Preoperative ultrasound in patients with breast cancer without evidence of clinical axillary disease represents an attempt to reliably identify axillary lymph node metastasis. However, the usefulness of ultrasound for the detection of axillary disease should be evaluated.
Materials and methodsThe study included a retrospective cohort of 826 patients with diagnosed invasive breast cancer, treated at the National Cancer Institute of Mexico, from 2014 to 2018. All patients underwent ipsilateral axillary ultrasound for staging purposes. Besides the descriptive analysis of the preoperative ultrasound, findings of the cohort were compared with their corresponding cytology and histopathology reports.
ResultsDiagnostic index for axillary ultrasound was calculated as follows: 32.8% sensitivity, 82.5% specificity, 37.1% positive predictive value (PPV), 79.6% negative predictive value (NPV), 70.6% diagnostic accuracy, 1.86 positive likelihood ratio (LR+), and 0.81 negative likelihood ratio (LR−). Loss of fatty hilum was associated with a higher risk of axillary metastasis on the multivariate analysis (OR 3.645; 95% CI, 1.664–7.985, p<0.001).
ConclusionsThe utility of axillary ultrasound as a method of determining the nodal status prior to surgery in patients with breast cancer without clinical evidence of axillary disease was not demonstrated in this study.
La ecografía preoperatoria en pacientes con cáncer de mama sin evidencia de enfermedad clínica axilar representa un intento de identificar de manera confiable metástasis a ganglios linfáticos axilares. Sin embargo, se debe evaluar la utilidad de la ecografía para la detección de la enfermedad axilar.
Material y métodosEl estudio incluyó una cohorte retrospectiva de 826 pacientes con cáncer de mama invasivo diagnosticado en el Instituto Nacional de Cancerología de México, de 2014 a 2018. Todos los pacientes se sometieron a una ecografía axilar ipsilateral con fines de estadificación. Además del análisis descriptivo de la ecografía preoperatoria, los resultados de la cohorte se compararon con sus correspondientes informes de citología e histopatología.
ResultadosLos índices diagnósticos para la ecografía axilar fueron: 32,8% de sensibilidad, 82,5% de especificidad, 37,1% de valor predictivo positivo (VPP), 79,6% de valor predictivo negativo (VPN), 70,6% de precisión diagnóstica, 1,86 de razón de verosimilitud positiva (LR+) y 0,81 de razón de verosimilitud negativa (LR−). La pérdida de hilio graso se asoció con un mayor riesgo de metástasis axilares en el análisis multivariado (RM: 3.645; IC al 95%: 1.664-7.985; p<0,001).
ConclusionesLa utilidad de la ecografía axilar como método para determinar el estado ganglionar antes de la cirugía en pacientes con cáncer de mama sin evidencia clínica de enfermedad axilar no se demostró en este estudio.
Breast cancer has become the leading cause of cancer deaths in women, with 626,679 deaths registered in 2018 and 2 million new cases diagnosed worldwide according to the GLOBOCAN database.1
Since the risk of finding nodal metastasis in patients with non-palpable axillary disease is about 20–40%,2–4 the comprehensive assessment of the axillary is mandatory, as it is one of the most important prognostic variables in the management of primary breast cancer; therefore, axillary ultrasound has emerged as an option to identify patients with risk of nodal involvement.3,5–7 Ultrasound findings related to malignant lymph nodes include variations in echogenicity, size, shape or borders, absence of uniformity, alterations in cortical thickness, and loss of fatty hilum on grayscale evaluation. Doppler analysis of suspicious lymph nodes can show abnormalities of the vascular pattern.8
The ultrasound diagnostic accuracy for staging axillary lymph nodes in breast cancer has been published before.5–11 The sensitivity and positive predicted value (PPV) usually varies from 40–86% and 66–70%, respectively. Ultrasound specificity is about 90–93%.5–11 The sensitivity and specificity of the axillary ultrasound findings associated with axillary lymph node metastasis reported in literature widely varies and this highlights the need to improve the criteria for the preoperative axillary evaluation of patients.6
Recently, the study Z0011 from the American College of Surgeons Oncology Group (ACOSOG) revealed practice-changing results, making the axillary lymph node dissection (ALND) an unnecessary procedure in patients with clinical T1-T2 tumors treated with breast-conserving therapy having less than three axillary lymph nodes with metastasis.12 The AMAROS trial results showed radiotherapy as a preferred alternative to ALND in this subset of patients. These studies questioned the usefulness of axillary ultrasound and preoperative fine needle aspiration biopsy (FNAB) as a less invasive axillary approach,5,12 and its utility is yet to be elucidated in Mexico.
Because in our country compiled data on the diagnostic accuracy of the ultrasound for pre-surgery evaluation of the axilla is scarce, this study analyzed the utility of the ultrasound as a tool for staging patients with newly diagnosed breast cancer and clinically negative axilla. The concordance among physical examination, axillary ultrasound, and cytology and histopathology studies was evaluated as well.
Materials and methodsA total of 826 women diagnosed and treated at the National Cancer Institute in Mexico City between 2014 and 2018 met the inclusion criteria. The data were extracted from medical records. All patients were classified according to the TNM (tumor, node, and metastasis) staging system. The patients’ demographic and clinical characteristics were recorded. The diagnostic approach included clinical evaluation. In addition, to the axillary ultrasound for all the patients through Aloka ProSound Alpha 7 equipment with 38mm linear transducer and 13.3–3.61MHz of the frequency range. Axillary ultrasound findings were classified either as “suspicious” or “non-suspicious” based on the cortical thickness, fatty hilum, and shape of lymph nodes. Only patients with suspicious ultrasound findings underwent pre-surgery FNAB. After breast surgery, malignant cell clusters larger than 0.2mm in extracted lymph nodes were considered as macrometastases or pN1a disease. The histopathology results were the standard of truth. The study meets ethical consideration and was approved by the Research Committee as required by the National Cancer Institute (INCAN/CI/0622/18).
Descriptive statistics, sensitivity, specificity, PPV, NPV, FN, FP and accuracy were obtained. Chi-square tests were used to analyze the categorical data between the differences of ultrasound findings and the histopathology reports. Positive likelihood ratio and negative likelihood ratio were calculated; diagnostic OR was calculated with a 95% confidential interval (CI). A logistic regression model was performed to identify the association between suspicious sonographic characteristics and metastatic lymph nodes. The statistical significance level was set at p<0.05. Statistical analysis was performed using the SPSS software V.22.
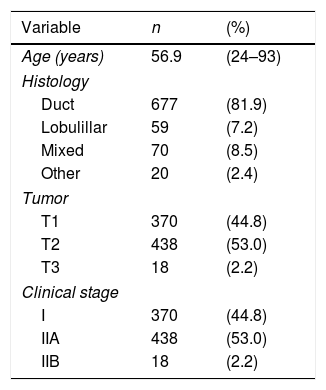
ResultsThe study population consisted of 826 women diagnosed with invasive breast carcinoma and non-palpable axillary disease, treated between 2014 and 2018. Patients who underwent neoadjuvant chemotherapy are excluded. The majority were postmenopausal women (mean age was 56.9 years). Ductal invasive breast carcinoma was the most common histology in 81.9% of the patients; 53% of the tumors were staged as T2. Demographic and tumor characteristics are shown in Table 1.
Clinical characteristics of the patients, and biological characteristics of the primary breast tumor.
| Variable | n | (%) |
|---|---|---|
| Age (years) | 56.9 | (24–93) |
| Histology | ||
| Duct | 677 | (81.9) |
| Lobulillar | 59 | (7.2) |
| Mixed | 70 | (8.5) |
| Other | 20 | (2.4) |
| Tumor | ||
| T1 | 370 | (44.8) |
| T2 | 438 | (53.0) |
| T3 | 18 | (2.2) |
| Clinical stage | ||
| I | 370 | (44.8) |
| IIA | 438 | (53.0) |
| IIB | 18 | (2.2) |
Data are presented as mean (maximum–minimum) or n (%).
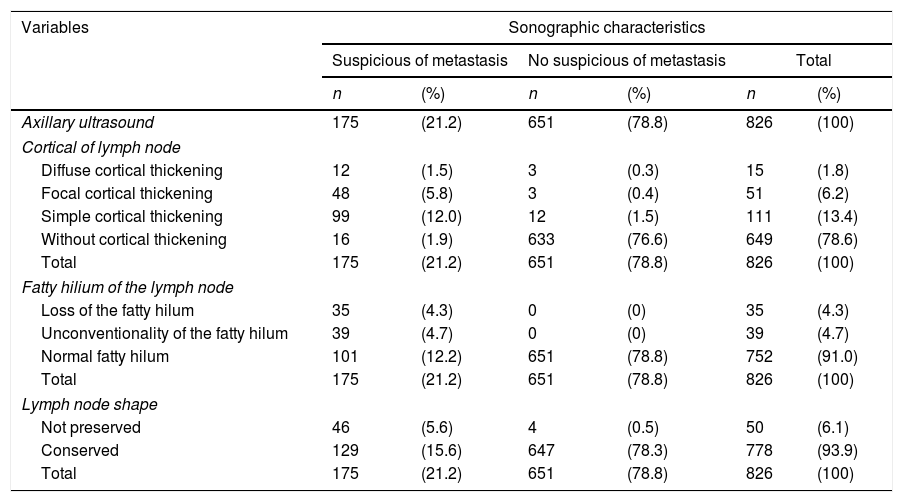
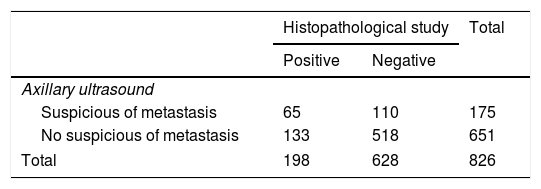
One hundred and seventy-five patients (21.2%) had suspicious lymph nodes by ultrasound (Table 2). One hundred and ninety-eight (24%) of the 826 women had breast surgery and axillary management showed metastatic lymph nodes, as it was determined by the final axillary histology (Table 3). In addition, micrometastases (<2mm) was reported in 37 cases (4.5%).
Characteristics of axillary lymph nodes observed by ultrasound.
| Variables | Sonographic characteristics | |||||
|---|---|---|---|---|---|---|
| Suspicious of metastasis | No suspicious of metastasis | Total | ||||
| n | (%) | n | (%) | n | (%) | |
| Axillary ultrasound | 175 | (21.2) | 651 | (78.8) | 826 | (100) |
| Cortical of lymph node | ||||||
| Diffuse cortical thickening | 12 | (1.5) | 3 | (0.3) | 15 | (1.8) |
| Focal cortical thickening | 48 | (5.8) | 3 | (0.4) | 51 | (6.2) |
| Simple cortical thickening | 99 | (12.0) | 12 | (1.5) | 111 | (13.4) |
| Without cortical thickening | 16 | (1.9) | 633 | (76.6) | 649 | (78.6) |
| Total | 175 | (21.2) | 651 | (78.8) | 826 | (100) |
| Fatty hilium of the lymph node | ||||||
| Loss of the fatty hilum | 35 | (4.3) | 0 | (0) | 35 | (4.3) |
| Unconventionality of the fatty hilum | 39 | (4.7) | 0 | (0) | 39 | (4.7) |
| Normal fatty hilum | 101 | (12.2) | 651 | (78.8) | 752 | (91.0) |
| Total | 175 | (21.2) | 651 | (78.8) | 826 | (100) |
| Lymph node shape | ||||||
| Not preserved | 46 | (5.6) | 4 | (0.5) | 50 | (6.1) |
| Conserved | 129 | (15.6) | 647 | (78.3) | 778 | (93.9) |
| Total | 175 | (21.2) | 651 | (78.8) | 826 | (100) |
Data are presented as n (%).
Contingency table that summarizes data of the axillary ultrasound and the histopathological study of the axillary lymph node.
| Histopathological study | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Axillary ultrasound | |||
| Suspicious of metastasis | 65 | 110 | 175 |
| No suspicious of metastasis | 133 | 518 | 651 |
| Total | 198 | 628 | 826 |
Data are presented as n.
From the 175 patients with suspicious axillary ultrasound, 65 (37.2%) patients had subsequent metastatic disease on axillary histopathology, thus corresponding to true positive cases (TP), and 110 (62.8%) patients had an absence of metastasis on histopathology, thus are false-positive cases (FP). On the other hand, of the 651 patients with non-suspicious axillary ultrasound, 133 patients (20.4%) had a positive histopathology report, thus corresponding to false-negative cases (FN), and 518 (79.6%) patients had an absence of metastasis on histopathology, true negative cases (TN).
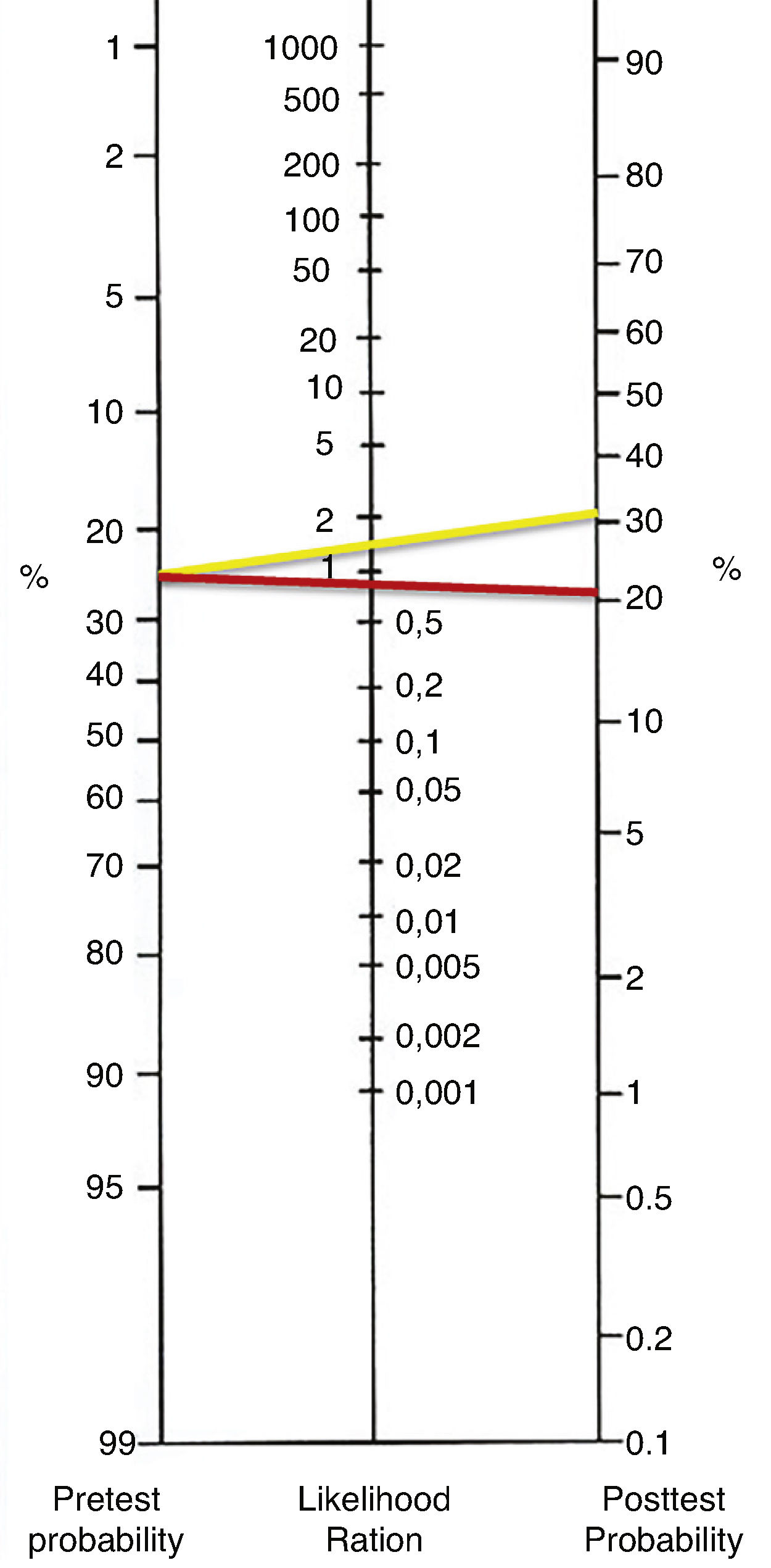
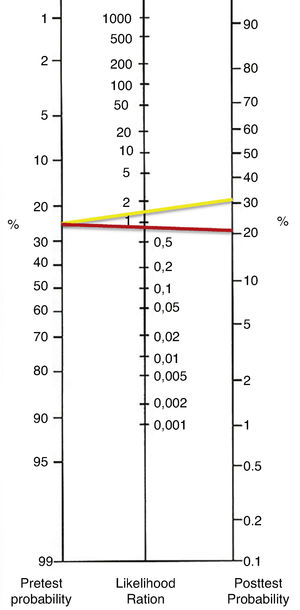
The axillary ultrasound findings were compared with their correspondent histopathology study. The positive likelihood ratio (LH+) was 1.86 and the negative likelihood ratio (LH−) was 1.23 (0.81). The diagnostic OR of the test was 2.28 with a 95% confidence interval (CI=1586–3299). According to the results of OR, patients with metastatic disease on axillary histopathology are only 2.28 times more likely to have suspicious axillary ultrasound than those without metastatic disease on axillary histopathology. The likelihood ratios were graphed in the Fagan Nomogram to obtain the post-test probability (Fig. 1).
According to review of axillary ultrasound findings, were reported 177 (21.4%) patients with cortical thickening, 74 (8.9%) patients showed abnormalities in the fatty hilum, and 50 (6.0%) patients not preserved the shape of the lymph node. A total of 62 patients (7.5%) had lymph nodes cortical thickening and abnormalities in the fatty hilum, 38 (4.6%) patients had cortical thickening and not preserved the shape of the lymph node, and 26 (3.1%) patients had abnormalities in the fatty hilum and not preserved the shape of the lymph node. Finally, 18 (2.1%) patients reported triple aberration in the axillary ultrasound report.
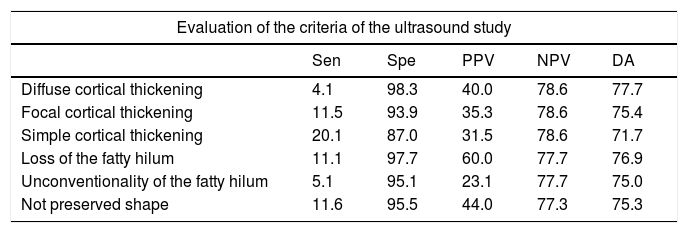
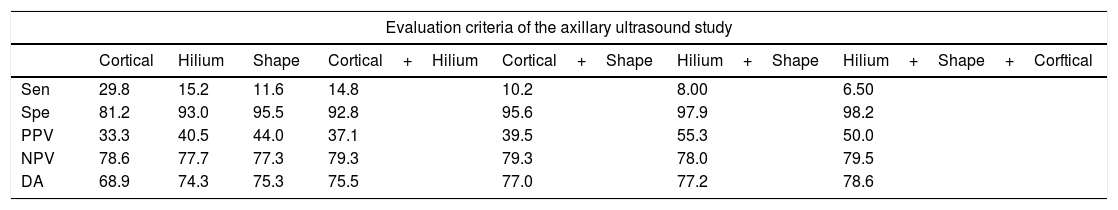
The analysis of sensitivity, specificity, Predictive Positive Value (PPV), Negative Predictive Value (NPV) and Diagnostic Accuracy (DA) of sonographic characteristics is reported in Tables 4 and 5.
Diagnostic indices of the alteration of each one of the characteristics of the axillary lymph nodes obtained by ultrasound.
| Evaluation of the criteria of the ultrasound study | |||||
|---|---|---|---|---|---|
| Sen | Spe | PPV | NPV | DA | |
| Diffuse cortical thickening | 4.1 | 98.3 | 40.0 | 78.6 | 77.7 |
| Focal cortical thickening | 11.5 | 93.9 | 35.3 | 78.6 | 75.4 |
| Simple cortical thickening | 20.1 | 87.0 | 31.5 | 78.6 | 71.7 |
| Loss of the fatty hilum | 11.1 | 97.7 | 60.0 | 77.7 | 76.9 |
| Unconventionality of the fatty hilum | 5.1 | 95.1 | 23.1 | 77.7 | 75.0 |
| Not preserved shape | 11.6 | 95.5 | 44.0 | 77.3 | 75.3 |
Data are presented as %.
Sensitivity (Sen), Specificity (Spe), Predictive Positive Value (PPV), Negative Predictive Value (NPV) and Diagnostic Accuracy (DA).
Diagnostic indices of the alteration of each one of the characteristics of the axillary lymph nodes obtained by ultrasound.
| Evaluation criteria of the axillary ultrasound study | |||||||
|---|---|---|---|---|---|---|---|
| Cortical | Hilium | Shape | Cortical+Hilium | Cortical+Shape | Hilium+Shape | Hilium+Shape+Corftical | |
| Sen | 29.8 | 15.2 | 11.6 | 14.8 | 10.2 | 8.00 | 6.50 |
| Spe | 81.2 | 93.0 | 95.5 | 92.8 | 95.6 | 97.9 | 98.2 |
| PPV | 33.3 | 40.5 | 44.0 | 37.1 | 39.5 | 55.3 | 50.0 |
| NPV | 78.6 | 77.7 | 77.3 | 79.3 | 79.3 | 78.0 | 79.5 |
| DA | 68.9 | 74.3 | 75.3 | 75.5 | 77.0 | 77.2 | 78.6 |
Data are presented as %.
Sensitivity (Sen), Specificity (Spe), Predictive Positive Value (PPV), Negative Predictive Value (NPV) and Diagnostic Accuracy (DA).
Loss of fatty hilum and focal cortical thickening can be associated with a higher risk of metastases lymph nodes: OR 5.214 with 95% CI=2.595–10.474, p<0.001 and OR 2.001 with 95% CI=1.094–3.662, p=0.024 respectively. For all other echographic criteria, the p value was not statistically significant (p>0.05).
But, in multivariate analysis of the suspicious sonographic characteristics of the axillary lymph nodes showed that only the loss of the fatty hilum could be associated with a higher risk of metastasis in lymph nodes: OR 3.645 with 95% CI=1.664–7.985 (p<0.001).
From the 175 patients with axillary ultrasound suspicious of metastasis, 156 patients underwent preoperative FNAB. The cytology results were negative in 103 patients (66.0%); the most common diagnoses in negative results were lymphoid hyperplasia, 7 (4.4%) failed procedures, and positive cytology in 46 patients (29.4%); from these, 41 had a positive histopathology. A correspondence of 30.8% was found between the axillary ultrasound and the cytology. Regarding the cytology study and the histopathology study, the correspondence was 86.9%. In addition, 6 patients who had a suspicious ultrasound and a positive FNAB for metastasis not had subsequent metastatic disease on histopathology.
In the axillary surgical approach, 669 patients underwent only sentinel lymph node biopsy (SLNB), 101 patients underwent SLNB and ALND, 19 cases due to a failed SLNB (2.4%), and 56 patients underwent immediate ALND. In our work, 198 patients (24%) had a positive histopathology report, but from these, only 33 patients had a pN2a–pN3a axillary disease.
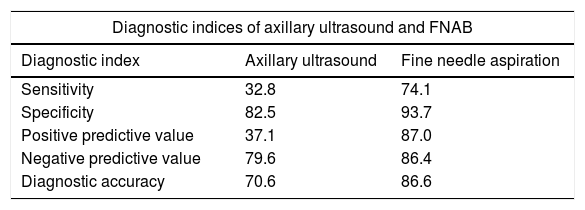
The findings obtained by ultrasound were not related to the pN2a–pN3a axillary disease (p>0.05). From the 33 patients with pN2a–pN3a disease, all cases are preserved shape of lymph node, 6 cases presented unconventionality in the fatty hilum and in 14 cases reported cortical thickening. The diagnostic indices of axillary ultrasound and the cytological study shown in Table 6.
Diagnostic indices of axillary ultrasound and fine needle aspiration biopsy for the diagnostic of axillary metastasis in patients with breast cancer.
| Diagnostic indices of axillary ultrasound and FNAB | ||
|---|---|---|
| Diagnostic index | Axillary ultrasound | Fine needle aspiration |
| Sensitivity | 32.8 | 74.1 |
| Specificity | 82.5 | 93.7 |
| Positive predictive value | 37.1 | 87.0 |
| Negative predictive value | 79.6 | 86.4 |
| Diagnostic accuracy | 70.6 | 86.6 |
Data are presented as %.
Axillary clinical examination is essential.13 However, have limitations: significant disparity of explorers, inaccurate appraisal of axillary lymph nodes, and lower sensitivity to a greater amount of adipose tissue, which results in up to 40% of false negatives in the clinical axillar evaluation.14 In our data, the prevalence of axillary lymph node metastasis was 24%, which places the National Cancer Institute of Mexico as one of the oncological centers with significant experience for axillary clinical examination, without leaving aside the need for strategies that complement it.
The diagnostic guidelines in breast cancer place the preoperative axillary ultrasound as part of the diagnostic approach. Patients with non-suspicious ultrasound benefit from SLNB, thus avoiding ALND, a surgery with high morbidity. On the other hand, axillary ultrasound guides FNAB of suspicious axillary lymph nodes toward correct staging of the disease and adjustment of the treatment. However, studies on the diagnostic accuracy and clinical utility of axillary ultrasound have presented inconsistent results in literature.15,16
Several studies have reported sensitivity for the axillary ultrasound ranges anywhere from 7.4% to 95%, and the specificity from 44% to 100%, in the diagnosis for metastasis in the axillary lymph nodes.5,9–11,15 In 2008, the sensitivity for the cohort of Cowher et al. was 18.2% and the specificity of 96.3%.17 In our study, the sensitivity was 32.8%. The diagnostic accuracy of ultrasound was not enough to perform the diagnosis of metastasis in this group of patients. In this regard, the sensitivity and PPV of axillary ultrasound will not reach 100%, but there is certainly room for improvement. Identification of morphologic determinants of axillary nodal metastasis will improve the sensitivity and PPV of axillary ultrasound, thereby increasing the certainty of the selection of surgical treatment and avoid unnecessary procedures.
Data on the specificity of axillary ultrasound is scarce due to the fact that several studies exclude patients without ultrasonographic findings of suspicion for metastasis, whereas patients with neoadjuvant chemotherapy are not excluded.7,8 In the present report, the specificity rendered a value of 82.5%.
In the present work, the positive predictive value of the axillary ultrasound was 37.1%. Consequently, this value did not allow confirming the presence of metastasis. However, the negative predictive value was 79.6%, which allowed us to consider a low probability for metastasis. In this sense, the hypothesis that SLNB could be omitted in patients with non-suspicious axillary ultrasound is important. The most recent data show that most of these patients will have low burden of tumor disease in the axillary lymph nodes in case of metastasis.18–20 The underlying reason for the diagnostic accuracy of the ultrasound (70.6%) in our data was mostly by the high negative predictive value.
False-positive cases of the axillary ultrasound were high percentage (62.8%). In addition, from patients submitted to FNAB due to a suspicious ultrasound, only 26.3% were positive for metastasis. These results show the high number of unnecessary procedures, the greater care that must be taken in the preoperative evaluation by ultrasound, and in the selection of patients who are chosen for FNAB, since all patients will be evaluated by SLNB and, if necessary, by ALND. The importance of the ultrasound findings with high sensitivity should be emphasized to bring patients with the extensive axillary disease to FNAB, selecting those who will surely require ALND and in whom SLNB could be omitted, avoiding a double surgical procedure, a greater impact on costs, and a longer hospital stay.5,21
The clinical impact of a diagnostic test not reported in the majority of the studies that evaluate axillary ultrasound. In our work, we obtained a positive likelihood ratio of 1.86 and, a negative likelihood ratio of 1.23 (0.81). Thus, the clinical utility of the ultrasound alone is insufficient for staging of the axillary lymph nodes.20,22 Besides, the value of our OR was 2.28, clinically effectiveness diagnostic tests report OR>20.19 Finally, according to the number necessary to diagnose (NND), must be done 7 axillary ultrasounds to obtain an accurate diagnosis of axillary metastasis.
Whitman et al. reported that a lymph node size greater than 2cm was associated with a higher probability of metastasis. However, in 2013, More et al. reported in a work with 110 women a greater size in axillary nodes without metastasis than in those with metastasis. The lymph node size was classified as a finding with low sensitivity for the diagnosis of metastasis in axillary lymph nodes7,23,24; probably for this reason, the size of axillary lymph nodes was not reported in the axillary ultrasound reports.
The aberrations in morphological characteristics are related to lymph node alteration.10 Several studies cite that the earliest finding in lymph nodes that could be related to metastasis is the cortical thickening with more than 2mm.7,14,25 In our work, cortical morphologic changes not were associated with axillary disease.
Nori et al. concluded that the most important morphological finding for lymph node metastasis was the loss of the fatty hilum, even with a better accuracy than cortical thickening (83.3% vs. 35%),10,24,26 finding that concordance with our study. The unconventionality of the fatty hilum was related with lymphoid hyperplasia. Loss of fatty hilum was associated with a higher risk of axillary metastasis and is the only ultrasound finding of high PPV for the diagnosis of axillary metastasis in our cohort. This indicates that replacement of the fatty hilum may be an effective indicator of axillary metastasis. However, at the time evaluate combined with more sonographic findings, the loss of the fatty hilum lowers accuracy diagnostic.
In 2006, Álvarez et al. reported, 60.9% sensitivity using size criteria compared with 43.9% using morphologic criterion, proposing that the diagnostic accuracy improves when a morphological criterion is used in addition to size.9 Houssami et al. determined that there was no difference in sensitivity using only size, morphological criteria, or both (79.6 vs. 79.6, p=0.99).27 In our work, 20 from 33 patients (60.6%) who presented the loss of the fatty hilum had axillary metastasis. In our work, two or more morphological findings did not improve the diagnostic indices.
To increase the diagnostic accuracy of the ultrasound, FNAB is used; however, operator experience remains a crucial factor, and the results could vary according to quality of the equipment and the sharpness of the images. Therefore, it is suggested that the biopsy should be taken in several identified suspicious areas, in addition to having a cytologist who approved the quality of the samples.28–30 In this study, we report a diagnostic concordance of 26.2% between the ultrasound and the cytological study, concluding that a suspicious axillary ultrasound is not a predictor of positive cytology.
Results of the cytological study should be taken into account to decrease the number of false negatives, failed procedures, or inadequate results. The use of core needle biopsy (CNB) can be considered superior to FNAB for the CNB has reported a higher sensitivity and a lower number of repeated biopsies, although a higher number of complications has been reported; morbidity can be reduced with trained human resources to perform the procedure.5,27,31
The global acceptance of the ACOSOG Z0011 study since its publication has influenced the decisions regarding the surgical treatment of the axilla. In our work, 97.8% of patients had T1-T2 lesions, with non-palpable axillary disease. The results obtained in this study make it possible to propose the following change in the axillary ultrasound approach: from using it in the diagnosis of all patients with axillary metastasis to only in the patients with a high burden of tumor disease in the axilla, because the use of FNAB as an indicator of ALND can increase its use up to 20.8%.4,5,12,32
Limitations to our retrospective study include that some ultrasonographic findings of axillary lymph nodes were not reported in clinical records. The study highlights the need to prospectively identify standard criteria to evaluate the axillary lymph nodes
ConclusionAxillary ultrasound is an accessible study. However, its utility as a method of determining the nodal status before surgery in patients with breast cancer without clinical evidence of axillary disease in the National Cancer Institute of Mexico was not demonstrated in this study due to low sensibility and clinical utility. It is recommends taking into only ultrasonographic findings of axillary lymph nodes with high PPV, in an attempt to try increase the utility of the study, and decrease unnecessary procedures.
Authors’ contributionsGerardo Cuamani Mitznahuatl and Rafael Vázquez Romo were the principal investigators and contributed to the conception and design of the study. GCM, RVR and CHFB prepared the main text, and the tables and figure with help of HIRG, GETD and MELH. JJOA and JGA were collaborated for collection of clinical data. All authors contributed to the content. All authors read and approved the final manuscript.
FundingThe authors declare that they have not received funding for this study.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to thank Mammary Tumors Department and Clinical Research Assistant of the Instituto Nacional de Cancerología of Mexico and the Escuela Superior de Medicina del Instituto Politécnico Nacional, Cuamani Mitznahuatl Gerardo is a Masters student and Conacyt fellowship from Programa de Ciencias de la Salud, Instituto Politécnico Nacional.