To study the correlation between radiologic response observed by late enhancement sequences in MRI and pathologic response after neoadjuvant chemotherapy in patients with breast cancer.
Material and methodsRetrospective observational study of 132 patients with 136 tumors (4 with bilateral disease), treated consecutively with neoadjuvant chemotherapy at our institution between 2011 and 2017. In all cases, we performed 3 breast MRI's, using late enhancement gadolinium sequences: the first prior to neoadjuvant chemotherapy, the second half way through treatment, and the third at the completion of therapy. Following treatment, contrast medium uptake in tumor bed was evaluated based on the Response Evaluation Criteria for Solid Tumors (RECIST).
All patients underwent conservative or radical surgery. We compared the radiologic response estimated by MRI, with the pathologic response observed in the surgical specimen, according to Miller and Payne grading system. We calculated the sensitivity, specificity, and predictive values of the test, and used the Spearman correlation coefficient to stablish correlations between the parameters analyzed.
ResultsComplete pathologic response (pCR) was observed in 58.1% (79/136). The percentage of global radio-pathologic correlation was 88.97%. MRI showed a sensitivity of 78.9%, a specificity of 79.7%, a positive-predictive value (PPV) of 73.8% and a negative-predictive value (NPV) of 84%. In patients with partial response, the Spearman correlation was positive (rho = 1, P < .001). According to surrogate subtypes of breast cancer, we observed moderate correlation for luminal tumors (rho = 0.63, P < .001) and poor correlation for non-luminal types (rho = 0,4, P < .01).
ConclusionsBreast MRI, using late enhancement sequences, accurately predicts tumor response to neoadjuvant chemotherapy, especially in cases of partial response to therapy and in luminal surrogate tumoral subtypes.
Estudiar la correlación entre la respuesta radiológica observada mediante secuencias de realce tardío en resonancia magnética y la respuesta patológica después de quimioterapia neoadyuvante en pacientes con cáncer de mama.
Material y métodosEstudio observacional retrospectivo de 132 pacientes con 136 tumores (cuatro con enfermedad bilateral), tratados consecutivamente con quimioterapia neoadyuvante en nuestra Institución entre 2011 y 2017. En todos los casos se realizaron tres resonancias magnéticas de mama, utilizando secuencias de realce tardío de gadolinio: la primera antes de la quimioterapia neoadyuvante, la segunda a mitad del tratamiento y la tercera al finalizar la terapia. Después del tratamiento, la captación media de contraste en el lecho tumoral se evaluó en función de los Criterios de Evaluación de la Respuesta para Tumores Sólidos (RECIST).
Todas las pacientes se sometieron a cirugía conservadora o radical. Comparamos la respuesta radiológica estimada por resonancia magnética, con la respuesta patológica observada en la pieza quirúrgica, valorada según clasificación de Miller y Payne. Se calcularon sensibilidad, especificidad, valores predictivos y correlacion de Spearman para establecer correlaciones entre los parametros analizados.
ResultadosSe observó respuesta patológica completa (pCR) en el 58,1% (79/136). El porcentaje de correlación radiopatológica global fue del 88,97%. La RM mostró una sensibilidad del 78,9%, una especificidad del 79,7%, un valor predictivo positivo (VPP) del 73,8% y un valor predictivo negativo (VAN) del 84%. En pacientes con respuesta parcial, la correlación de Spearman fue positiva (rho = 1, p < 0,001). De acuerdo con los tipos subrogados de cáncer de mama, observamos una correlación moderada para los tumores luminales (rho = 0,63, p < 0,001) y una correlación deficiente para los tipos no luminales (rho = 0,4, p < 0,01).
ConclusionesLa resonancia magnética de mama, utilizando secuencias de realce tardío, predice con precisión la respuesta del tumor a la quimioterapia neoadyuvante, especialmente en casos de respuesta parcial y especialmente en subtipos luminales.
Currently, neoadjuvant chemotherapy is considered a first-line treatment for specific breast cancers. Among the possible benefits of this strategy, we can highlight the following: the under-staging of the initial tumor, the achievement of a more conservative surgery, and the verification of tumor chemo-sensitivity in vivo.1,2 This approach has also been used to treat initially operable cancers, but with a high probability of early systemic spread. Moreover, evaluation of tumor response in the surgical specimen, allows a more effective estimation of risk of recurrence, especially in tumors that reach a pathologic complete response (pCR).3–5
Given that MRI is superior to both mammography and ultrasound for the evaluation of tumor size and disease extension, as well as to the response to neoadjuvant chemotherapy,6 we decided to estimate the predictive capacity of this technique. This was performed by comparing the correlation between the radiologic response observed in the pre-surgical MRI, using late enhancement sequences,7 and the pathologic response observed in the surgical specimen. Theoretically, because of the antiangiogenic activity attributed to anthracycline and taxane-based chemotherapy schemes,8 the contrast medium used in the MRI takes longer time to reach the tumor bed. This potentially results in a more reliable determination of its presence or absence in the initial tumor site, thus facilitating surgical planning after neoadjuvant chemotherapy. The goal of this study was to investigate the correlation of the radiologic tumor response to neoadjuvant chemotherapy, using late enhancement sequences in MRI, with the pathological response observed in the surgical specimen.
Material and methodsStudy population and selection of patientsWe designed a retrospective observational study, including breast cancer patients who had received neoadjuvant chemotherapy at our institution between 2011 and 2017. A total of 132 patients with 136 tumors (4 with bilateral disease of different histology) were enrolled. Patients with breast MRI performed in another center or with follow-up shorter than 7 months, were excluded from the study.
Data inclusionAn encrypted database was designed to collect clinical and pathologic data. Patients were restaged using the 8th edition of the TNM classification of the American Joint Committee on Cancer (AJCC).9
DiagnosisThe diagnosis was made using image-guided core needle biopsy (CNB) or vacuum aspiration biopsy (VAB). The histopathological report included data on histologic type and grade according to the Nottingham Prognostic Index.10 Hormonal receptor status was informed according to the Allred Scoring System.11 Immunohistochemistry was used to determine both the Ki-67 proliferation index score and the degree of Her-2/neu expression. Equivocal Her-2 cases were clarified using FISH (Fluorescence In Situ Hybridization). Tumors were reassigned to one of the currently accepted surrogate subtypes of breast cancers, equivalent to the molecular types described by Perou.12 Namely, Luminal A (positive hormonal receptors, negative Her-2/negative neu and Ki-67 < 15%), Luminal B Her-2 negative (positive hormonal receptors, Her-2/neu negative and Ki-67 ≥ 15%), Luminal B Her-2 positive (positive hormonal receptors and positive Her-2/neu), Triple negative (negative hormonal receptors and negative Her-2/neu), and pure overexpressed Her-2/neu (negative hormonal receptors and positive Her-2/neu).
Metastatic workup and chemotherapy regimensTo determine cancer spread prior to chemotherapy, all patients underwent computed tomography of the chest, abdomen, and pelvis; a bone scan; an axillary ultrasound and a diffusion-weighted MRI of the breast. Patients with suspicious lymph nodes on ultrasound had a fine needle aspiration biopsy (FNAB) performed.
The medical treatment scheme was adjusted according to surrogate cancer subtype, including anthracyclines, taxanes, carboplatin,13 and trastuzumab. Other chemotherapy regimens were also used based in ongoing clinical trials.
MRI diagnosisWe performed 3 breast MRI's: the first prior to neoadjuvant chemotherapy, the second half way through treatment, and the third at the completion of therapy.
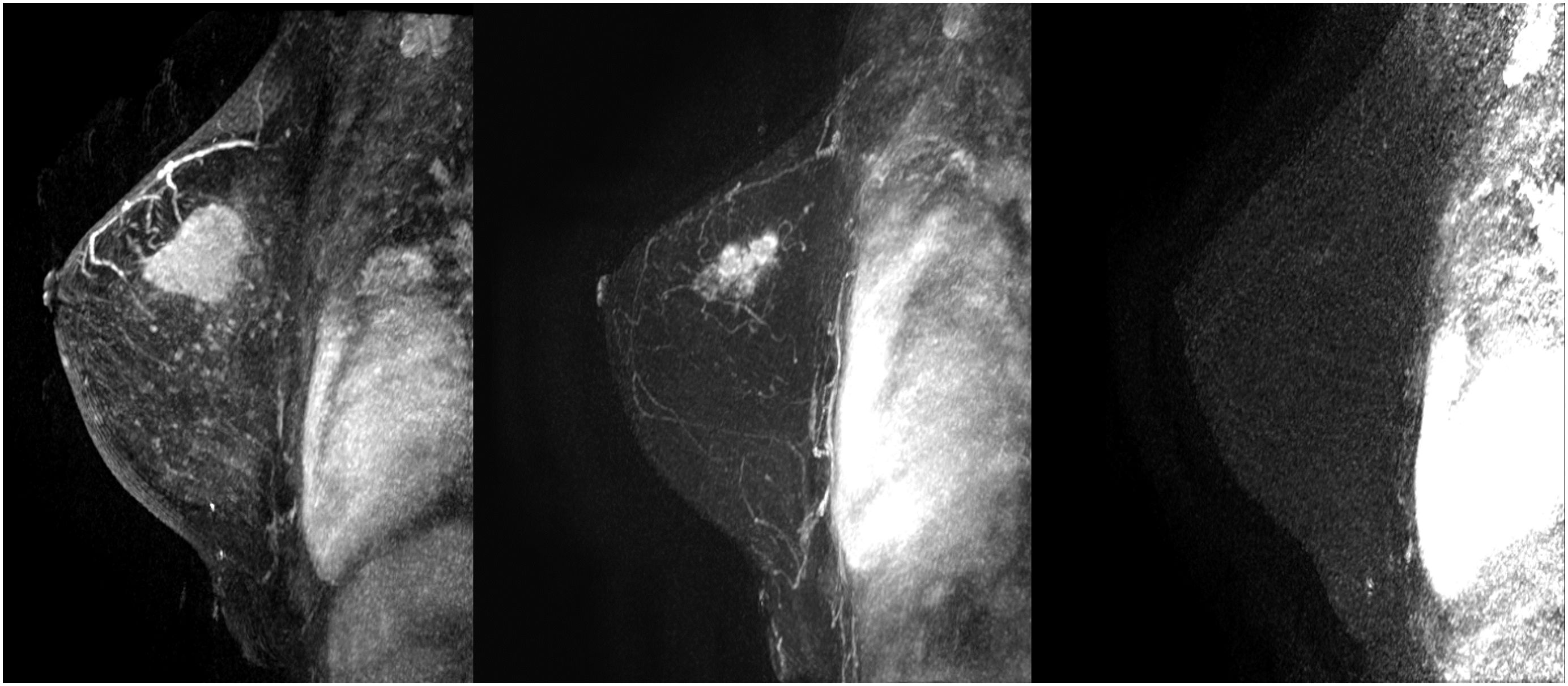
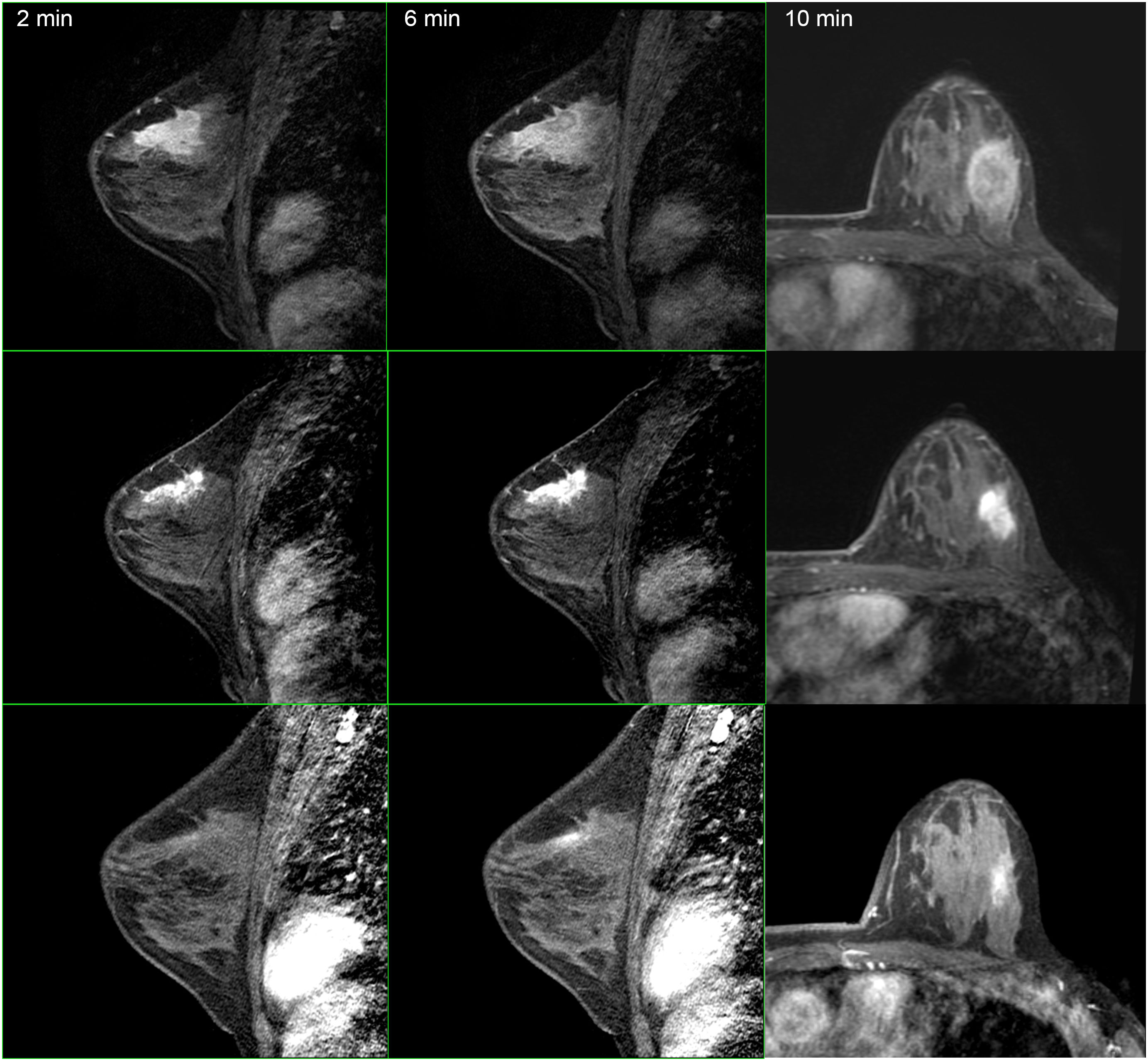
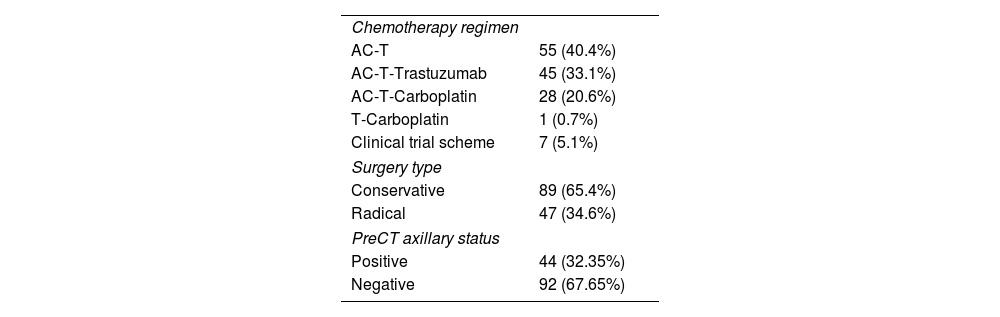
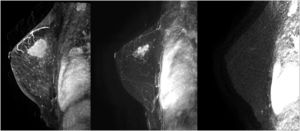
The studies were performed in decubitus prone position, using a 3.0 Tesla device (General Electric Medical Systems®), sequences enhanced in T1 and T2 in sagittal plane followed by diffusion sequence in axial plane. These were followed by the dynamic study with 3D T1-enhanced gradient echo sequences (Fast Spoiled Gradient Echo:FSPGR) with fat suppression in the bilateral sagittal plane. Images were obtained before and after intravenous injection of gadopentate dimeglumine, using an automatic injector (Magnevist®), delivering 0.1 mmol/l per kg weight, followed by a 20 ml bolus of saline solution to flush the intravenous cannula, at an injection rate of 3 ml/s. Slice thicknesses of 2.5–3 mm were used, depending on breast size, with an in-plane resolution of 0.8*0.6 mm. Temporal resolution of 60 s was achieved per 3D sequence. A total of seven 3D sequences were acquired, the first without contrast medium and the following 6 after administration of the medium. The total duration for the dynamic study was 7 min (Fig. 1). Finally, after the dynamic study, late enhancement was performed in the axial plane (3D VIBRANT) with fat suppression, achieving a resolution of 0.5*0.5 mm, acquiring a slice thickness of 2 mm for subsequent study. Examinations were interpreted by radiologists with more than 10 years of experience in breast MRI. The response was analyzed using late enhancement sequences, obtaining images at 2, 6 and 10 min. (Fig. 2). This protocol is used just when evaluating patients treated with neoadjuvant chemotherapy.
Following neoadjuvant therapy, contrast medium uptake in tumor bed was evaluated based on the Response Evaluation Criteria for Solid Tumors (RECIST),14 categorizing the response as:
Complete Radiologic Response (CRR): no uptake.
Partial Response divided in:
Major Partial Response (MAJPR): minor focal uptakes of approx. 4 mm.
Minor Partial Response (MINPR): focal uptake greater than 4 mm.
No response (NR): no response or progression.
Localization techniquePatients with clinically negative lymph nodes prior to neoadjuvant chemotherapy, had the residual tumor bed marked and the sentinel lymph node identified, 24 h prior to surgery. MRI-guided Radioguided Occult Lesion Localization/Sentinel Node Occult Lesion Localization (ROLL/SNOLL) techniques, previously described by our group,15 were used.
Breast and axillary surgeryBoth the conservative and the radical surgery were carried out by breast surgeons with over 10 years of experience. Following our clinical guidelines,16 all patients with clinically negative axilla on starting neoadjuvant chemotherapy, underwent sentinel lymph node biopsy (SLNB) always after treatment. In cases with initial axillary involvement, a standard lymphadenectomy was performed.
Pathologic responsePathologic response to treatment was determined by Miller and Payne's grading system.17 This classification includes the following categories:
G1: minimal changes without significant reduction in invasive tumor cellularity.
G2: discrete decrease in invasive cellularity, less than 30% of tumor mass.
G3: significant decrease in invasive cellularity, between 30% and 90% of the tumor mass.
G4: marked decrease in invasive cellularity, greater than 90% of tumor mass.
G5: absence of invasive cellularity.
So, this classification considers pCR only when there was absence of infiltrating disease, allowing “in situ” disease.
Treatment and follow-upAll patients treated with breast conservative surgery received post-operative radiotherapy. In addition, adjuvant hormone therapy was prescribed to all hormone-dependent tumors; as well trastuzumab for 1 year to Her-2/neu positive cancers.
Our follow-up protocol consisted in 3 months check-ups during the first year, every 6-months for the following 4 years, and annually from then after, including annual breast imaging studies.
The study was closed February 2020.
Statistical analysisPatients' characteristics were determined by a descriptive statistical study and they were classified according to the surrogate subtype of the tumor. Frequencies were expressed as absolute numbers and percentages for qualitative variables. Data corresponding to quantitative variables, were summarized as means ± standard deviation, if they followed a normal distribution; and median and interquartile range, for variables with non-normal distributions. The Kolmogorov–Smirnov test was applied to check for normality. Qualitative variables were compared using Pearson's χ2 test. The variables analyzed correspond to the clinical and pathological characteristics of the patients in the study, as well as the percentages of response obtained after neoadjuvant chemotherapy of the different surrogate subtypes. Percentage of global radio-pathologic concordance, were calculated including all patients of the study, in addition to sensitivity, specificity, positive-predictive value (PPV), and negative-predictive value (NPV). Spearman's correlation coefficient was used to analyze the correlation between residual tumor size variable, found in the surgical specimen and the one observed by MRI. Given that the variables did not follow a normal distribution, we used Spearman's rho, instead of Pearson's correlation coefficient.
P-values lower than .05 were considered as statistically significant.
Statistical analysis was performed using the software SPSS version 21 for Windows (IBM Corporation, Chicago, IL, USA).
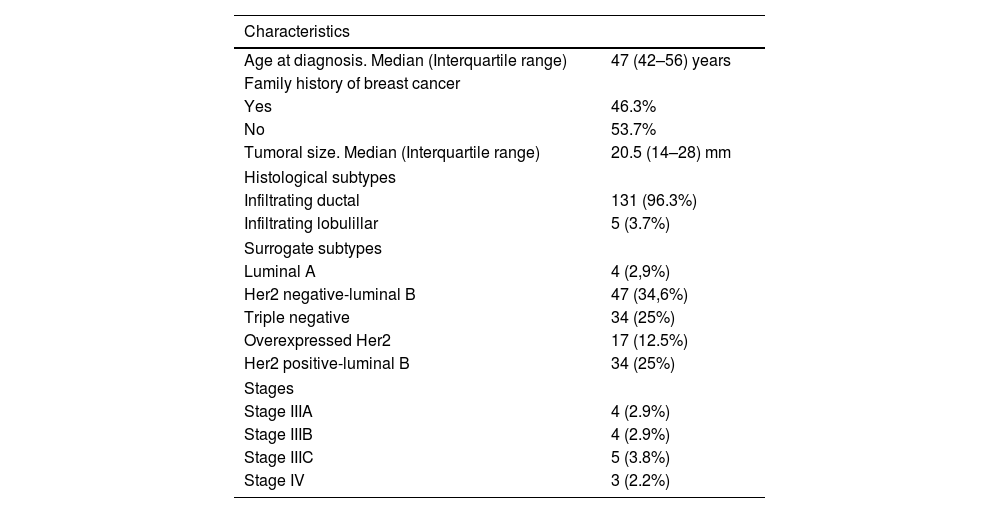
ResultsTable 1 summarizes the principal clinic-pathologic characteristics of our patients. Most tumors corresponded to early stages (88.2%). Table 2 summarizes the chemotherapy regimens used, the type of surgery performed and the pre-surgical axillary status. Finally, the majority of patients had tumors with luminal phenotypes (62.5%). The median follow-up was 50 months (37–61).
Clinical and pathological characteristics.
| Characteristics | |
|---|---|
| Age at diagnosis. Median (Interquartile range) | 47 (42–56) years |
| Family history of breast cancer | |
| Yes | 46.3% |
| No | 53.7% |
| Tumoral size. Median (Interquartile range) | 20.5 (14–28) mm |
| Histological subtypes | |
| Infiltrating ductal | 131 (96.3%) |
| Infiltrating lobulillar | 5 (3.7%) |
| Surrogate subtypes | |
| Luminal A | 4 (2,9%) |
| Her2 negative-luminal B | 47 (34,6%) |
| Triple negative | 34 (25%) |
| Overexpressed Her2 | 17 (12.5%) |
| Her2 positive-luminal B | 34 (25%) |
| Stages | |
| Stage IIIA | 4 (2.9%) |
| Stage IIIB | 4 (2.9%) |
| Stage IIIC | 5 (3.8%) |
| Stage IV | 3 (2.2%) |
CT regimen, surgery, preCT axillary status.
| Chemotherapy regimen | |
| AC-T | 55 (40.4%) |
| AC-T-Trastuzumab | 45 (33.1%) |
| AC-T-Carboplatin | 28 (20.6%) |
| T-Carboplatin | 1 (0.7%) |
| Clinical trial scheme | 7 (5.1%) |
| Surgery type | |
| Conservative | 89 (65.4%) |
| Radical | 47 (34.6%) |
| PreCT axillary status | |
| Positive | 44 (32.35%) |
| Negative | 92 (67.65%) |
CT: chemotherapy.
We observed a G5, pathologic response after neoadjuvant therapy (absence of invasive cellularity), in 58.1% of cases, and a G4 response (marked decrease in invasive cellularity, greater than 90% of tumor mass), in 20.6%; hence 78.7% of cases showed a very important response to treatment. When analyzing the G5 response in relation to surrogate marker types, we found that tumors with overexpression of pure Her-2/neu disappeared in 94.1% of cases, triple negatives in 82.4%, Her-2 positive Luminal B in 55.9%, Her-2 negative Luminal B in 31.9%, and Luminal A in 25%. The differences in the response rates obtained reached statistical significance (P < .05).
Concerning tumor bed assessment, CRR was observed in 75 tumors (55.1%), MAJPR in 51 (37.5%), MINPR in 9 (6.6%) and NR in 1 tumor (0.7%).
Considering pathologic response, we obtained the following radiologic response results: sensitivity 78.9%, specificity 79.7%, PPV 73.8%, and NPV 84%. The MRI underestimated the residual lesion in 9 cases (6.6%) and overestimated in 6 (4.4%). We found a correct radio-pathologic correlation in 88.97% of cases.
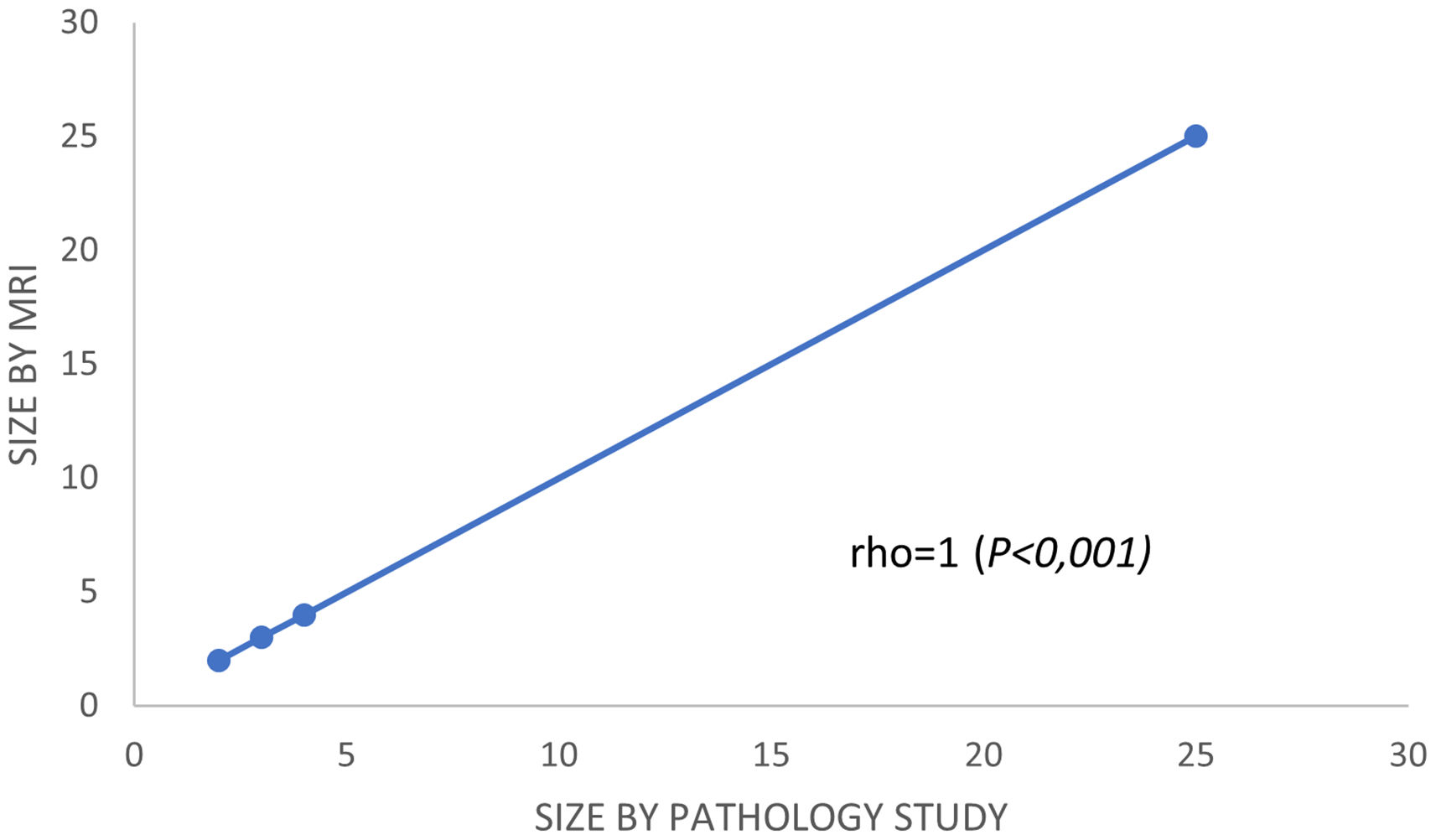
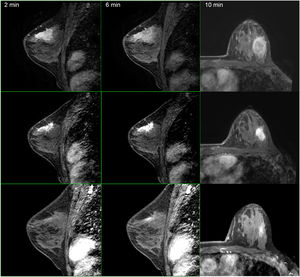
In the subgroup of patients with a partial response, Spearman's correlation coefficient was used to calculate and compare the residual tumor size observed in the pathology specimen with that observed in the post-chemotherapy MRI. Spearman's correlation was lineal and positive (rho = 1, P < .001) (Fig. 3). Analyzing it by surrogate subtypes, the coefficient reflected a moderate correlation for the luminal cancers (rho = 0.63, P <.001); and a poor correlation for the non-luminal types (rho = 0.4, P < .01).
When analyzing the 15 discordant cases, different responses were observed in the pre-surgical MRI compared to the final pathology. Ten (46.7%) corresponded to luminal tumors and the MRI overestimated the response in the majority of these cases (6). However, in 2 triple-negative tumors and in 3 pure Her-2 positive cancers, the MRI underestimated the response.
Only 3 patients had positive margins (2.2%) after conservative surgery and none required mastectomy. They all underwent a re-operation to expand margins.
DiscussionIn this study, we investigated the predictive capacity of MRI using late gadolinium enhancement MRI sequences7 and pathologic response evaluation. An excellent correlation is observed between the residual tumor size after neoadjuvant chemotherapy measured in MRI and the size measured in the surgical specimen, which allows a better estimate of the real response obtained, and, therefore, a greater precision in the surgical design. So, our results suggest that the use of late sequences in pre-surgical MRI is a useful tool to evaluate tumor response following neoadjuvant chemotherapy.
Previously, our group had performed a preliminary small size pilot study, analyzing late enhancement sequences for a cohort of breast cancer patients treated with neoadjuvant chemotherapy. In this series, we obtained a NPV of 100%.18 In the light of these promising results, in this study, we included a larger number of patients and all surrogate breast cancer subtypes.
First, a clinical feature to highlight in our study is the high pCR rate (58.1%) observed. This value is slightly higher compared to other similar studies observed in the literature, ranging between 12% and 52%.19–21 This could partly be due to the way of evaluating response that differs among the different studies. Not everyone uses Miller and Payne grading system that classifies a G5 response as an absence of infiltrating component but allows the presence of in situ carcinoma. In addition, we believe that the systematic use of targeted treatment in tumors overexpressing Her-2/neu, and the addition of carboplatin in cases of triple-negative tumors, probably contributed to reach this high pCR rate. Another factor that may have increased our percentage of pCR were our indications for neoadjuvant chemotherapy, based mainly on surrogate subtype and axillary status, resulting in more than 50% of our cases corresponding to initial stages.
Overall, the values of sensitivity, specificity, PPV, and NPV we obtained are within the ranges published in the systematic review by Lobbes et al.6
With a similar casuistic to our study, Weber et al.22 report a PPV of 63.4% and a NPV of 84.1%, although these authors consider a pCR as the absence of both an infiltrating component and in situ carcinoma. This, once again, introduces a differential factor between their study and ours, given that the definition we used for pCR, according to the Miller and Payne grading system permits the presence of in situ carcinoma.
Choi et al.23 use a software designed to estimate residual tumor volume to distinguish between the pCR group and those with partial response, with sensitivity and specificity values of 77.3 and 95%, respectively. This same group also published a good radio-pathologic correlation using the Residual Cancer Burden Index (RCB).24 Comparatively, in our study, we obtain lower values of specificity and similar sensitivity, bearing in mind that pathologic responses were evaluated using different scales.
Second, and from our perspective, the most important finding in our study was the results obtained using Spearman rho analysis for cases with incomplete pCR. These presented a lineal curve, reaching a statistically significant correlation between residual tumor sizes in the pre-surgical MRI and in the surgical specimen (Fig. 3). Therefore, in our experience and in patients with partial pathologic response, late enhancement sequences MRI accurately predicts the residual tumor size in breast cancer patients, following chemotherapy. This may be an important factor to avoid positive margins in patients undergoing conservative surgery. In fact, we only observed a 2.2% of affected margins in this series. As we can see in Fig. 2 compared to Fig. 1 (both from the same patient), the fact of obtaining late enhancement sequences allows to avoid a false assessment of complete response by image if only the dynamic study had been taken into account, visualizing gadolinium enhancement in the images obtained at 6 and 10 min.
When analyzing the RR in the different surrogate tumor subtypes, we found that the luminal subtypes, had a moderate but statistically significant correlation, which was slightly higher than that reported by other authors.25 We should also take into account that these tumor subtypes usually have a higher percentage of partial responses. Therefore, MRI with late enhancement sequences, has a greater predictive capacity in these type of breast cancers. In fact, in the radiologic assessment by MRI, luminal subtypes have a tendency to present more late enhancement phenomena.26
The main limitations of the study include its retrospective design, its small sample size and the fact that it was carried out in single center. We also found some differences in the evaluation and interpretation of the pathologic response depending on which scale you use: Miller and Payne or RCB. Hence, evaluations were more similar for tumors with pCR than in those with a partial response, probably because of the inclusion of axillary status in the RCB classification in the latter cases.27
Prospective randomized studies comparing the use of late enhancement sequences with conventional imaging acquisition protocols in MRI (Fig. 1) are needed to conclusively establish their potential usefulness; and possibly, in a near future, radiologic evaluation of malignant breast neoplasms by MRI will be implemented by machine and deep learning methods, which are already starting to be used in other imaging techniques.28
ConclusionsBreast MRI, using late enhancement sequences, accurately predicts tumor response to neoadjuvant chemotherapy, especially in cases of partial response to therapy and in luminal surrogate tumoral subtypes.
FundingNo source of funding has been received.
EthicsThe work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
As this was a retrospective study conducted in patients, informed consent was waived, according to recommendations of Institutional Review Board.