Triple-negative breast cancer (TNBC) is an aggressive subtype, where no effective therapies have been established for it. Searching for therapeutic targets for TNBC patients is the aim of the current research. Poly adenosine diphosphate ribose polymerase (PARP1) inhibitors are promising antitumor therapy that have a high potency in BRCA1-deficient breast cancers.
Materials and methodsForty TNBC patients who received neoadjuvant chemotherapy (NAC) were enrolled in this study, and evaluated for PARP1, BRCA1, and Androgen receptor (AR) immunohistochemical expression before and after receiving NAC. Data of patients' clinical and pathological responses to the chemotherapy were collected and finally analyzed.
ResultsThe immunohistochemical results revealed 10 cases (25%) positive for AR, while 18 cases (45%) and 22 cases (55%) expressed PARP1 at low and high levels, respectively. Twelve cases (30%) and 28 cases (70%) expressed BRCA1 at low and high levels, respectively. There was a significant difference between PARP1 expression in normal and malignant tissues (P < 0.001). Higher PARP1 expression was correlated with a better overall clinical response (OAR) and pathological complete response (pCR) (P = 0.018, 0.01 respectively). Co-expression of both PARP1 & BRCA1 was correlated with OAR and pCR. Chemotherapy decreases PARP1 protein levels in matched patient samples (P = 0.015). Positive AR expression was correlated with BRCA1 overexpression.
ConclusionPARP1 is highly expressed in TNBC with a better OAR and pCR especially in cases with high BRCA1, so it might be considered as a therapeutic target for this risky group.
El cáncer de mama triple negativo (TNBC) es un subtipo agresivo, donde no se han establecido terapias efectivas para este cáncer. La búsqueda de dianas terapéuticas para pacientes con TNBC es el objetivo de la investigación actual. Los inhibidores de la poli adenosina difosfato ribosa polimerasa (PARP1) son prometedores terapia antitumoral que tienen una alta potencia en los cánceres de mama deficientes de BRCA1.
Materiales y métodosCuarenta pacientes de TNBC que recibieron quimioterapia neoadyuvante (NAC) se inscribieron en este estudio y se evaluaron para la expresión inmunohistoquímica de PARP1, BRCA1 y receptores de andrógenos (AR) antes y después de recibir NAC. Se recogieron y finalmente se analizaron los datos de las respuestas clínicas y patológicas de los pacientes a la quimioterapia.
ResultadosLos resultados inmunohistoquímicos revelaron 10 casos (25%) positivos para AR, mientras que 18 casos (45%) y 22 casos (55%) expresaron PARP1 en niveles bajos y altos, respectivamente. Doce casos (30%) y 28 casos (70%) expresaron BRCA1 en niveles bajos y altos, respectivamente. Hubo una diferencia significativa entre la expresión de PARP1 en tejidos normales y malignos (P 0.001). Una mayor expresión de PARP1 se correlacionó con una mejor respuesta clínica global (OAR) y una respuesta patológica completa (pCR) (P = 0,018, 0,01 respectivamente). La co-expresión de ambos PARP1 y BRCA1 se correlacionó con OAR y pCR. La quimioterapia disminuye los niveles de proteína PARP1 en muestras de pacientes emparejadas (P = 0,015). La expresión positivos de AR se correlacionó con la expresión de BRCA1.
ConclusiónEl PARP1 está muy expresado en TNBC con una mejor OAR y pCR especialmente en casos con BRCA1 alto, por lo que podría ser considerado como una diana terapéutica para este grupo de riesgo.
Poly-(adenosine diphosphate (ADP)-ribose) polymerase (PARP) is a family of enzymes performing multiple cellular functions, including repair of damaged DNA. Binding of PARP1 to DNA strand breaks is essential for the resealing of DNA single-strand breaks (SSB) during base excision repair1.
Unlike PARP1, breast cancer susceptibility gene 1 (BRCA1) is a tumor suppressor gene which maintains genomic stability through repairing double-stranded DNA breaks by homologous recombination repair (HRR) pathway in which BRCA1 has a major role, also through regulation of cell cycle and apoptosis2. Germ-line mutations in BRCA1 cause mutant BRCA1 that causes genomic instability, and development of breast and ovarian cancer. Triple-negative breast cancer (TNBC) occurs in BRCA1 mutation carriers3.
Despite having higher rates of clinical response to neoadjuvant chemotherapy (NAC), TNBC is associated with a higher recurrence rate, poor prognosis and more aggressive than receptor-positive tumors. More extensive molecular analyses of TNBCs are needed to explain tumor heterogeneity and complexity of this disease and identify recent molecular biomarkers for development of new targeted therapy. Androgen receptor (AR) is one member of the steroid receptor family with considerable expression in TNBC patients with BRCA1/2 mutations with more favorable prognosis in TNBC. So, AR blockade might be a potential effective endocrine therapy for TNBC4–7.
Whenever, HRR pathway is impaired as in BRCA1-mutated cells, PARP inhibition in these tumor cells can produce accumulation of DNA damage and lead to cell death because of impaired DNA repair from both SSB excision repair and HRR8. In BRCA1-mutant TNBCs, a clinical use of PARP inhibitors as a targeted therapy could be provided1.
Material and methodsPatients and specimen selectionForty TNBC invasive duct carcinomas (No special type) were enrolled in this prospective study. Full lab investigation was done to all patients to assess their tolerability to neoadjuvant chemotherapy; ECHO was done before starting therapy, patients >60 years was offered epirubicine as alternative to doxorubicine. The diagnosis was set through clinical examination followed by mammography, ultrasonography, and eventually core biopsies were obtained at surgical oncology unit, faculty of Medicine, Zagazig University, Egypt. Institutional Review Board (IRB) of faculty of Medicine, Zagazig University confirmed the study protocol (No. 5738). The core biopsies from 40 patients were diagnosed at Pathology department then the patients received neoadjuvant chemotherapy at Medical Oncology department, Zagazig University Hospitals, Egypt. ER and PR were determined by immunohistochemistry (IHC) (SP1 and PgR636 clones, respectively; Dako, Carpinteria, CA) establishing positivity criteria in ≥1% of nuclear tumor staining. Her2/neu was considered positive if complete and intense circumferential membranous staining in >10% of tumor cells was found (according to ASCO/CAP HER2 Testing Guideline Update, 2013). Ki-67 was studied by IHC (MIB1 clone; Dako). The eligible criteria were applied on operable TNBC cases with enough available tissue, and clinical follow-up data. Patients who have other malignancies or ineligible for neoadjuvant chemotherapy were excluded. After NAC, surgical intervention was done for all patients (29 cases who had mass less than 3 cm were marked with clipping and prepared for breast conservative surgery however other (11) cases had modified radical mastectomy). Patients who did not achieve pathological CR will receive capecitabine adjuvant chemotherapy for 6 months and radiotherapy according to international guidelines. Patients who achieved pathological CR will receive radiotherapy according to international guidelines at clinical oncology and nuclear medicine department Zagazig University Hospitals.
Evaluation of response to neoadjuvant chemotherapyAll patients had received neoadjuvant chemotherapy. The used regimen at our institute was AC (Adriamycin/Cyclophosphamide), every 21 days x 4 cycles followed by paclitaxel weekly x12 weeks. The responses to NAC were evaluated both clinically and pathologically. Clinical response was assessed 3 times by ultrasonography examination: before and after neoadjuvant chemotherapy. Tumor size was measured as tumor length × width (cm2). Clinical responses were categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR was achieved by disappearance of all palpable tumor masses. PR was achieved if >50% tumor volume reduction has occurred. Tumor reduction <50% or an increase in volume up to 25% was considered as SD. An increase of >25% of tumor volume was scored as PD9. Pathological response was graded as complete pathological response (pCR) or residual disease (RD). pCR was defined as the absence of invasive tumor in the breast and axillary lymph nodes.
Immunohistochemistry procedureForty TNBC patients who received neoadjuvant chemotherapy (NAC) were enrolled in this study, and evaluated for PARP1, BRCA1, and androgen receptor (AR) immunohistochemical expression before NAC on 40 core biopsies and after receiving therapy on 32 surgical specimens of patients who had residual tissue masses. to evaluate possible changes in biomarker expression, induced by chemotherapy. Immunohistochemical staining was carried out using the polymer Envision detection system; the Dako EnVision ™ kit (Dako, Copenhagen, Denmark). Tissue sections (3–5 μm) were deparaffinized in xylene and rehydrated in graded alcohol. To block endogenous peroxidase, slides were incubated for 10 min in hydrogen peroxide 3%. Dako target antigen retrieval solution (pH 6.0) was used. Then slides were incubated with polyclonal rabbit anti-human antibody against PARP1, monoclonal mouse anti-BRCA1 antibody [MS110], and monoclonal rabbit anti-Androgen receptor antibody [EPR1535(2)] (ab6079, ab16780, ab133273, respectively; Abcam, Cambridge, UK). The reaction was visualized by incubating the sections with diaminobenzidine (DAB) for 15 min then Mayer's hematoxylin was used.
Interpretation of immunostainingPARP1nuclear expression was evaluated based on positive cells distribution. The percentage of positivity was scored as: “0” (<5%, negative), “1” (5–25%, sporadic), “2” (25–50%, focal), or “3” (>50%, diffuse). For the statistical analysis, negative and sporadic staining was combined as low staining, however focal and diffuse nuclear staining as high staining 10.
The percentage of positive cells for nuclear BRCA1 expression was scored on a scale of 0 to 4 as: 0 (< 1%), 1 (1–24%), 2 (25–49%), 3 (50–74%), and 4 (75–100%). The staining intensity was scored from 0 to 3 as: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The scores for percentages of positive cells and staining intensities were then multiplied to generate an immunoreactivity score (IS). The IS ranged from (0–12). Cutoff values for this scoring system were assigned as: high expression of BRCA1 was defined if IS of ≥4, while low expression was defined if IS of <411.
AR nuclear expression was semi-quantitatively scored using an H-score method described by Niemeier et al. An immunohistochemical score >10 was considered as positive12.
Statistical analysisContinuous variables were expressed as the mean ± SD & median (range), and the categorical variables were expressed as a number (percentage). Continuous variables were checked for normality by using Shapiro–Wilk test. Mann Whitney U test was used to compare between two groups of non-normally distributed variables. Kraskall Wallis H test was used to compare between more than two groups of non-normally distributed variables. Wilcoxon signed ranks test was used to compare between two dependent groups of non-normally distributed variables. Percent of categorical variables were compared using Pearson's Chi-square test or Fisher's exact test when was appropriate. Trend of change in distribution of relative frequencies between ordinal data were compared using Chi-square test for trend. All tests were two-sided. P-values <0.05, <0.01 were considered significant, highly significant, respectively. All statistics were performed using SPSS 22.0 for windows (SPSS Inc., Chicago, IL, USA).
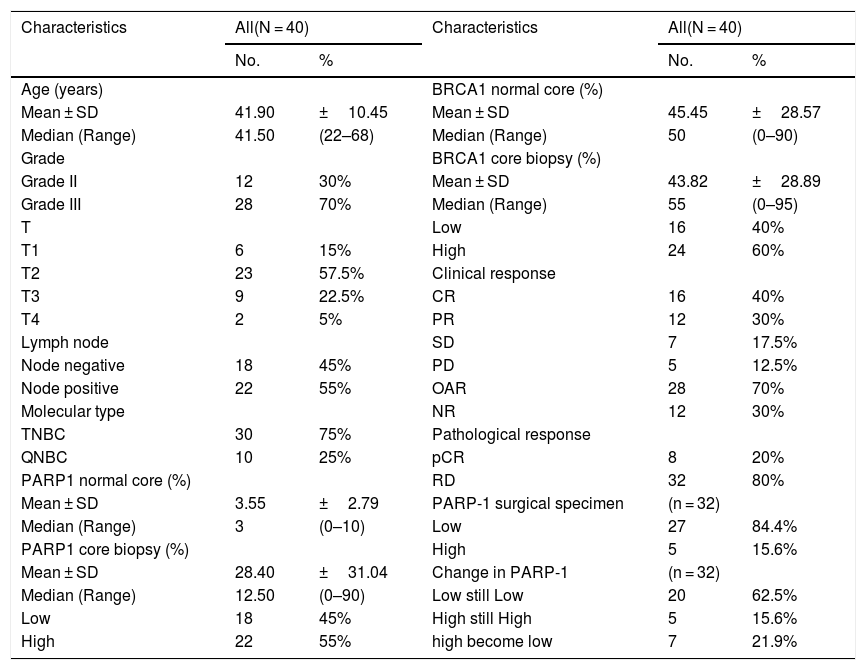
ResultsPatients' characteristicsThe median age of the patients was 41.5 years. About 70% of patients had poorly differentiated invasive duct carcinoma (NOS), according to Scarff-Bloom-Richardson modified by Elston13. Tumor staging was classified using the American Joint Committee on Cancer staging system with 57.5% of the cases were stage T2. Lymph node positive cases were 55%. Patients who achieved OAR and pCR were 70%, 20%, respectively. Table 1.
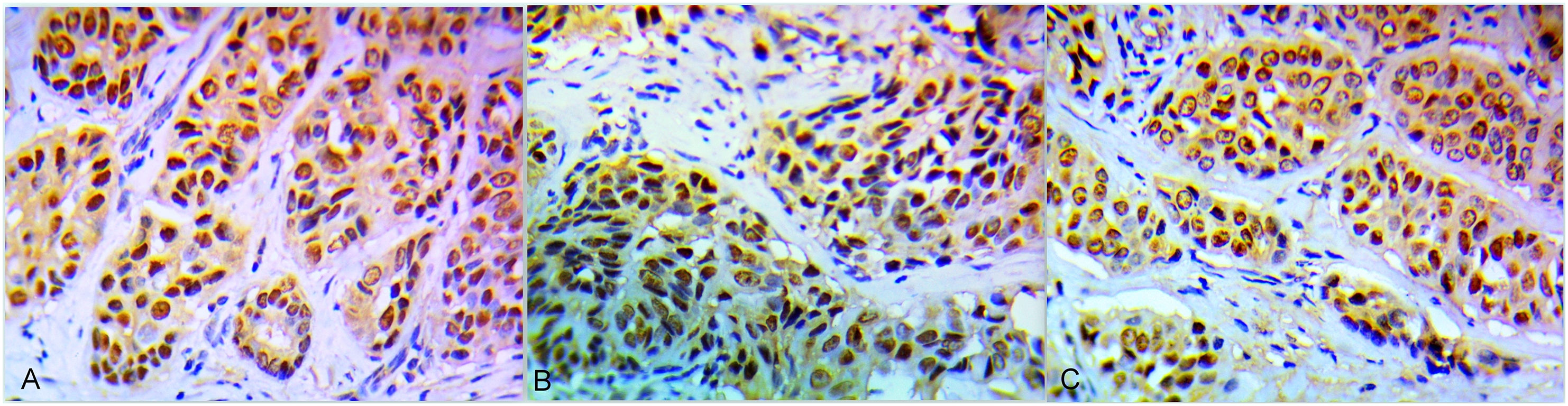
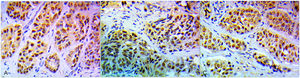
Association between PARP1, BRCA1 and AR expression in core biopsies and the clinicopathological parametersThe immunohistochemical results revealed 22 (55%) cases high PARP1 (Fig. 1A). Twenty-eight cases (70%) expressed BRCA1 high levels (Fig. 1B) and 10 cases (25%) AR positive expression (Fig. 1C). There was a significant difference between PARP1 expression in normal and malignant tissues (P < 0.001). Higher PARP1 expression was correlated with OAR and pCR (P = 0.018, 0.01, respectively). Coexpression of both PARP1 & BRCA1 was correlated with OAR and pCR. Chemotherapy decreases PARP1 protein levels in matched patient samples (P = 0.015).Tables 1, 2, 3. Positive AR expression was significantly correlated with BRCA1 expression in core biopsy (P = 0.029) Table 4.
Clinicopathological features of triple negative breast cancer patients (N = 40).
| Characteristics | All(N = 40) | Characteristics | All(N = 40) | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (years) | BRCA1 normal core (%) | ||||
| Mean ± SD | 41.90 | ±10.45 | Mean ± SD | 45.45 | ±28.57 |
| Median (Range) | 41.50 | (22–68) | Median (Range) | 50 | (0–90) |
| Grade | BRCA1 core biopsy (%) | ||||
| Grade II | 12 | 30% | Mean ± SD | 43.82 | ±28.89 |
| Grade III | 28 | 70% | Median (Range) | 55 | (0–95) |
| T | Low | 16 | 40% | ||
| T1 | 6 | 15% | High | 24 | 60% |
| T2 | 23 | 57.5% | Clinical response | ||
| T3 | 9 | 22.5% | CR | 16 | 40% |
| T4 | 2 | 5% | PR | 12 | 30% |
| Lymph node | SD | 7 | 17.5% | ||
| Node negative | 18 | 45% | PD | 5 | 12.5% |
| Node positive | 22 | 55% | OAR | 28 | 70% |
| Molecular type | NR | 12 | 30% | ||
| TNBC | 30 | 75% | Pathological response | ||
| QNBC | 10 | 25% | pCR | 8 | 20% |
| PARP1 normal core (%) | RD | 32 | 80% | ||
| Mean ± SD | 3.55 | ±2.79 | PARP-1 surgical specimen | (n = 32) | |
| Median (Range) | 3 | (0–10) | Low | 27 | 84.4% |
| PARP1 core biopsy (%) | High | 5 | 15.6% | ||
| Mean ± SD | 28.40 | ±31.04 | Change in PARP-1 | (n = 32) | |
| Median (Range) | 12.50 | (0–90) | Low still Low | 20 | 62.5% |
| Low | 18 | 45% | High still High | 5 | 15.6% |
| High | 22 | 55% | high become low | 7 | 21.9% |
Continuous variables were expressed as mean ± SD & median (range); categorical variables were expressed as number (percentage).
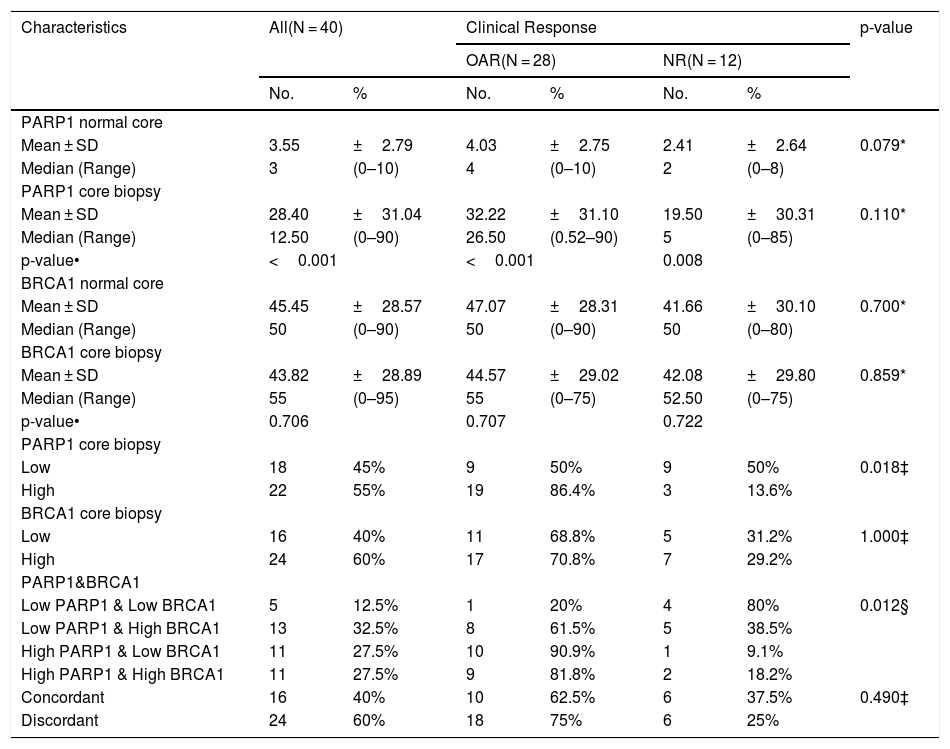
Relationship between PARP1, BRCA1 and clinical response among triple negative breast cancer patients (N = 40).
| Characteristics | All(N = 40) | Clinical Response | p-value | ||||
|---|---|---|---|---|---|---|---|
| OAR(N = 28) | NR(N = 12) | ||||||
| No. | % | No. | % | No. | % | ||
| PARP1 normal core | |||||||
| Mean ± SD | 3.55 | ±2.79 | 4.03 | ±2.75 | 2.41 | ±2.64 | 0.079* |
| Median (Range) | 3 | (0–10) | 4 | (0–10) | 2 | (0–8) | |
| PARP1 core biopsy | |||||||
| Mean ± SD | 28.40 | ±31.04 | 32.22 | ±31.10 | 19.50 | ±30.31 | 0.110* |
| Median (Range) | 12.50 | (0–90) | 26.50 | (0.52–90) | 5 | (0–85) | |
| p-value• | <0.001 | <0.001 | 0.008 | ||||
| BRCA1 normal core | |||||||
| Mean ± SD | 45.45 | ±28.57 | 47.07 | ±28.31 | 41.66 | ±30.10 | 0.700* |
| Median (Range) | 50 | (0–90) | 50 | (0–90) | 50 | (0–80) | |
| BRCA1 core biopsy | |||||||
| Mean ± SD | 43.82 | ±28.89 | 44.57 | ±29.02 | 42.08 | ±29.80 | 0.859* |
| Median (Range) | 55 | (0–95) | 55 | (0–75) | 52.50 | (0–75) | |
| p-value• | 0.706 | 0.707 | 0.722 | ||||
| PARP1 core biopsy | |||||||
| Low | 18 | 45% | 9 | 50% | 9 | 50% | 0.018‡ |
| High | 22 | 55% | 19 | 86.4% | 3 | 13.6% | |
| BRCA1 core biopsy | |||||||
| Low | 16 | 40% | 11 | 68.8% | 5 | 31.2% | 1.000‡ |
| High | 24 | 60% | 17 | 70.8% | 7 | 29.2% | |
| PARP1&BRCA1 | |||||||
| Low PARP1 & Low BRCA1 | 5 | 12.5% | 1 | 20% | 4 | 80% | 0.012§ |
| Low PARP1 & High BRCA1 | 13 | 32.5% | 8 | 61.5% | 5 | 38.5% | |
| High PARP1 & Low BRCA1 | 11 | 27.5% | 10 | 90.9% | 1 | 9.1% | |
| High PARP1 & High BRCA1 | 11 | 27.5% | 9 | 81.8% | 2 | 18.2% | |
| Concordant | 16 | 40% | 10 | 62.5% | 6 | 37.5% | 0.490‡ |
| Discordant | 24 | 60% | 18 | 75% | 6 | 25% | |
Categorical variables were expressed as number (percentage); Continuous variables were expressed as mean ± SD & median (range); *Mann Whitney U test;• Wilcoxon signed ranks test; ‡ Chi-square test; § Chi-square for trend test; p < 0.05 is significant.
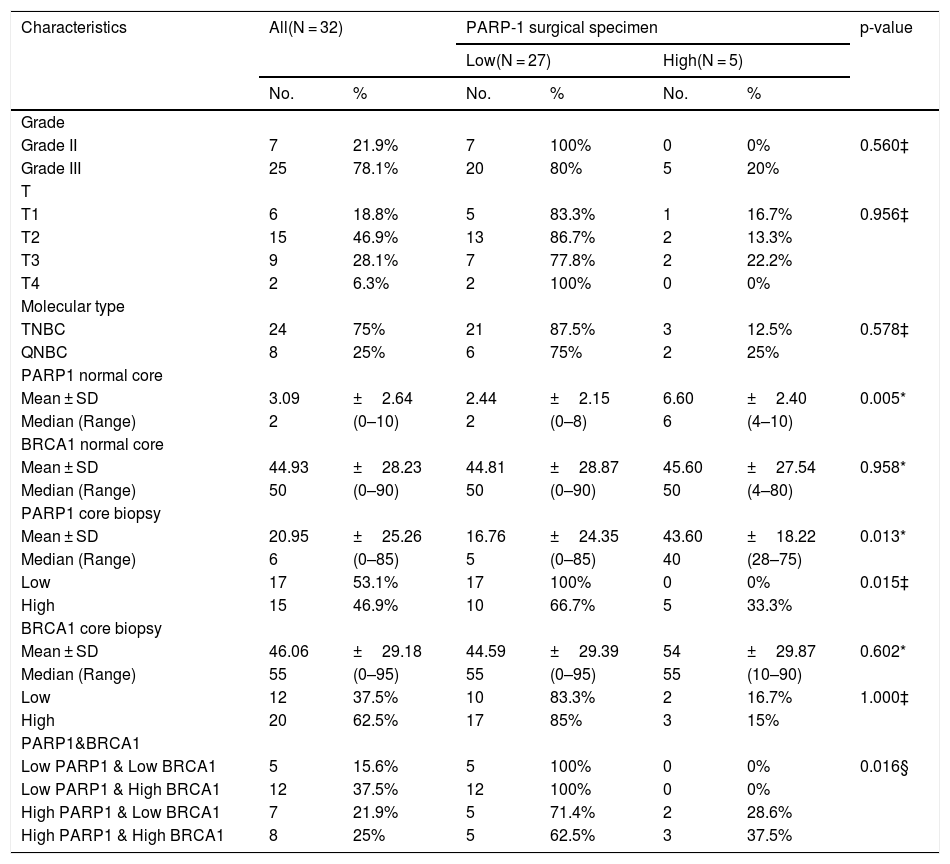
Predictors of low post-neoadjuvant PARP1 expression among triple negative breast cancer patients (N = 40).
| Characteristics | All(N = 32) | PARP-1 surgical specimen | p-value | ||||
|---|---|---|---|---|---|---|---|
| Low(N = 27) | High(N = 5) | ||||||
| No. | % | No. | % | No. | % | ||
| Grade | |||||||
| Grade II | 7 | 21.9% | 7 | 100% | 0 | 0% | 0.560‡ |
| Grade III | 25 | 78.1% | 20 | 80% | 5 | 20% | |
| T | |||||||
| T1 | 6 | 18.8% | 5 | 83.3% | 1 | 16.7% | 0.956‡ |
| T2 | 15 | 46.9% | 13 | 86.7% | 2 | 13.3% | |
| T3 | 9 | 28.1% | 7 | 77.8% | 2 | 22.2% | |
| T4 | 2 | 6.3% | 2 | 100% | 0 | 0% | |
| Molecular type | |||||||
| TNBC | 24 | 75% | 21 | 87.5% | 3 | 12.5% | 0.578‡ |
| QNBC | 8 | 25% | 6 | 75% | 2 | 25% | |
| PARP1 normal core | |||||||
| Mean ± SD | 3.09 | ±2.64 | 2.44 | ±2.15 | 6.60 | ±2.40 | 0.005* |
| Median (Range) | 2 | (0–10) | 2 | (0–8) | 6 | (4–10) | |
| BRCA1 normal core | |||||||
| Mean ± SD | 44.93 | ±28.23 | 44.81 | ±28.87 | 45.60 | ±27.54 | 0.958* |
| Median (Range) | 50 | (0–90) | 50 | (0–90) | 50 | (4–80) | |
| PARP1 core biopsy | |||||||
| Mean ± SD | 20.95 | ±25.26 | 16.76 | ±24.35 | 43.60 | ±18.22 | 0.013* |
| Median (Range) | 6 | (0–85) | 5 | (0–85) | 40 | (28–75) | |
| Low | 17 | 53.1% | 17 | 100% | 0 | 0% | 0.015‡ |
| High | 15 | 46.9% | 10 | 66.7% | 5 | 33.3% | |
| BRCA1 core biopsy | |||||||
| Mean ± SD | 46.06 | ±29.18 | 44.59 | ±29.39 | 54 | ±29.87 | 0.602* |
| Median (Range) | 55 | (0–95) | 55 | (0–95) | 55 | (10–90) | |
| Low | 12 | 37.5% | 10 | 83.3% | 2 | 16.7% | 1.000‡ |
| High | 20 | 62.5% | 17 | 85% | 3 | 15% | |
| PARP1&BRCA1 | |||||||
| Low PARP1 & Low BRCA1 | 5 | 15.6% | 5 | 100% | 0 | 0% | 0.016§ |
| Low PARP1 & High BRCA1 | 12 | 37.5% | 12 | 100% | 0 | 0% | |
| High PARP1 & Low BRCA1 | 7 | 21.9% | 5 | 71.4% | 2 | 28.6% | |
| High PARP1 & High BRCA1 | 8 | 25% | 5 | 62.5% | 3 | 37.5% | |
Categorical variables were expressed as number (percentage); Continuous variables were expressed as mean ± SD & median (range); *Mann Whitney U test; ‡ Chi-square test; § Chi-square for trend test; p < 0.05 is significant.
Comparison between AR positive and AR negative among TNBC (N = 40).
| All(N = 40) | AR negative(N = 30) | AR positive(N = 10) | p-value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age (years) | |||||||
| Mean ± SD | 41.90 | ±10.45 | 41.70 | ±11.45 | 42.50 | ±7.13 | 0.673* |
| Median (Range) | 41.50 | (22–68) | 40 | (22–68) | 44.50 | (33–53) | |
| PARP1 normal core | |||||||
| Mean ± SD | 3.55 | ±2.79 | 3.16 | ±2.61 | 4.70 | ±3.12 | 0.181* |
| Median (Range) | 3 | (0–10) | 2 | (0–10) | 5 | (0–9) | |
| BRCA1 normal core | |||||||
| Mean ± SD | 45.45 | ±28.57 | 39.96 | ±29.28 | 61.90 | ±19.36 | 0.040* |
| Median (Range) | 50 | (0–90) | 40 | (0–90) | 60 | (30–90) | |
| PARP1 core biopsy | |||||||
| Mean ± SD | 28.40 | ±31.04 | 23.17 | ±28.03 | 44.10 | ±35.76 | 0.174* |
| Median (Range) | 12.50 | (0–90) | 8 | (0–90) | 42.50 | (0–90) | |
| Low | 18 | 45% | 15 | 50% | 3 | 30% | 0.464‡ |
| High | 22 | 55% | 15 | 50% | 7 | 70% | |
| BRCA1 core biopsy | |||||||
| Mean ± SD | 43.82 | ±28.89 | 37.46 | ±26.80 | 62.80 | ±27.64 | 0.029* |
| Median (Range) | 55 | (0–95) | 52.50 | (0–75) | 65 | (4–95) | |
| PARP1&BRCA1 | |||||||
| Low PARP1 & Low BRCA1 | 5 | 12.5% | 4 | 13.3% | 1 | 10% | 0.329‡ |
| Low PARP1 & High BRCA1 | 13 | 32.5% | 11 | 36.7% | 2 | 20% | |
| High PARP1 & Low BRCA1 | 11 | 27.5% | 9 | 30% | 2 | 20% | |
| High PARP1 & High BRCA1 | 11 | 27.5% | 6 | 20% | 5 | 50% | |
| Concordant | 16 | 40% | 10 | 33.3% | 6 | 60% | 0.159‡ |
| Discordant | 24 | 60% | 20 | 66.7% | 4 | 40% | |
| PARP1 | (N = 32) | (N = 24) | (N = 8) | ||||
| Low | 27 | 84.4% | 21 | 87.5% | 6 | 75% | 0.578‡ |
| High | 5 | 15.6% | 3 | 12.5% | 2 | 25% | |
| Change in PARP1 | |||||||
| Low still Low | 20 | 62.5% | 17 | 70.8% | 3 | 37.5% | 0.240‡ |
| High still High | 5 | 15.6% | 3 | 12.5% | 2 | 15.6% | |
| low become high | 7 | 21.9% | 4 | 16.7% | 3 | 37.5% | |
Categorical variables were expressed as number (percentage); Continuous variables were expressed as mean ± SD & median (range); *Mann Whitney U test; ‡ Chi-square test; p < 0.05 is significant.
Those who had lack of ER, PR expression and absence of HER-2 amplification (TNBC) cannot benefit from hormonal therapy or anti-HER2 targeted treatment. Many researches have shown AR may be a hormonal therapy for TNBC14–16. AR expression may reach 60–90% among all types of breast cancer10,14 and reaches between 7–75% among TNBC patients14,15,17,18,19.
The role of AR expression as a prognostic biomarker in TNBC is not clear. Some studies reported it as a favorable prognostic facto19,20, others reported its association with increased mortality, and poor overall survival21,22. In this study, positive AR expression was significantly correlated with BRCA1 expression so high AR expression may be favorable prognostic marker. Activation of androgen by dihydrotestosterone (DHT) may promote cell proliferation and conversely inhibition of this receptor by bicalutamid could inhibit cell proliferation and activates cell apoptosis in AR-positive TNBC. This work revealed that 25% of TNBC cases expressed androgen and it goes with the study done by Luo et al., (2016) who found 28.8% of TNBC expressing AR23. Also, we agreed with a study reported 27.2% AR positive in 77 TNBC patients24. However, they found an association between AR expression and high grade, high KI 67, positive nodal status, and CA15-3. Along with this study, no patient died in AR positive cases, those patients received bicalutamide 50 mg once daily over 2 years as treatment duration with better 2–3 year OS which were 85% and 78% with p values of <0.001, 0.0005 respectively; bicalutamide was well-tolerated toxicity with better OS and DFS outcome24.
BRCA1 acts as a regulator for genomic stability through a major role in DNA repair. Also it is found to be a co-activator of androgen receptor and can increase the expression of AR target genes25,26. In this study positive AR expression was correlated with high BRCA1 expression (P = 0.029). This disagree with Zhang et al., 201624 who stated that overexpression of BRCA1 is significantly associated with decreased expression of AR by activation of SIRT1, a mammalian homolog of yeast Sir2 that belongs to the seven-member family of (NAD+)‑dependent III histone and protein deacetylases, suggesting that BRCA1 attenuates AR-stimulated proliferation of breast cancer cells via SIRT1 mediated pathway11.
BRCA1 and BRCA2 are frequently mutated in sporadic breast cancers. However, the extent to which the expression of these two genes modulates a significant role in sporadic cancer is still unclear24. Several studies have revealed that the promoter region of BRCA1 gene has been detected to be hypermethylated in most of the sporadic breast cancer cases. This means that the interference with the expression of the gene may lead to the abnormal expression of the protein27,28. In the present work, a tumor tissue-specific decline in the protein expression of BRCA1 than normal tissue was found but this result does not reach a significant level.
Ossovskaya et al., 201029 stated that PARP1 expression was significantly increased in several primary malignant tumors, including breast carcinomas, in this study the mean PARP1 expression in malignant core specimens was 28.40 ± 31.04 compared to 3.55 ± 2.79 in normal breast tissue cores and this goes with the study done by Ossovskaya et al., 2010 who found that PARP1 expression was increased >2-fold in more than 70% of breast duct carcinomas, compared with normal tissues.
Some previously reported data about the role for PARP1 in the survival of BRCA1/2-deficient cells, in addition to the high rates of BRCA1 mutations in TNBC; However, this study was interested in comparing the levels of PARP1 and BRCA1 expression by immunohistochemistry in TNBC and QNBC that showed better OAR in both high expression of PARP1 and BRCA1. So, the increased susceptibility of BRCA-associated malignant tumors to PARP1 inhibition has prompted growing increased attention toward the development of PARP inhibitors as a therapeutic target for the treatment of breast cancer. Interestingly, Most of TNBCs have a defect in DNA repair, identifiable by mutational signature analysis that may be targetable with PARP inhibitors30.
ConclusionPARP1 is highly expressed in TNBC with a better OAR and pCR especially in cases with high BRCA1, so it might be considered as a therapeutic target for this risky group. As Positive AR expression was correlated with BRCA1 overexpression, it can be considered as a favorable prognostic factor.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical considerationInstitutional Review Board (IRB) of the faculty of Medicine Zagazig University confirmed the study protocol (No. 5738). Written informed consent was obtained from all participants.