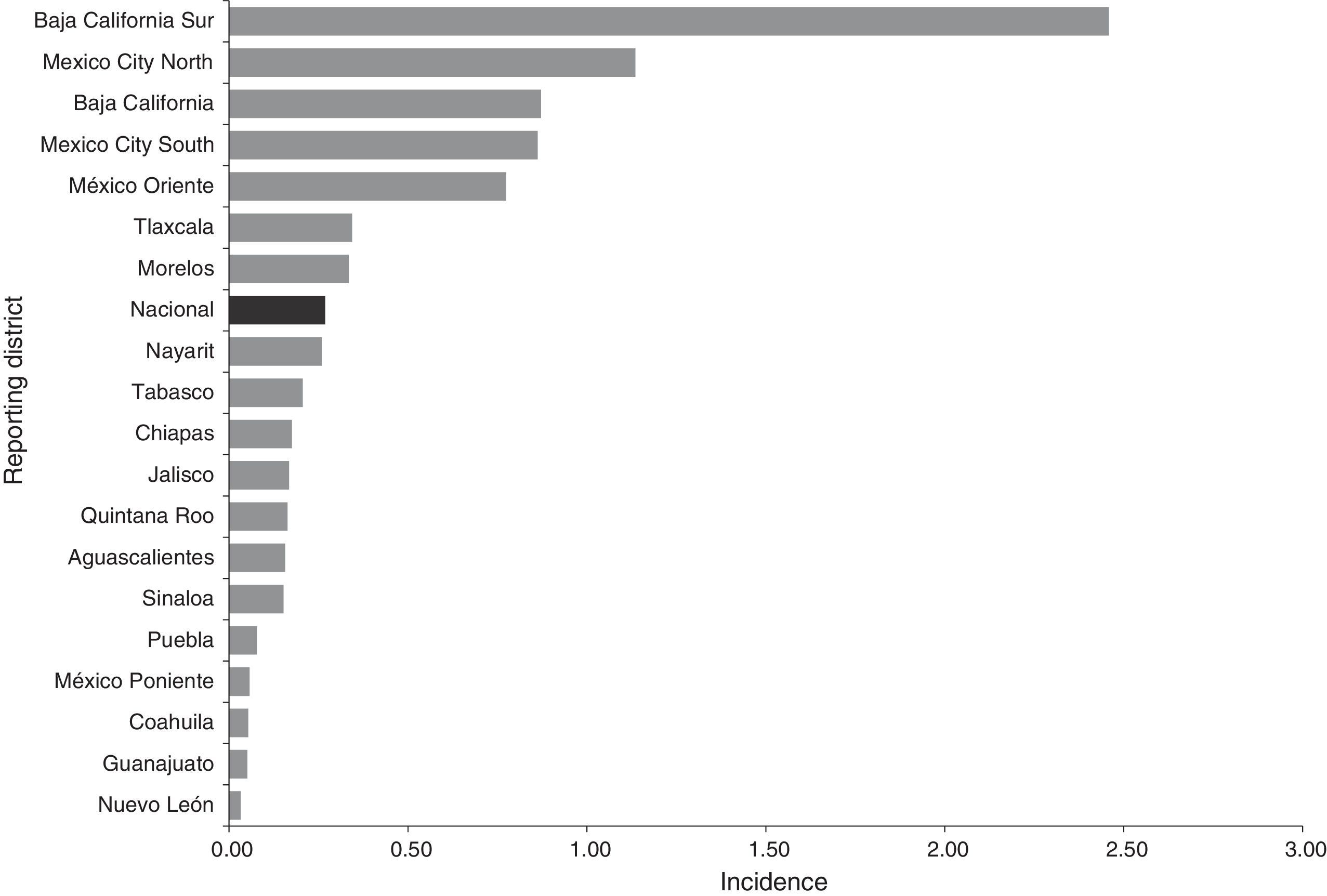
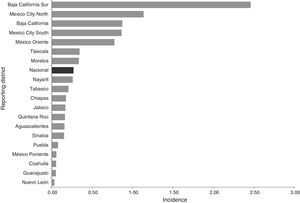
ResultsEpidemiological situation during the periodBetween 2012 and 2014, 113 probable cases of meningococcal disease were reported: 35 in 2012, 30 in 2013 and 48 in 2014. The overall crude incidence during the period was 0.27 cases per 100,000 social security beneficiaries who saw their GP. The crude incidence showed an upward trend. This disease was reported in 19 states (Chart 1).
Distribution by age and sexIn total, 72 cases in men and 41 in women were reported, and the age range was from 2 days to 66 years. The ratio of men to women was 1:1.8. In all, 47.8% (95% CI, 38.8, 56.92, p=0.6381) of the cases were in children under 10. In most of the age groups, men made up the majority, except for the ages from 45 to 54 and from 65 to 69. Children under one year of age were the most affected, with 3.20 cases per 100,000 social security beneficiaries, followed by the population from 1 to 4 years of age, with 0.72 cases. In each year, those most affected were children under one year of age.
Cases with a positive result for meningococcus in this period showed a general crude incidence rate of 0.02 cases per 100,000 social security beneficiaries, and this was an upward trend between 2012 and 2013. No cases of meningococcus were identified by the laboratory in 2014. Those most affected were between the ages of 15 and 19, with 0.16 cases per 100,000 social security beneficiaries. The median age for confirmed cases of meningococcus was 13.5 years (ranging from 5 to 47).
Behaviour and seasonCases were reported year-round. In the last three years, the disease was predominant in winter, with more cases between November and January.
Level of medical careThroughout the period, 78 cases were reported in secondary care hospitals (69.0%, 95% CI, 59.99, 76.81, p<0.0001) and 31.0% (95% CI, 23.19, 40.01, p<0.0001) in highly specialised care units (UMAE).
Initial assessmentGenerally, the initial diagnosis in 69% (95% CI, 60.92, 77.6, p<0.0001) was meningitis of an aetiology to be determined, followed by viral encephalitis. The average number of days till seeking medical care was four (ranging from 0 to 32; median of two days). Two outliers were excluded, as well as one for which the date of onset was not known.
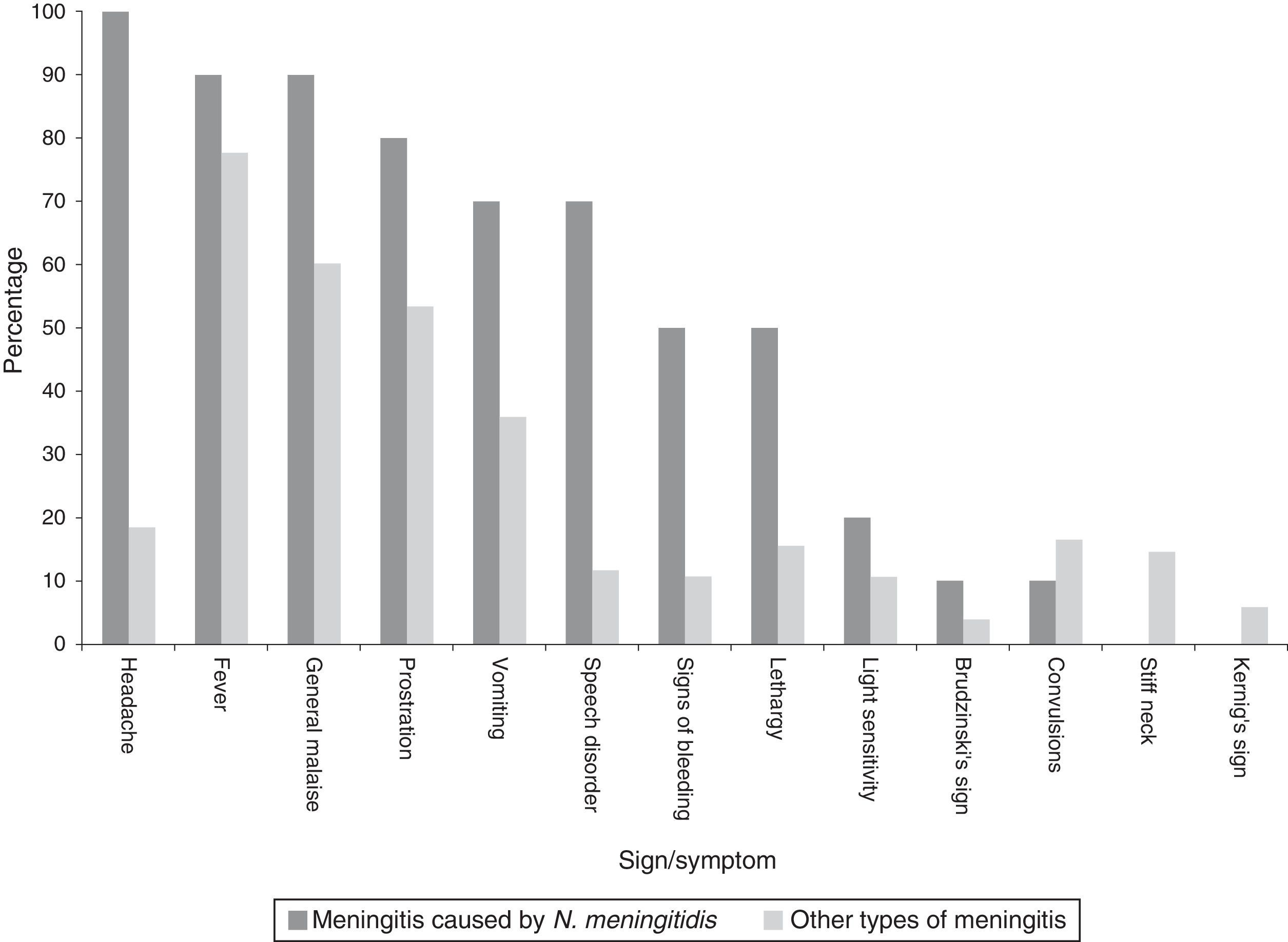
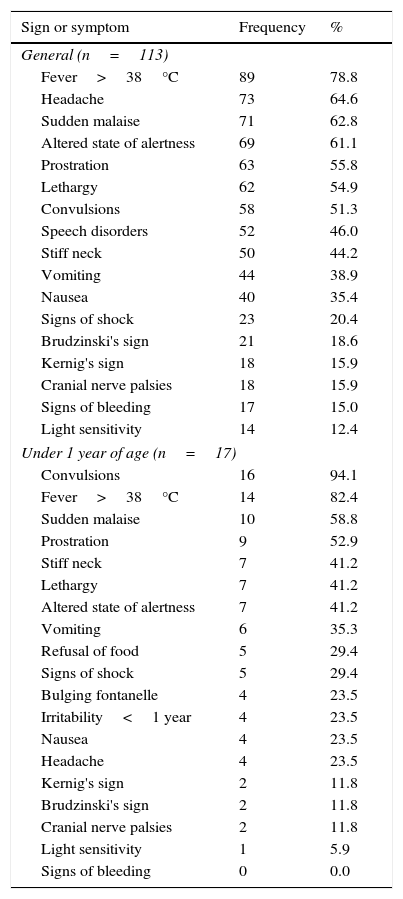
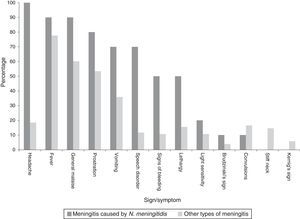
Clinical manifestationsAmongst all cases the main sign was fever. However, in characterising the clinical manifestations in children under one year of age, convulsions predominated, whereas, amongst all cases, convulsions occurred in half (Table 1). In describing the clinical symptoms of confirmed cases of meningococcal disease (n=10), the main symptoms were headache, fever and sudden malaise (Chart 2).
Clinical manifestations of probable cases of meningococcal disease, 2012–2014.
| Sign or symptom | Frequency | % |
|---|---|---|
| General (n=113) | ||
| Fever>38°C | 89 | 78.8 |
| Headache | 73 | 64.6 |
| Sudden malaise | 71 | 62.8 |
| Altered state of alertness | 69 | 61.1 |
| Prostration | 63 | 55.8 |
| Lethargy | 62 | 54.9 |
| Convulsions | 58 | 51.3 |
| Speech disorders | 52 | 46.0 |
| Stiff neck | 50 | 44.2 |
| Vomiting | 44 | 38.9 |
| Nausea | 40 | 35.4 |
| Signs of shock | 23 | 20.4 |
| Brudzinski's sign | 21 | 18.6 |
| Kernig's sign | 18 | 15.9 |
| Cranial nerve palsies | 18 | 15.9 |
| Signs of bleeding | 17 | 15.0 |
| Light sensitivity | 14 | 12.4 |
| Under 1 year of age (n=17) | ||
| Convulsions | 16 | 94.1 |
| Fever>38°C | 14 | 82.4 |
| Sudden malaise | 10 | 58.8 |
| Prostration | 9 | 52.9 |
| Stiff neck | 7 | 41.2 |
| Lethargy | 7 | 41.2 |
| Altered state of alertness | 7 | 41.2 |
| Vomiting | 6 | 35.3 |
| Refusal of food | 5 | 29.4 |
| Signs of shock | 5 | 29.4 |
| Bulging fontanelle | 4 | 23.5 |
| Irritability<1 year | 4 | 23.5 |
| Nausea | 4 | 23.5 |
| Headache | 4 | 23.5 |
| Kernig's sign | 2 | 11.8 |
| Brudzinski's sign | 2 | 11.8 |
| Cranial nerve palsies | 2 | 11.8 |
| Light sensitivity | 1 | 5.9 |
| Signs of bleeding | 0 | 0.0 |
Of the 113 reported cases, 57 of them had no underlying condition (50%), while in 43 it was not known (39%); 13 had a comorbidity (11%). The most common comorbidity was hypertension (4), followed by diabetes mellitus (3). As regards the hypertension, three of the cases were women and one a man, with an average age of 51; while in the case of diabetes, two of the cases were women, aged 15 and 66, and one an 18-year-old man.
TreatmentTreatment was with antibiotics, antifungals and antivirals, mainly in double and triple regimens and with combinations of vancomycin, ceftriaxone and cefotaxime. In total, 24 patients were prescribed monotherapy, especially with ceftriaxone. Acyclovir was prescribed to 17 patients, and prescribed as monotherapy in five patients. All those treated with fluconazole were also given at least one antibiotic.
The average time between the onset of drug therapy and the onset of symptoms (n=109) was six days (ranging from 0 to 64 days; median of three days); while from the first contact with health services to prescription (n=111), the average was two days (ranging from 0 to 22; median of zero days).
Additional findings from the laboratory diagnosesOf the 113 cases, 93 of them underwent a lumbar puncture to study the CSF (82.3%, 95% CI, 74.24, 88.24, p<0.0001), the results of which showed turbidity in 47.4% (95% CI, 37.47, 57.36, p<0.6041), transparency in 45.2% (95% CI, 35.44, 55.27, p<0.3507) and blood-count issues in 7.4% (95% CI, 3.694, 14.73, p<0.0001). 39 cases or 41.9% (95% CI, 32.42, 52.09, p<0.1198) showed bacterial growth.
The CSF glucose report was present in 73 cases, with a mean of 47.8mg/dl, WBC count 58.4% in 44 cases, and the identification and recording of proteins was very inconsistent. In that sense, in cases in which meningococcus was identified, the mean percentage of WBCs was 91.2% (ranging from 80 to 99; median of 92.5), while glucose was 18.6mg/dl (ranging from 2 to 37; median of 20).
According to the Gram stain, 11 were Gram-positive and 18, Gram-negative. By grouping by bacteria only, the main agent was N. meningitidis with 33.3%, followed by Streptococcus pneumoniae with 16.7%. It should be noted that on identifying N. meningitidis, five were type C; two, type A; and three, not determined.
In 96 cases the date of the puncture and CSF sample was recorded; of these, 67 of the cases first had a lumbar puncture and then started antibiotics, ranging from 0 to 11 days. In 18 cases the report showed no bacterial growth, where the average time between the puncture and the start of treatment was 15h (SD=2.16), whereas for those showing bacterial growth it was 20h on average (ranging from 0 to 11 days, SD=2.23). To identify meningococcus, only in one case was the drug prescribed first and then the lumbar puncture performed, with an interval of two days. The rest of the cases were done in the reverse order (seven on the same day).
Reason for hospital dischargeIn all, 91 cases indicated the reason for hospital discharge, amounting to an average hospital stay of 16 days (ranging 0 to 93; median of 11 days). Most were discharged due to improvement (67%, 95% CI, 56.86, 75.83, p<0.0011) or due to death (21.9%, 95% CI, 14.7, 31.52, p<0.0001).
On including the agents identified by the lab and the clinical diagnostic tests (n=38), the aetiology was classed as follows: 10 cases caused by meningococcus (26.3%); five each by S. pneumoniae (13.2%) and varicella, group B Streptococcus and herpes; two each by Acinetobacter baumannii and Staphylococcus aureus (5.3%). The other agents were classed and/or identified in only one patient.
ChemoprophylaxisChemoprophylaxis for contacts was given in 20 cases with ciprofloxacin or rifampicin. In nine of these cases, meningococcus was identified by the lab, and in another two, other bacterial agents were found. In the rest of the cases, the agent was not identified. Broken down by initial diagnostic test, meningococcal meningitis was recorded in five cases; in three of these chemoprophylaxis was performed.
DeathsThroughout the period, 20 deaths were recorded: five in 2012, seven in 2013 and eight in 2014. The average age of those who died was 25.5 years (ranging from 1 month to 64 years), with hospital care requested within two days (ranging from 0 to 10; median of one day), and the start of antibiotic treatment was four days on average from the onset of symptoms (ranging from 0 to 13; median of two days). Those who died had on average 16 days of symptoms (ranging from 1 to 65; median of 13 days, with one outlier excluded).
The bacteria were isolated in seven of these cases, with the main aetiological agent being type C N. meningitidis, and S. pneumoniae, Cryptococcus neoformans, Staphylococcus haemolyticus, St. aureus and A. baumannii also isolated.
The general three-year crude mortality rate was 0.05 deaths per 100,000 social security beneficiaries who saw their GP, whereas for confirmed cases of meningococcus it was 0.005 deaths. In children under one year of age, a crude mortality rate of 0.19 was recorded for the period.
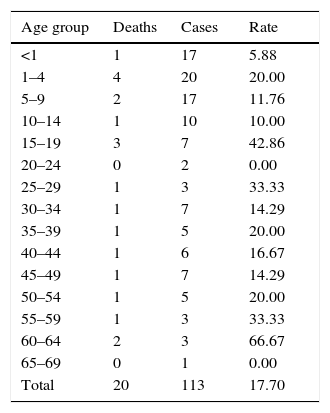
The mortality rate for the entire period was 17.7% (95% CI, 11.76, 25.76, p<0.0001), whereas for the cases in which meningococcus was identified it was 20.0% (95% CI, 5.669, 50.98, p<0.0577). The mortality rate varied: 14.3 (95% CI, 6.261, 29.37, p<0.0001) in 2012, 23.3 (95% CI, 11.79, 40.93, p<0.0034) in 2013 and 16.7 (95% CI, 8.696, 29.58, p<0.0001) in 2014. By age group, of the total number of cases, the highest mortality rate was seen in those aged 60–64 (Table 2).
Deaths and mortality rate of probable cases of meningococcal disease by age group, 2012–2014.
| Age group | Deaths | Cases | Rate |
|---|---|---|---|
| <1 | 1 | 17 | 5.88 |
| 1–4 | 4 | 20 | 20.00 |
| 5–9 | 2 | 17 | 11.76 |
| 10–14 | 1 | 10 | 10.00 |
| 15–19 | 3 | 7 | 42.86 |
| 20–24 | 0 | 2 | 0.00 |
| 25–29 | 1 | 3 | 33.33 |
| 30–34 | 1 | 7 | 14.29 |
| 35–39 | 1 | 5 | 20.00 |
| 40–44 | 1 | 6 | 16.67 |
| 45–49 | 1 | 7 | 14.29 |
| 50–54 | 1 | 5 | 20.00 |
| 55–59 | 1 | 3 | 33.33 |
| 60–64 | 2 | 3 | 66.67 |
| 65–69 | 0 | 1 | 0.00 |
| Total | 20 | 113 | 17.70 |
Population in June 2013, Division of Health Information.
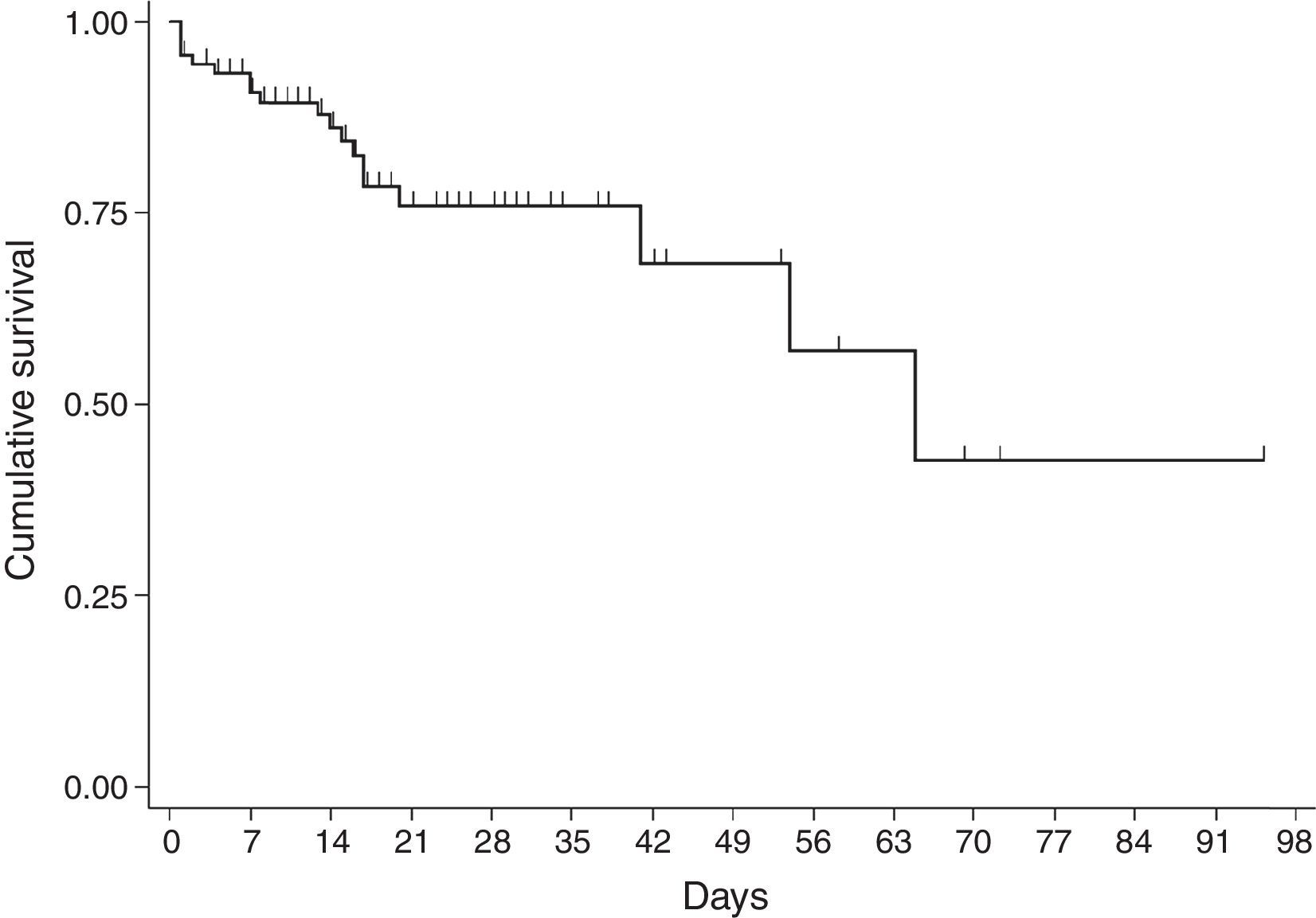
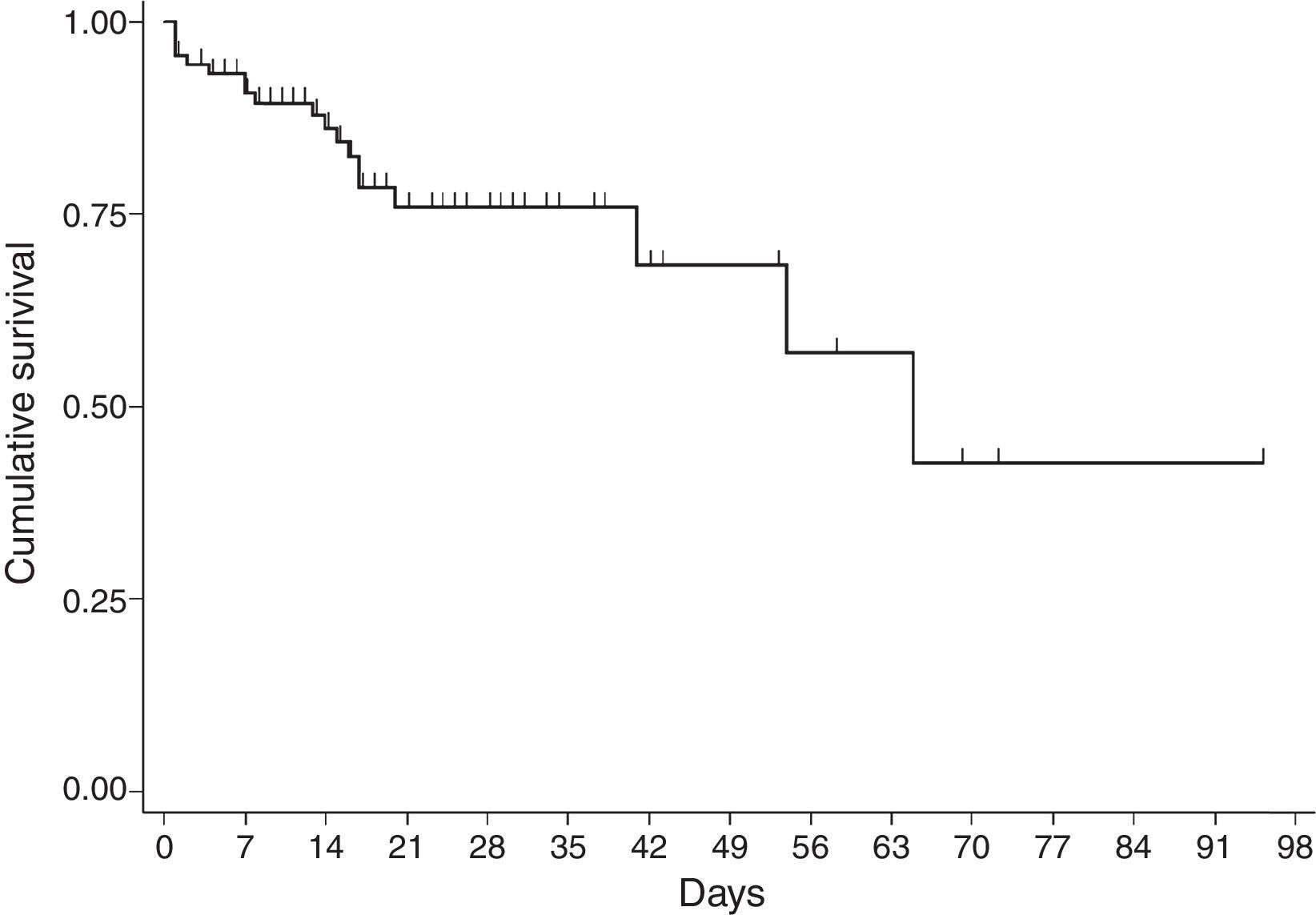
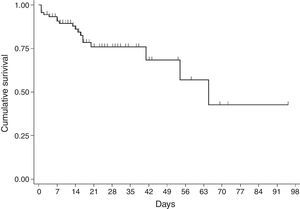
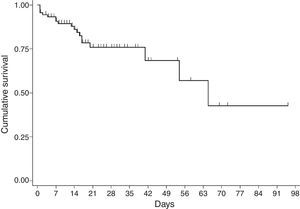
For the survival analysis, in total, 90 cases were analysed, 19 of whom died (one outlier was excluded); the average estimated time of survival was 61.6 days (95% CI, 45.08, 75.18) and the median, 65 days (95% CI, 41.39, 88.61). The probability of surviving meningitis 2 days after the onset of symptoms was 0.944; after 20 days, it was 0.758 and after 87 days it was 0.427 (Chart 3). Survival models were used, stratified by sex and age, with no statistically significant differences. Using the agent identified, amongst those with meningococcus, compared with the other agents (Chart 4), no statistical difference was found (p=0.4975).
Epidemiological surveillance systemThe average number of days in which cases were reported was five (ranging from 0 to 62; median of two days); 47.78% (95% CI, 38.8, 56.92, p<0.6381) were reported within 24h.
In 2012 the average was five days (ranging from 0 to 46). In all, 46% were reported in a timely manner. The following year, reporting improved, with an average of 24h (ranging from 0 to 8 days), and in 2014 the average was six days (ranging from 0 to 62), where four in ten cases were reported in a timely manner.
DiscussionThe incidence of bacterial meningitis in adults in different countries varies from 1.0 to 7.2 per 100,000 inhabitants.17–19 However, the crude incidence rate of meningococcus was very low, lower even than that indicated in the most recent annual report by the European Centre for Disease Prevention and Control (ECDC).20 In Europe, it was a rare condition in 2011, with 0.75 cases per 100,000 inhabitants, with 4,121 reported cases and 3814 confirmed cases, mainly serotype B and C meningococcus.20 In Burkina Faso, in 2012, 5,807 cases of meningitis were reported, 2,352 of which were laboratory-confirmed,21 and in the United States, in children under 1 year of age, the meningococcus incidence rate between 2006 and 2012 was 2.74 children.22 In this respect, the number of reported and studied cases, and the crude incidence rate is lower than in these publications, a fact which reflects the capacity for identification and diagnosis of cases, to be studied in an epidemiological surveillance system.
Invasive meningococcal disease in Mexico has been documented to be a common health problem23,24; however, the monitoring of this disease in Mexico is passive, and underreporting is a subject of concern, a situation reflected in this study. In the past two decades, and earlier in this decade, the reporting of cases of meningococcal meningitis varied. From 2001 to 2010, 474 cases were reported, with an average of 47 cases per year, specifically 45 cases in 2011 and 43 cases the following year.25,26 For the diagnosis of meningitis (G00-G03, except G00.0 and G01), 732 cases were reported, and in 2013, 895.27
In Europe, babies and children under five years of age have the highest risk of developing the disease, followed by those 15–19 years of age.20 In Mexico, according to information from the Epidemiological Surveillance Single Information System, between 2002 and 2010, the incidence rate was highest in children under 10.28 Similarly, in an outbreak of meningococcal meningitis in Tijuana, Baja California, 73.7% of the cases were in children under 13.29 This shows that the predominance of the disease continued in children, a situation documented in this study, where the child population was the most affected.
According to cases reported through the special system in the past three years, the disease shows a seasonal prevalence in winter, with more cases between November and January. This situation is similar to that recorded in Europe,13 as well as in the sector's case series in Mexico.28
Most cases were concentrated in four states, initially in the Baja California Peninsula and centre of the country. Thus the following assumptions can be made: (1) in the Baja California Peninsula an outbreak of this disease occurred in 2012 and 2013, and in addition, this is considered to be an endemic area for meningococcal disease29,30; (2) in the Peninsula there is probably a better understanding in terms of detecting and reporting cases; (3) there may also be other influencing factors, such as the geographic conditions (dry season/wind)29; (4) in Mexico City, 66% of all cases were reported by a UMAE (mainly the Siglo XXI Children's Hospital UMAE), which are major regional and reference centres in other districts. In this sense it is essential to strengthen epidemiological surveillance in children, because half of the reported cases were in children under 14. Furthermore, although the largest number of cases were reported by second-level medical units, particularly Mexico City, there is an indication that the demand for health care was (1) in very advanced stages due to the lack of a timely diagnosis (up to 20 days; on average five days), (2) limitations or deficiencies in the management in second-level care, and (3) additive to a defective culture in education, prevention and health promotion in the population. In addition, HIV results in infectious and parasitic diseases that manifest neurologically, and this is a factor in the leading causes of diseases affecting the nervous system.31 Although this condition was not found in this study (only one case was reported with HIV/AIDS as a comorbidity), it is essential to communicate and identify these cases, since the neurological manifestations associated with HIV/AIDS prevailing in industrialising countries are secondary to opportunistic infections.32,33
In a study conducted in Mexico in children under 18, it was found that the characteristics of the clinical symptoms at hospital admission included infectious syndrome (87%) and meningeal syndrome (74%),2 similar to our study, in which in seven out of every 10 cases the initial diagnosis was meningitis.
Half of the cases of meningococcal meningitis were reported in Baja California between 2012 and 2013. Furthermore, in 2013 an outbreak of serotype C meningococcal meningitis was reported in Tijuana, Baja California, with 19 cases.29 This year, the cases reported and confirmed by labs in this report correspond to the same serotype reported in Tijuana. The circulation of this serotype has been identified before,34 and so it has been considered endemic to the area,30 exacerbated by the presence of a highly virulent strain (ST-11 clonal complex) detected in Baja California.31
The Baja California Peninsula has shown specific determinants in terms of its inhabitants acquiring the disease. Low socio-economic levels prevail in this disease.28,29,31 Though not possible to describe this condition in this study, it represents a flashpoint for identifying cases in which medical attention is required. In a seven-year study in a tertiary care hospital specialising in neurology and neurosurgery in Mexico City, the main causes of medical care subject to epidemiological surveillance were acute inflammatory polyneuropathy (19.7%), unspecified viral encephalitis (18.5%) and neurocysticercosis (17.1%)31 which reinforces this condition. Moreover, there have been recent outbreaks of this disease in Chile; 75 cases were reported in 2010, 68 in 2011 and 108 in 2012, most of which were type B.35
There are several determinants in the identification of the bacteria, in terms of diagnostic capacity: geographic area, study time and age of the case. For example, the frequency in Hong Kong between 1992 and 2001 differs, where Mycobacterium tuberculosis (46%), S. pneumoniae (11%), Streptococcus suis (9%) and Klebsiella pneumoniae (8%) were the most significant, whereas N. meningitidis and H. influenzae were the rarest.36 Moreover, K. pneumoniae was reported as a major causative agent in Taiwan,37,38 but not by Hui36 or in this study. In 2012, in Burkina Faso, 62% of meningitis cases were due to serotype W N. meningitidis.21 In a study conducted in the UK and the Republic of Ireland during 2010–2011, in children under 90 days of age, it was observed that 50% of the cases were due to group B Streptococcus, 14% Escherichia coli, 9% S. pneumoniae, and 8% N. meningitidis.39 During 2012–2013, Cuba reported 10 isolated cases of meningitis due to S. pneumoniae, which amounted to 55% of confirmed cases40; the situation was similar in Colombia in 2011.41 In Mexico, at Mexico City Children's Hospital, over 21 years of study, it was found that H. influenzae was the main bacterium, followed by S. pneumoniae with 46 (55.42%) and 15 (18.07%)2; this situation can be contrasted with this report, as 26.3% were due to N. meningitidis, followed by S. pneumoniae. Apart from these differences, this reveals the need to standardise the pre-testing, testing and post-testing stages in the clinical laboratories of medical units.
Despite significant progress of hospital care, life support and the development of antibiotics, acute meningitis has been a devastating disease with long-lasting effects,42 attributed to the host's systemic inflammatory response to the agent. In one systematic review it was observed that the mortality rate in African children hospitalised with acute bacterial meningitis caused by S. pneumoniae was 35%, H. influenzae 25% and N. meningitidis 4%.42 This condition also results in various complications, such as ventriculitis, hydrocephalus and brain abscesses. Although one limitation in this study was not having this information, the calculated crude rate was high, if lower than that found in other studies36,42; it was also lower than that reported in the meningococcal meningitis outbreak in Tijuana, Baja California, which was 36.8%.31
Regarding comorbidities, diabetes was present before admission in between 7% and 10% of adult patients with community-acquired bacterial meningitis,1,43 and in some this figure was 48.1%46 which, contrasted with this study, was lower. These differences may be attributable to (1) the number of cases studied, (2) the fact that most of the cases were in children or (3) inconsistencies in recording the comorbidities of this study. Despite this, however, it is important to note that a large proportion of patients with diabetes mellitus hospitalised for bacterial meningitis have metabolic disorders, and comprehensive care and life support are therefore vital.43
The survival analysis was used mainly to compare treatments and/or interventions,45,46 as well as the clinical changes of the microorganisms.44,47 Therefore this study provides survival data on this disease based on the cases reported and studied in an epidemiological surveillance system for social security beneficiaries, and this is a rarity in the scientific literature.
The limitations of this study may be attributed to the mixed collection of information due to changes in the format of the epidemiological case study during the study period; likewise, the CSF lab results for cytological, cytochemical and microbiological testing in the analytical and post-analytical stage are heterogeneous in each hospital unit, in which this variable cannot be controlled and that is reflected in the recording of the testing in the epidemiological case study. Lastly, regarding the incomplete recording of the date, reason for hospital discharge (mainly in 2012) and comorbidities, this system is “young”, given that it was started in 2010 and is therefore in the process of maturing.
Epidemiological surveillance provides information on specific health problems so that decisions can be made and risks prevented. This work provides an overview of the disease that can guide, deepen or rectify preventive or control-related actions. It is necessary to strengthen epidemiological surveillance in terms of coverage across hospital units and timely reporting, directly involving the healthcare personnel who treat patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestThe authors declare that they have no conflict of interests.