Clozapine is an antipsychotic drug that has shown to be more effective than other antipsychotics in the treatment of schizophrenia, but its use is limited due to its side effects, particularly by the risk of causing agranulocytosis. A study was made on the variations in white cell and neutrophil counts in patients treated with Clozapine in a Long-term Psychiatric Unit.
MethodsA retrospective observational study was conducted with a sample of women of our long-term psychiatric care unit who had been treated with Clozapine. A study was made on the variations in white cell and neutrophil counts during the first 18 weeks of treatment, as well as the onset of leukopenia, neutropenia, agranulocytosis, and the influence of concomitant drugs.
Results and conclusionsThe study included 55 patients on treatment with Clozapine. The incidence rate of neutropenia was 1.82% (95% CI: 0.05–10.13). The incidence rate of leukopenia and agranulocytosis was 0%. An increase in white cell and neutrophil counts from baseline to week 3–4 was observed. Only small variations were observed after this time, but the counts remained higher than the initial values. These changes were statistically significant in the white cell count: One-way repeated ANOVA with Greenhouse–Geisser correction F (11.47, 37)=2.114 (P=.011); and in neutrophils: One-way repeated ANOVA with Greenhouse–Geisser correction F (10.3, 37)=3.312 (P=.0002), and MANOVA F (18, 37)=2.693 (P=.005), ηP2=0.567. The influence of concomitant drugs (lithium, valproic and biperiden) was not significant on the overall increase found in white cells or neutrophils (MANOVA).
La clozapina es un antipsicótico que ha demostrado mayor eficacia que el resto de antipsicóticos en el tratamiento de la esquizofrenia, pero su uso está restringido por sus efectos secundarios, especialmente por su riesgo de agranulocitosis. Nos propusimos estudiar las variaciones en los leucocitos y neutrófilos en pacientes en tratamiento con clozapina e ingresadas en hospitalización psiquiátrica prolongada.
Material y métodosSe estudió una muestra de mujeres ingresadas en nuestra UCPP y en tratamiento con clozapina. Se estudió la variación de los recuentos de leucocitos y neutrófilos durante las primeras 18 semanas de tratamiento, la aparición de leucopenia, neutropenia y agranulocitosis, así como la influencia de los fármacos empleados de forma concomitante.
Resultados y conclusionesSe obtuvo una tasa de incidencia de neutropenia de 1,82% (IC 95%: 0,05-10,13) y ningún caso de leucopenia ni agranulocitosis (0%). En el análisis cuantitativo de leucocitos y neutrófilos durante las 18 semanas de tratamiento, se observó un aumento hasta la semana 3-4, tendiendo después a la estabilización de las cifras alcanzadas, pero manteniendo siempre cifras superiores a las de los valores iniciales. Estas diferencias resultaron estadísticamente significativas para los leucocitos en el ANOVA de medidas repetidas con la corrección de Greenhouse-Geisser F (11,47, 37)=2,114 (p=0,011), ŋ2P=0,038. También resultó significativo para los neutrófilos el ANOVA con la corrección de Greenhouse-Geisser F (10,33, 37)=3,312 (p=0,0002), y el MANOVA F (18, 37)=2,693 (p=0,005), ŋ2P=0,567). La influencia de los fármacos estudiados de forma concomitante (litio, valproico y biperideno) no resultó globalmente significativa (MANOVA) sobre el aumento hallado en los leucocitos y neutrófilos.
Clozapine is the only drug indicated specifically for the treatment of treatment-refractory schizophrenia. It has been shown to achieve a greater improvement than other antipsychotic drugs for positive, negative and cognitive symptoms. Its side effects profile means that its role as the first line antipsychotic in first-time neuroleptic treatment patients is questionable. Nevertheless, over the years major variations have been observed in different populations in the risk of Clozapine-induced agranulocytosis. The appearance of agranulocytosis seems to be a non-dose dependent side effect, and although there are different hypotheses about its physiopathology and possible associations with other trigger factors,1 conclusive data are still lacking. Although the initial studies found an incidence of agranulocytosis of from 1% to 2%,2 subsequent studies undertaken after the introduction of monitoring of leucocyte and neutrophil counts have shown a lower risk. In this respect it is more similar to other antipsychotic drugs (one study performed in Spain from 1993 to 1998 calculated an incidence of 0.2% during the first 3 years of treatment, CI 95%: 0.1–0.6).3 Given that patients with treatment-refractory schizophrenia form a large proportion of patients with prolonged psychiatric hospitalisation, and given the lack of specific data on the haematological effects of Clozapine in this population, we decided to study the variations in leucocyte and neutrophil counts in patients with these characteristics.
Material and methodsOur study has an observational, analytical, longitudinal and retrospective design, with repeated measurements of white cell and neutrophil counts during the first 18 weeks of treatment with Clozapine. A sample of women admitted to a long-term psychiatric care unit (LTPCU) of the Complejo Asistencial Benito Menni, Ciempozuelos (Madrid) was selected on 31 December 2012. They were treated with Clozapine during admission, and were selected independently of their age, clinical diagnosis, reason for commencing treatment or other variables. The exclusion criteria were: having started treatment with Clozapine prior to admission in the unit, or presenting an incomplete protocol of haematological monitoring due to an error in compliance (but not if the interruption were for a clinical reason). Patient sociodemographic data were collected during the study, including their age and psychiatric diagnoses coded according to the CIE-10.
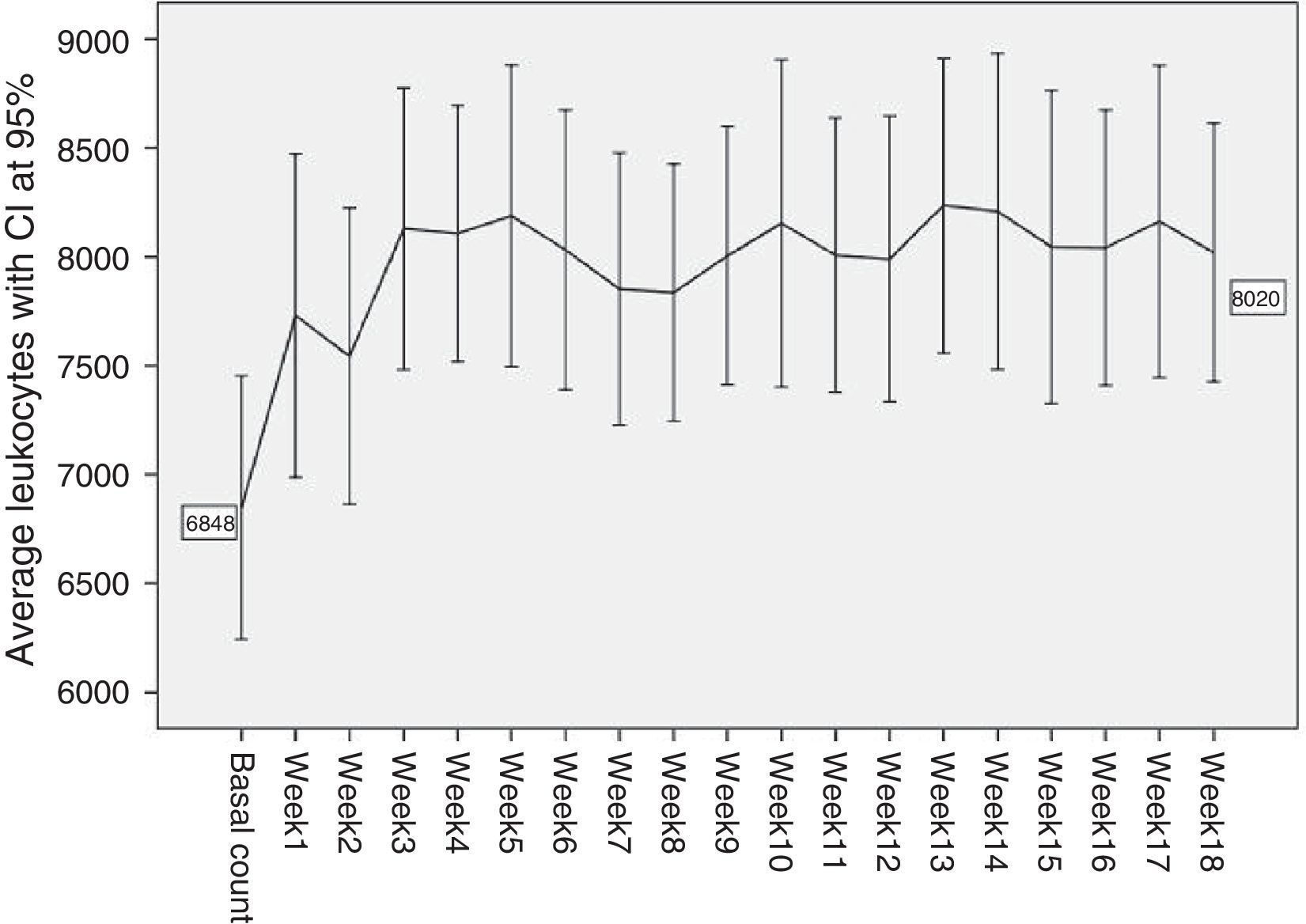
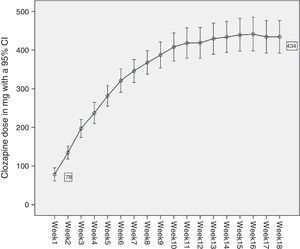
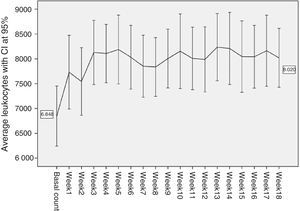
To gather data the haematological monitoring checks during follow-up were used (according to the regulations in force4). In our case data gathering was restricted to the basal count (prior to starting Clozapine treatment) and the count after the first 18 weeks of treatment. The dose of Clozapine administered each week to each patient was recorded, together with the doses of the other drugs administered to each patient concomitantly. The period of time during which these counts took place amounted to 34 years, as the oldest values correspond to a patient who started treatment in 1978, and the most recent figures date from 2012. The usual descriptive statistics were calculated for patients’ ages and the Clozapine dose administered each week. The frequencies of the different diagnoses present in the sample were calculated (Table 1 and Fig. 1). The accumulated incidences of leukopenia, neutropenia and agranulocytosis present in our study were calculated with confidence intervals of 95%. The internationally accepted cut-off points were used as the threshold at which it becomes advisable to withdraw Clozapine: leukopenia with a leucocyte count of <3×109/l, neutropenia with a neutrophil count of from .5 to 1.5×109/l, and agranulocytosis with a neutrophil count of <.5×109/l.5–7 The average counts of neutrophils and leucocytes in the different weeks were shown graphically with their corresponding confidence intervals at 95% (Figs. 2 and 3), as an initial quantitative illustration of the changes which arose in the leucocyte and neutrophil counts. Univariate ANOVA was used to check whether these changes were significant, while single factor MANOVA was used (multivariate according the Pillai trace) for repeated measurements using general lineal model (GLM) equations. The main effects at the different weeks studied were also compared 2 by 2. This was carried out for the leucocyte and neutrophil counts with the Bonferroni correction (basal count-week 1 count, basal count-week 2, etc.). The most relevant drugs in terms of their possible influence on leucocyte and neutrophil level were selected from those administered concomitantly Clozapine to our sample. This was because the bibliography suggested that they were relevant (and in our case lithium was studied8–10), or because they were often administered in the sample (valproic acid and biperiden in our case). To analyse them an intersubject multiple factor MANOVA was applied for leukocytes as well as neutrophils (exposure to Clozapine and to each one of the drugs studied), and mixed analysis was performed (Clozapine alone and Clozapine+another drug).
Diagnoses in the sample with their absolute frequencies and the percentages they represent of the total.
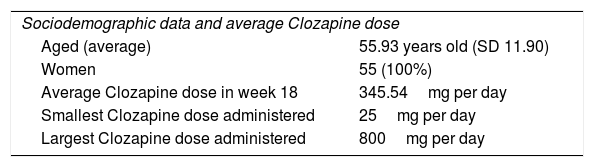
| Sociodemographic data and average Clozapine dose | |
| Aged (average) | 55.93 years old (SD 11.90) |
| Women | 55 (100%) |
| Average Clozapine dose in week 18 | 345.54mg per day |
| Smallest Clozapine dose administered | 25mg per day |
| Largest Clozapine dose administered | 800mg per day |
| Diagnoses | Patients | Percentage |
| Schizophrenia | 42 | 76.4 |
| Schizoaffective disorder | 3 | 5.4 |
| NS psychotic disorder | 4 | 7.3 |
| Obsessive-compulsive disorder | 1 | 1.8 |
| NS personality disorder | 3 | 5.5 |
| Delusional disorder | 1 | 1.8 |
| Recurring depressive disorder | 1 | 1.8 |
To make it possible to include patients whose treatment with Clozapine had been interrupted due to leukopenia or neutropenia during the 18 week follow-up in the ANOVA and MANOVA, it was decided to use the last values obtained for leukocytes and neutrophils before the withdrawal of Clozapine in the remaining weeks. In our case this was only applied in the case of a single patient who had leukopenia in week 3. For all of the tests checks were performed to ensure that they fulfilled the statistical conditions for their application. The level of statistical significance was set at the presence of a probability of random error lower than 5% (P<.05). The SPSS v. 18.0.0 and EPIDAT v. 3.0 computer programs were used for data analysis.
ResultsFifty-five women were selected from the 273 patients admitted to the LTPCU. The sociodemographic data of the sample, the doses of Clozapine administered and the psychiatric diagnoses of the patients are shown in Table 1. Fig. 1 shows a curve with the average doses of Clozapine in each of the first 18 weeks, with a CI of 95%.
During the first 18 weeks of treatment with Clozapine only one patient fulfilled the criteria for neutropenia (1445neutrofilos/mm3) without fulfilling those for leukopenia (3300leucocitos/mm3) in week 3, at a Clozapine dose of 150mg/day when it was withdrawn. The neutropenia incidence rate during the first 18 weeks was 1.82% (CI 95%: 0.05–10.13). In the case of leukopenia and agranulocytosis the incidence rate was 0% as there was no case in our sample (and for both conditions it was impossible to calculate the confidence interval). Figs. 2 and 3 show a very striking increase in leukocytes and neutrophils from the start of treatment until week 3–4, after which they tend to stabilise at the levels attained, showing smaller oscillations with several smaller increases and falls, although always at figures higher than initial values. In our sample the average basal counts were 6848leucocytes/mm3 and 4014neutrophils/mm3, with a maximum peak of leucocytes in week 13 (8235leucocitos/mm3), and the neutrophil peak in week 10 (5579neutrophils/mm3).
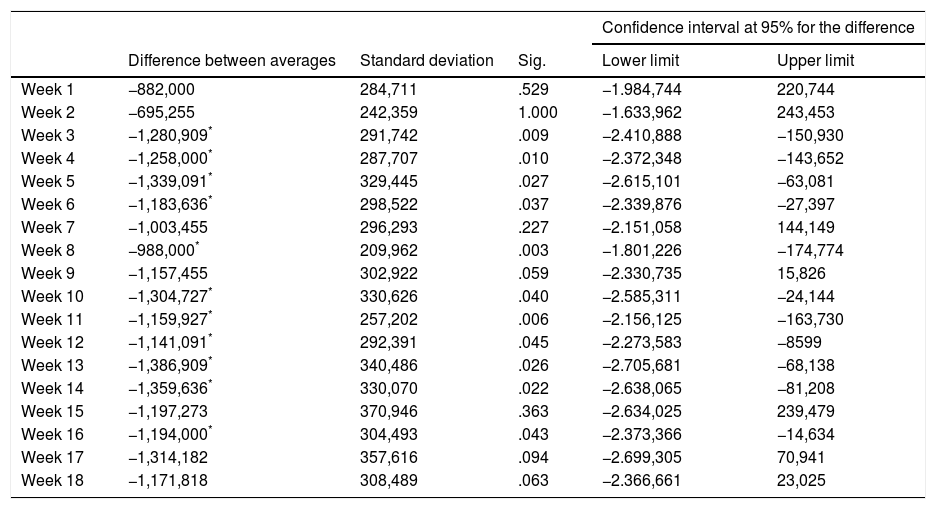
When ANOVA (univariate) was applied to the leucocyte counts during the 19 weeks of the study (the basal count and each one of the counts in the 18 subsequent weeks) the variables do not fulfil the sphericity criterion (Mauchly's W=0.007; P=.001). However, when the Greenhouse–Geisser (G–G epsilon 0.637) was applied statistically significant differences were found F (11.47, 37)=2.114 (P=.011), ηP2=0.038. No statistically significant differences were found in the MANOVA (multivariate) F (18, 37)=1.743 (P=.075), ηP2=0.459. In the comparisons between pairs of weeks the differences of the main effects were significant between the basal count and weeks 3, 4, 5, 6, 8, 10, 11, 12, 13, 14 and 16 of the treatment (but not the other weeks 1, 2, 7, 9, 15, 17 and 18) (Table 2).
Differences in the average number of leukocytes between initial counts and the first 18 weeks of treatment con Clozapine.
| Confidence interval at 95% for the difference | |||||
|---|---|---|---|---|---|
| Difference between averages | Standard deviation | Sig. | Lower limit | Upper limit | |
| Week 1 | −882,000 | 284,711 | .529 | −1.984,744 | 220,744 |
| Week 2 | −695,255 | 242,359 | 1.000 | −1.633,962 | 243,453 |
| Week 3 | −1,280,909* | 291,742 | .009 | −2.410,888 | −150,930 |
| Week 4 | −1,258,000* | 287,707 | .010 | −2.372,348 | −143,652 |
| Week 5 | −1,339,091* | 329,445 | .027 | −2.615,101 | −63,081 |
| Week 6 | −1,183,636* | 298,522 | .037 | −2.339,876 | −27,397 |
| Week 7 | −1,003,455 | 296,293 | .227 | −2.151,058 | 144,149 |
| Week 8 | −988,000* | 209,962 | .003 | −1.801,226 | −174,774 |
| Week 9 | −1,157,455 | 302,922 | .059 | −2.330,735 | 15,826 |
| Week 10 | −1,304,727* | 330,626 | .040 | −2.585,311 | −24,144 |
| Week 11 | −1,159,927* | 257,202 | .006 | −2.156,125 | −163,730 |
| Week 12 | −1,141,091* | 292,391 | .045 | −2.273,583 | −8599 |
| Week 13 | −1,386,909* | 340,486 | .026 | −2.705,681 | −68,138 |
| Week 14 | −1,359,636* | 330,070 | .022 | −2.638,065 | −81,208 |
| Week 15 | −1,197,273 | 370,946 | .363 | −2.634,025 | 239,479 |
| Week 16 | −1,194,000* | 304,493 | .043 | −2.373,366 | −14,634 |
| Week 17 | −1,314,182 | 357,616 | .094 | −2.699,305 | 70,941 |
| Week 18 | −1,171,818 | 308,489 | .063 | −2.366,661 | 23,025 |
Note: Sig. indicates the level of statistical significance.
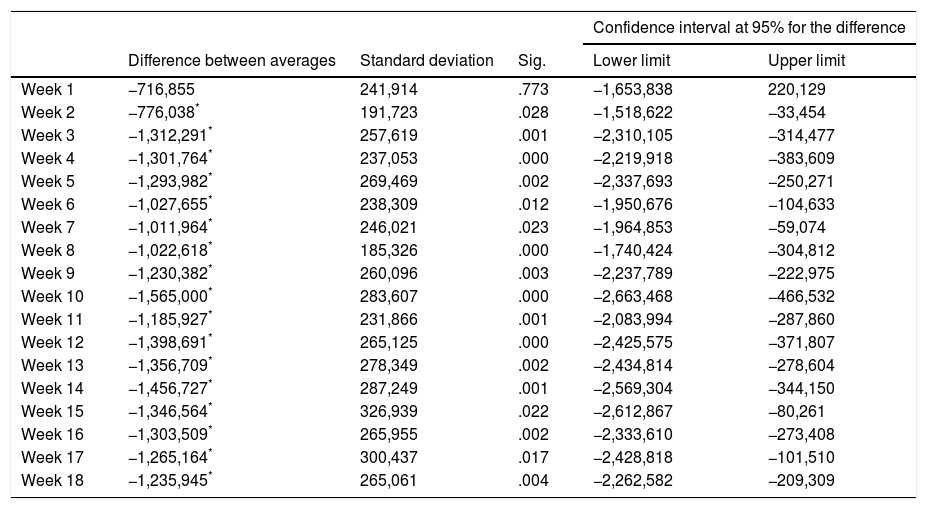
Nor did the ANOVA (univariate) for neutrophils fulfil the supposition of sphericity (Mauchly's W=.002; P<.001) and this too required the Greenhouse–Geisser (G–G epsilon .574) correction for calculation, and it was statistically significant F (10.33, 37)=3.312 (P=.0002), ηP2=.058. Statistically significant differences were also found in the MANOVA (multivariate) for neutrophils F (18, 37)=2.693 (P=.005), ηP2=.567. The differences in the main effects between the basal week and weeks 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and 18 were also significant (but not in week 1) (Table 3).
Differences between average numbers of neutrophils between the initial counts and the first 18 weeks of treatment con Clozapine.
| Confidence interval at 95% for the difference | |||||
|---|---|---|---|---|---|
| Difference between averages | Standard deviation | Sig. | Lower limit | Upper limit | |
| Week 1 | −716,855 | 241,914 | .773 | −1,653,838 | 220,129 |
| Week 2 | −776,038* | 191,723 | .028 | −1,518,622 | −33,454 |
| Week 3 | −1,312,291* | 257,619 | .001 | −2,310,105 | −314,477 |
| Week 4 | −1,301,764* | 237,053 | .000 | −2,219,918 | −383,609 |
| Week 5 | −1,293,982* | 269,469 | .002 | −2,337,693 | −250,271 |
| Week 6 | −1,027,655* | 238,309 | .012 | −1,950,676 | −104,633 |
| Week 7 | −1,011,964* | 246,021 | .023 | −1,964,853 | −59,074 |
| Week 8 | −1,022,618* | 185,326 | .000 | −1,740,424 | −304,812 |
| Week 9 | −1,230,382* | 260,096 | .003 | −2,237,789 | −222,975 |
| Week 10 | −1,565,000* | 283,607 | .000 | −2,663,468 | −466,532 |
| Week 11 | −1,185,927* | 231,866 | .001 | −2,083,994 | −287,860 |
| Week 12 | −1,398,691* | 265,125 | .000 | −2,425,575 | −371,807 |
| Week 13 | −1,356,709* | 278,349 | .002 | −2,434,814 | −278,604 |
| Week 14 | −1,456,727* | 287,249 | .001 | −2,569,304 | −344,150 |
| Week 15 | −1,346,564* | 326,939 | .022 | −2,612,867 | −80,261 |
| Week 16 | −1,303,509* | 265,955 | .002 | −2,333,610 | −273,408 |
| Week 17 | −1,265,164* | 300,437 | .017 | −2,428,818 | −101,510 |
| Week 18 | −1,235,945* | 265,061 | .004 | −2,262,582 | −209,309 |
Note: Sig. indicates the level of statistical significance.
Two patients in the sample were taking lithium, so it was ruled out as a possible cause that would influence the result of the sample. In MANOVA (18 weeks of exposure to Clozapine×presence or not of lithium associated with the treatment) for leucocytes F (17, 37)=1.393 (P=.195), and for neutrophils F (17, 37)=1.025 (P=.456). Valproic acid was administered to 26 patients. The MANOVA (18 weeks of treatment×the presence or not of valproic acid associated with Clozapine) was not statistically significant either, in the case of the leucocyte counts F (17, 37)=.717 (P=.766), and for the neutrophils F (17, 37)=.657 (P=.822). Biperiden was administered to 18 patients. The MANOVA (18 weeks of treatment×the presence or not of biperiden associated with Clozapine) was not statistically significant either, neither for the leucocyte counts F (17, 37)=.631 (P=.845), nor for neutrophils F (17, 37)=.1004 (P=.476).
DiscussionAs was pointed out in the above section, in our study an increase in the average counts of leukocytes and neutrophils occurred from the start of treatment with Clozapine. This increase was very pronounced at first, and it stabilised after week 3–4. Levels then remained at values higher than the initial ones until the end of follow-up (week 18), with a maximum peak in week 13 for leukocytes and week 10 for neutrophils. The influence of the drugs administered concomitantly (lithium, valproic acid and biperiden) was not significant.
A recent study with characteristics similar to those of this work11 also found higher levels of leukocytes and neutrophils than basal levels during the first 5 weeks of treatment (with a maximum peak in week 2). However, the levels then fell gradually and more slowly to under basal levels, reaching a minimum level in week 18. In spite of the similarities in the design of both studies, there are also major differences between them that may explain these results (Table 4).
Summary of main characteristics.
| Pons et al. (2012)8 | This study | |
|---|---|---|
| Sample size | 271 patients | 55 patients |
| Sex | Men (63.5%) and women (36.5%) | Only women |
| Age | 32.3-years-old (SD 10.5) | 55.93-years-old (SD 11.90) |
| Average dose of Clozapine over 18 weeks | 227.6mg per day | 345.54mg per day |
| Plasmatic level of Clozapine and norclozapine | In a sub-group of 112 patients (41.33% of the total sample): - Men: 274.7ng/ml and 166.3ng/ml respectively (with an average dose of 256mg per day) - Women: 239.1ng/ml and 138.2ng/ml, respectively (with an average dose of 207.6mg per day) | Not tested |
| Form of treatment | Out-patient | Long-term psychiatric hospitalisation unit |
| Time sequence of study | Prospective | Retrospective |
| Appearance of leukopenia, neutropenia and agranulocytosis during the first 18 weeks of treatment | 3 cases of leukopenia (1.3%), 7 cases of neutropenia (3.0%) and none of agranulocytosis | One case of neutropenia (1.82%) and none of agranulocytosis or leukopenia |
Note: Comparison between our study and another study with a similar design.
Although the general tendency for leukocytes and neutrophils to increase in our sample may be interpreted as a protective factor against leukopenia or neutropenia, it is not possible to infer this due to the lack of conclusive data on the appearance of leukopenia, neutropenia and agranulocytosis in our study. Thus the rate of incidence of neutropenia found in our study (1.82%) was similar the rates found in other previous studies (.93%,3 3.0%11), although the confidence interval for this parameter was very broad (CI 95%: .05–10.13).
Clozapine is a known cause of increased and decreased levels of leukocytes and neutrophils. Although there are different hypotheses, some authors have found that this may be due to Clozapine acting at 2 levels: acting directly by increasing the number of free radicals that stimulate the proapoptotic genes (p53, bax alpha and bik), while indirectly stimulating the liberation of cytokines (TNFa, IL-2, IL-6 and G-CSF) that would increase the expression of antiapoptotic proteins, thereby inducing the differentiation and maturing of the myelocytes. The balance between the proapoptotic and antiapoptotic factor may therefore be decisive in the final count of leukocytes and neutrophils. Genetic factors would determine the appearance of severe leukopenia and agranulocytosis in an unpredictable and non-dosage dependent way.12 Although agranulocytosis is the consequence that has been studied the most, due to its severity, several studies have found the possible appearance of leukocytosis; and in fact, its frequency seems to be higher than that of neutropenia and leukopenia.1
Previous studies have indicated that there is an increased risk of leukopenia and agranulocytosis in elderly patients (above 64-years-old), with the administration of myelosuppressor drugs and female sex,3 so that our sample would be a population at high risk of suffering leukopenia and neutropenia. Further prospective studies would be necessary that included men and women, to show whether there is an increase in leucocyte and neutrophil counts in patients with prolonged psychiatric hospitalisation, and if this is associated in any way with a higher or lower risk of leukopenia or neutropenia.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed obey the ethical norms of the responsible human experimentation committee and are according to the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they followed the protocols of their centre of work on patient data publication.
Right to privacy and informed consentThe authors declare that no patient data appear in this paper.
FinancingNo financing by any institution was required for this work.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the Complejo Asistencial Benito Menni, Ciempozuelos, and the Hermanas Hospitalarias del Sagrado Corazón de Jesús.
Please cite this article as: Capllonch A, de Pablo S, de la Torre A, Morales I. Aumento en los recuentos de leucocitos y neutrófilos durante las primeras 18 semanas de tratamiento con clozapina en pacientes ingresadas en una unidad de cuidados psiquiátricos prolongados. Rev Psiquiatr Salud Ment (Barc.). 2018;11:94–100.