Communication and collaboration between radiologist and surgeon has a key role for planning oncolpastic surgery in order to choose the best option for the patient. Radiology furthermore has also a pivotal role prior to surgery itself and during follow up for early detection of recurrence.
In this paper, we provide an overview of conventional and emerging breast imaging techniques in oncoplastic surgery, considering the preoperative peri-operative and post-operative pivotal role of breast radiologists.
La comunicación y la colaboración entre el radiólogo y el cirujano tienen un papel esencial en la planificación de la cirugía oncoplástica, a fin de elegir la mejor opción para el paciente. Además, la radiología tiene también un papel fundamental previo a la cirugía y durante el periodo de seguimiento para la detección temprana de recidivas.
En este documento aportamos una visión general de las técnicas de imagen de mama convencionales y emergentes para la cirugía oncoplástica, considerando el papel esencial de los radiólogos especialistas en mama a nivel preoperatorio, perioperatorio y postoperatorio.
Breast cancer (BC) is a heterogeneous disease and the leading cause of cancer-related death among women world-wide.1 Prognosis and treatment are currently determined largely based on breast cancer stage. BC treatment involves a multidisciplinary approach and surgery is usually the primary local treatment for early-stage. Staging information will help in choosing between breast conservation and mastectomy, preoperative and postoperative chemotherapy or hormonal therapy, sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND), and radiation therapy. The surgical strategy depends on several factors, including the clinical and radiological evaluation of the stage of the disease, and patient's preferences. Communication between radiologist and surgeon is essential to select the correct treatment. Ensuring adequate surgical margins is fundamental to breast-conserving surgery (BCS) for both invasive BC and ductal carcinoma in situ (DCIS).2
With the advent of cutting-edge techniques for breast surgery, patients have a wider range of surgical options, and the role of the radiologist in breast imaging has become increasingly important over the years not only in screening and in the pre-operative setting but also in the follow-up of breast cancer survivors.3 Loco-regional recurrences and new ipsilateral or contralateral BCs during 15–20 years after the first diagnosis of breast cancers are expected with an annual rate of 1.0–1.5%.4,5 Post-operative imaging can be changeling due to post-treatment changes such as fat necrosis, surgical distortion, metallic clips, and scars and DM alone might not be sufficient.6,7 Hence, different international guidelines suggest that women with personal history of breast cancer with a more than 20% lifetime risk of a second breast cancer or with mammographically dense breast tissue may benefit from additional imaging with magnetic resonance imaging (MRI), particularly survivors.4,8,9
In this paper, we provide an overview of conventional and emerging breast imaging techniques in oncoplastic surgery, considering the preoperative peri-operative and post-operative pivotal role of breast radiologists.
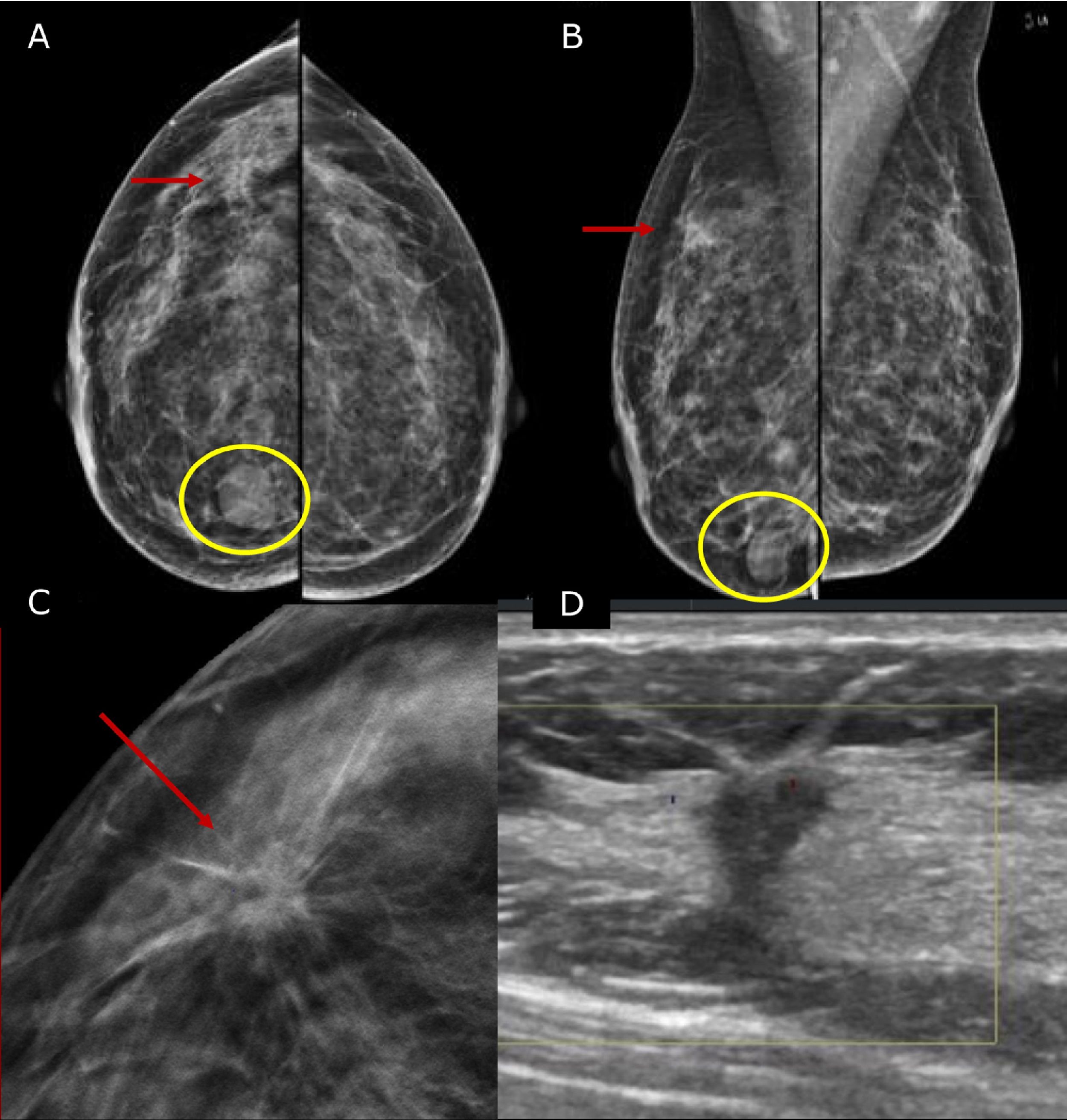
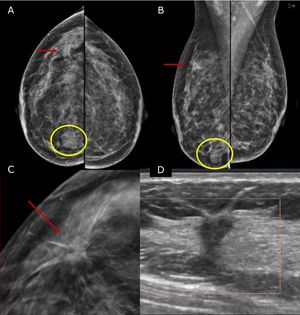
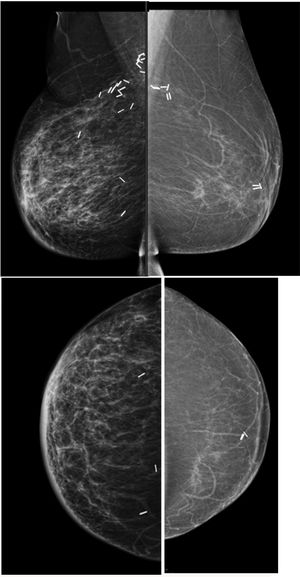
Pre-operative stagingBreast imaging with a multimodality approach is pivotal in the management of breast cancer patients and has replaced the role of clinical breast examination (CBE). CBE is not an accurate methods for assessing tumor size, in fact when lesion are palpable it tends to overestimate it.10 Hence, the role of DM and ultrasonography (US) is firmly established in preoperative staging. DM often in conjunction with specialized views – latero-medial (LM) and mediolateral (ML), extended CC, magnification, spot compression, and other view- is the first step to estimate tumor size and evaluate additional finding, such as the presence of microcalcifications.11 Digital breast tomosynthesis (DBT) overcome some of the limitation of standard two-dimensional DM by using a pseudo-three-dimensional reconstruction that allows for overcoming fibro-glandular tissue overlapping. Multiple low-dose variably angled projections of the compressed breast are obtained while the cathodic tube moves along an angular direction.12 In this setting DBT has proved to improve lesion visibility helping detect multifocal, multicentric, or contralateral disease and, improve assessment of lesion size.12–14 Several studies have shown that DBT measurement correlates well with pathologic analysis in masses measuring up to 20mm15–18 and is more accurate than DM alone, even in women with dense breasts16,19 (Fig. 1).
45 yo patient.first screening mammography. Digital Mammography in cranio caudal (A) and medio-lateral oblique (B) shows heterogeneously dense breasts with an oval shaped, well circumscribed margins, mass in the inner-inferior quadrant of the right breast (yellow circle, BI-RADS 2) and in the upper-outer quadrant of the same breast an architectural distortion which is better depicted in Digital Breast Tomosynthesis (C). The lesion had a sonographic appearance on B-mode (D) of a hypo-echoic mass, with micro-lobulated margins, posterior acoustic shadowing, and not-parallel orientation, highly suggestive for breast cancer (BI-RADS 5). The lesion was biopsied and histology revealed Invasive Ductal Cancer (ER+, PgR+, Her2neu−).
Both, DM and DBT can be used as stereotactic guidance to assess microcalcifications. However, in case microcalcifications are associated with an asymmetry or mass, further evaluation with US is needed. US is widely used not only to confirm a diagnosis of cancer but also to look for additional disease in the breast which is found in up to 20% cases, especially in the nipple/areola region and in the same quadrant of the index lesion.11 US is also the method of choice worldwide to assess lymph node involvement in BC patients. Morphologic characteristics predictive of malignancy such as cortical thickness greater than 2.5–3.0mm, focal cortical lobulation, loss of the fatty hilum, a round shape, and abnormal cortical blood flow (non-hilar flow) can be depicted in US with a specificity ranging between 88 and 98%.20–22 US can be used also for sampling, such as fine-needle aspiration (US-FNA) and core-needle biopsy (US-CNB), indispensable for confirming the presence of suspicious lesion on imaging. US-FNA is quick, well-tolerated, and associated with minimal morbidity. However, US-FNA has a moderate sensitivity compared to US-CNB that has been shown to be equally safe as US-FNA but has a higher sensitivity, reaching 94% in some cases, several institutions have abandoned US-FNA in favor of US-CNB.23,24
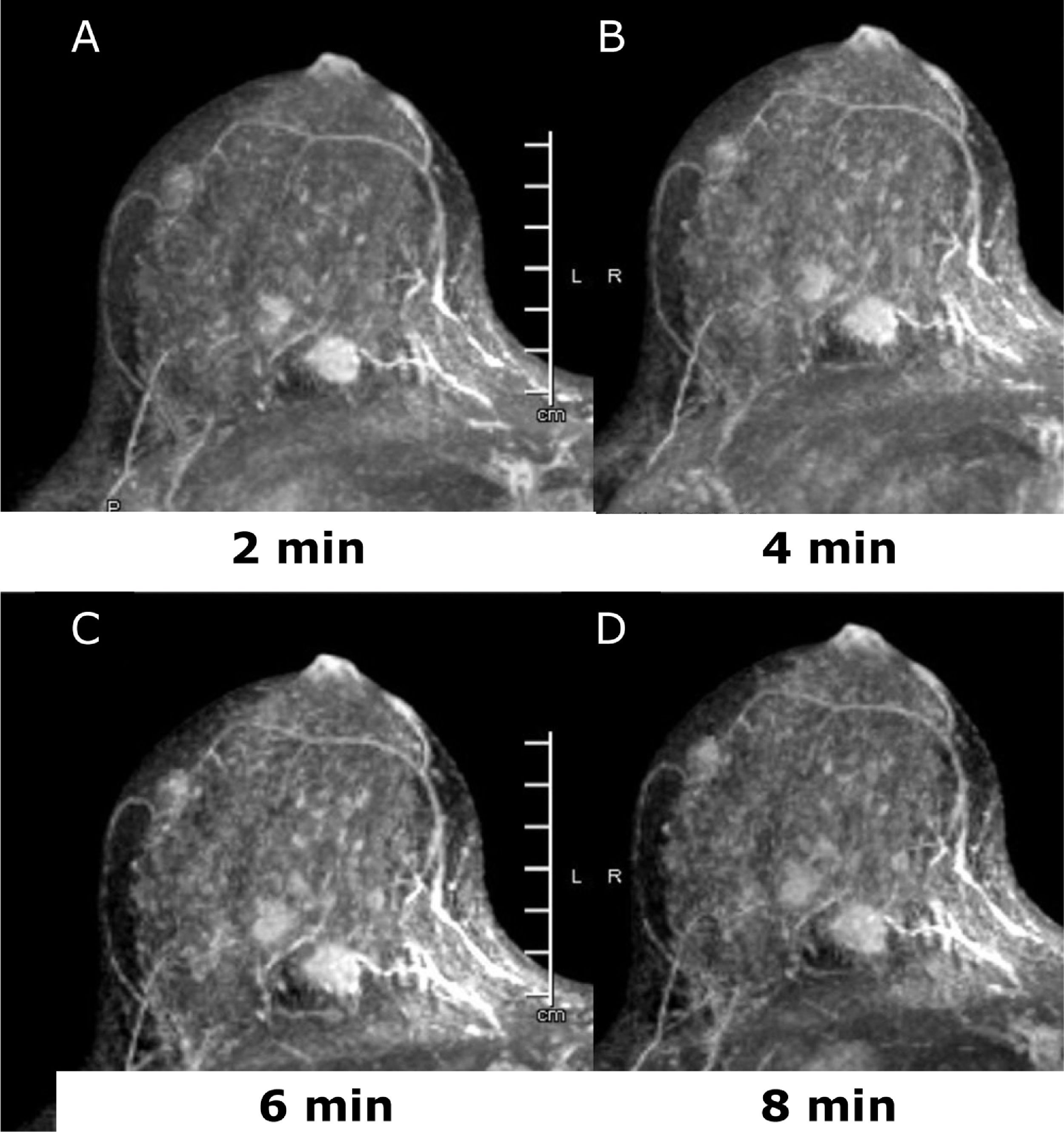
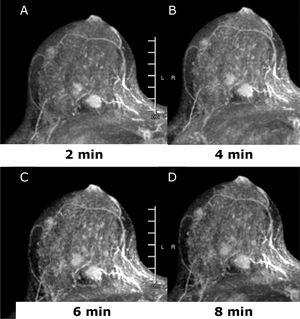
Dynamic-contrast enhanced MRI (DCE-MRI) of the breast is an established non-invasive breast imaging modality with several indications, such as pre-operative staging, the monitoring and assessment of treatment response, the differentiation of scar versus recurrence, the evaluation of breast implants, the screening of high-risk women, and for the assessment of patients with cancers of unknown primary (CUP) syndrome, and is a reliable problem-solving tool to accurately exclude malignancy.25–29 The role of preoperative MRI in newly diagnosed patients is still controversial and yet unsolved with arguments in favor30–33 and against.34,35 Main drawbacks of pre-operative breast MRI are its high cost, limited availability, and false-positive findings that can affect the surgical planning leading to additional biopsies, patient anxiety, and in some cases, unnecessary mastectomy.34–36 Nevertheless, there is a compelling body of evidence that breast MRI is the imaging tool with the highest diagnostic accuracy for the detection and staging of breast cancer both invasive and not invasive.25,28,33,37–41 Good candidates for preoperative breast MRI are women with BC at a young age; when breast cancer is occult on mammography or there is discrepancy between mammographic and sonographic size; women with hormone receptor – negative cancers, invasive lobular cancers or dense breasts; those undergoing breast conservation without radiation therapy and in those selected for neo-adjuvant chemotherapy (NAC)38,42,43 (Fig. 2). Breast DCE-MRI is the most sensitive modality currently available for identifying DCIS exceeding mammography in assessing the extent of DCIS (92% vs 56%) and is particularly sensitive for identifying high-grade and intermediate-grade DCIS.33,44
45 yo patient. (A–D) Dynamic contrast enhanced MRI at 2min (A), 4min (B), 6min (C) and 8min (D) after administration of gadolinium of the right breast. There are at least three oval shaped masses, with irregular margins and homogeneous contrast uptake with fast intake in the initial phase and wash-out in the delayed phase (BI-RADS 5). The lesions were histologically proven multifocal Invasive Lobular Cancer with small foci of lobular carcinoma in situ. Of note, only one lesion was identified at conventional imaging, mammography, and ultrasound (not shown).
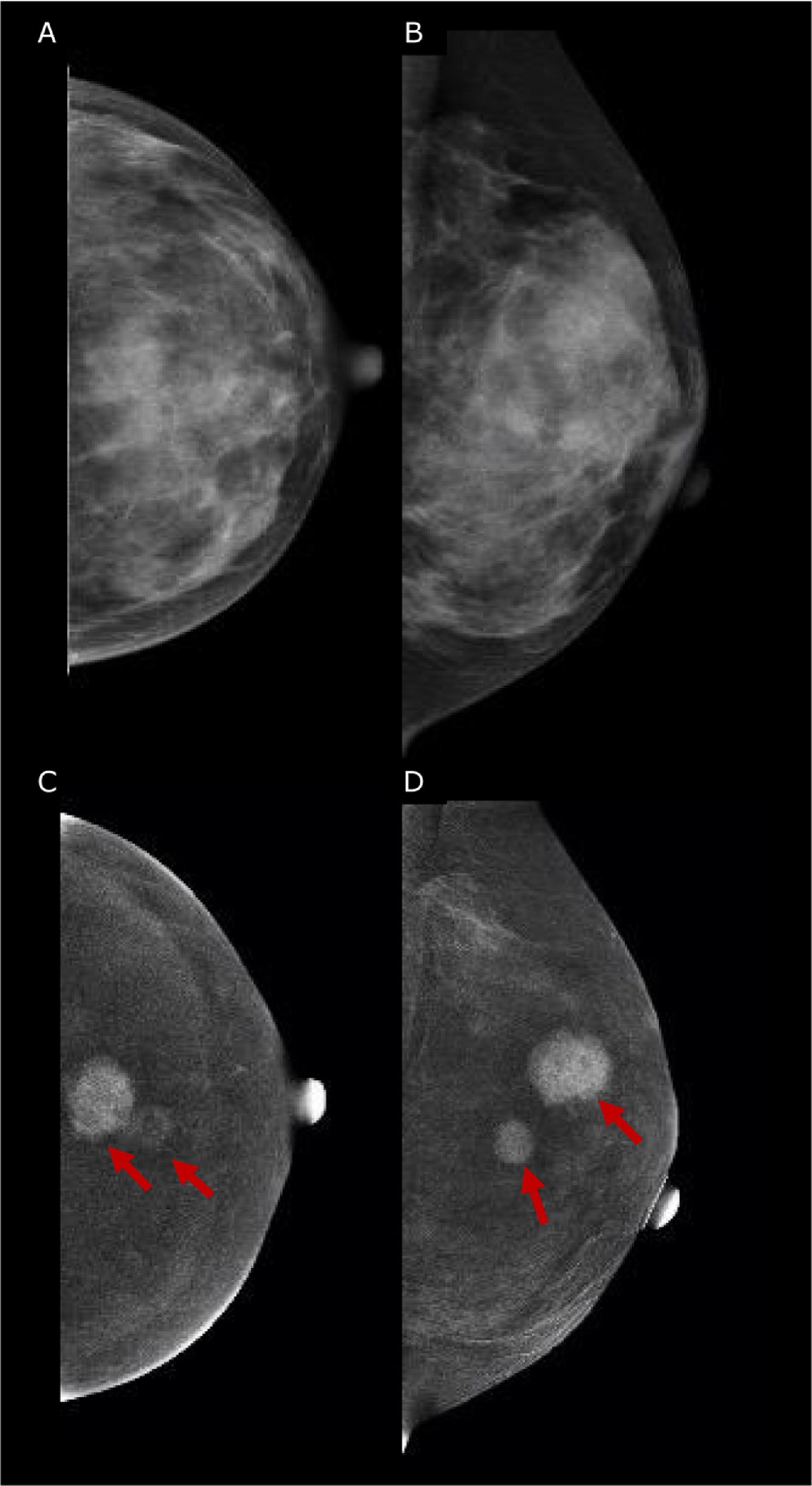
Similar to DCE-MRI, Contrast-Enhanced Mammography (CEM) provides functional information on neoplastic neo-angiogenesis, thereby achieving improved sensitivity compared to mammography, maintained in dense breasts, and providing staging information that is at least equivalent to DCE-MRI.45–47 Emerging evidences have shown promising results for CEM in the preoperative setting.45 CEM has also shown high accuracy both in assessing the size of the target lesion (with a difference ranging between 0.03mm and 5mm compared to the gross specimen46,48,49) and in identifying additional lesions, in multifocal and multi-centric disease50–52 (Fig. 3). Hence, current European guidelines officially recognized CEM as an alternative imaging tool to MRI for pre-operative surgery.53 The main limitation of the CEM is the field of view which, compared to MRI, cannot allow the assessment of the axilla.
45 yo patient with histologically proven multifocal IDC of the Left Breast. (A, B) Low-energy digital mammography of the left breast, cranio-caudal (A) and medio-lateral oblique projections, shows dense breasts (ACR d): no suspicious mass or microcalcifications are detected. (C, D) Contrast-enhanced mammography in cranio-caudal (C) and medio-lateral oblique of the left breast shows two enhancing lesions in central-superior quadrants, demonstrating multifocal disease. The lesions were biopsied, and the histopathology proved Invasive Ductal Cancers (ER+, PgR+, Her2neg).
Imaging-guided pre-operative localization of nonpalpable breast lesions has a fundamental role in guiding the surgeon in lesions removal; it can be performed with many techniques and is often required in cases of breast conservation surgery to remove only the index lesion with adequate surgical margins and reducing the extent of surgical treatment.54 Many different image-guided localization procedures are available nowadays.55 Wire localizations have been for decades the standard for pre-operative percutaneous localizations. Recently, different non-wire localization techniques have been introduced overcoming some of the limits imposed by the wires.54–58 In Table 1 the current options for image-guided localizations are summarized with their own strengths and weaknesses. However, no single localization tool or technique proved better for achieving adequate surgical margins, hence each multidisciplinary team should adopt the most effective localization based on the skills and technologies available.54,59
Summary of various localization methods.54,55,58,72
| Localization technique | Strengths | Disadvantages |
|---|---|---|
| Wire localization | Mostly used and least expensiveNo depth limitationNo minimal spacing for lesion bracketingCan be placed with MRI guidance | Scheduling inflexibilityPossible vasovagal reactions, wire rupture or migration.PneumotoraxPotential patient discomfort while waiting for surgery |
| Carbon marking | Old method; however, needing specific local experience. Surgery up to1 month after. | Foreign-body reactions can mimic malignancy. |
| Radio-guided localization | Specific local experience is required.Surgery within 24h after.Radiation exposure (low dose) | Expense, need for nuclear medicine laboratory, intraoperative tools for surgeons, intraductal injection of 99 Technetium disperses radiotracerHigher cost than wire localization. |
| Radioactive (125I) seed localization | Can be used for sentinel lymph node biopsyBreast and axillary node localizationNo depth limitationSmall size Outcomes similar or better than those with wire localization | Radiation exposure to patient and personnel Lengthy process to begin programRegulatory requirements, licensing, need for radiation safety officer supervision |
| Magnetic seed localization | Surgery up to1 month after.Breast and axillary node localizationDeployment similar to biopsy clipSmall sizeSame console and probe can be used for sentinel lymph node biopsy | High costSusceptibility artifact at MRIContraindicated in patients with implanted cardiac devicesNonmagnetic surgical tools are needed because ferromagnetic instruments interfere with signal |
| Radar reflector | Long-term implantationBreast and axillary node localizationMinimal susceptibility artifact at MRI | Multiple factors may limit signal detection Potential for allergic reaction for patients with known nitinol allergy |
| RFID tag | Long-term implantationEach tag has a unique identification number that is visible on the consolePencil-sized surgical probe | Axillary node localization considered off labelSingle-use surgical probe |
Placement of multiple localizing wires or non-wire systems (bracketing) may be useful for larger lesions, multifocal tumors, or extensive DCIS60 when performing BCS. Once the localization technique has been chosen, the radiologist will decide the best imaging modality for the procedure which should be the easiest method whereby the lesion or marker can be certainly identified.58 Typically, sonographic, mammographic and, more recently, DBT are the most used imaging-guide while less frequently MRI guidance are employed. Regardless of the localization device, usually after its placement, a postprocedural mammogram is performed to confirm its location and as guidance for the surgeon in theater. Additionally, a preoperative skin tattoo directly over the nonpalpable breast lesion and the measurement of the depth of the lesion in the supine operative position using US could be performed to help the surgeon to access the lesion accurately.58 Intraoperative imaging aims to evaluate the removed targeted nonpalpable lesion before the patient leaves the operating room. Gross specimen X-rays are usually performed in two 90° orthogonal radiographs for a better assessment of margins compared to one view only. Prompt communication between radiologists and surgeons can ensure that no residual disease remains in the breast and, if needed, additional tissue can be removed.55,60 Other modalities, such as DBT, and US can be used for specimen imaging.
Post- operative benign changes and possible complicationsImaging plays a pivotal role in the detection and management of oncoplastic surgery complications, and it is fundamental that the radiologist is familiar with the appearance of them.
Post-surgical complications include seromas, hematomas, and infections. The most common benign changes seen in breast reconstructed breast are edema, fat necrosis, epidermal inclusion cyst and fibrosis.61
In patients who undergo radiation therapy diffuse thickening of the skin and trabeculae may be seen usually in the first 6 months, which gradually resolve within 2–3 years.
Seroma or hematoma may appear immediately after breast reconstruction and gradually resolve, being replaced by scarring and fibrosis within 1 to 1 ½ years after surgery.
US is the first imaging choice has can help in diagnosis and guide drainage. Seroma may appear as anechoic fluid collection with multiple internal septa and sometimes with fluid-debris level. After aspiration of seroma the fluid can be sent for microbiological analysis to guide specific antibiotic treatment. MR imaging identified seroma as high signal intensity on T2-weighted images.62
Israeli et al.63 found that LD flaps are more prone to seromas than TRAM flaps, where TRAM are associated with more perfusion-related complications such skin necrosis and fat necrosis.
Hematomas are also seen in the early post-operative period, with sonographic appearance that depends on the state of degradation of the blood products. The sonographic appearance is varied including hyperechoic, cystic and complex cystic masses with internal debris and echogenic walls.62,64 In MR imaging hematomas have variable signal intensity on the amount of time that has elapsed since surgery. MRI it is not the exam of choice to investigate seroma or hematoma.
Infection ultrasound is usually required to differentiate benign mastitis and abscess. Abscess on ultrasound is shown as fluid collection with irregular, oval, cystic masses with internal debris and surrounding subcutaneous edema. Ultrasound is an important tool to guide percutaneous drainage of abscess leading to microbiological analysis of the fluid content.
Fat necrosis is the most common cause of palpable and non-palpable lesions in the reconstructed breast, particularly frequent after fat transfer. It is caused by an inadequate blood supply to the tissue as the flap, where they are usually located at the periphery due to reduced vascularity.
Clinical examination in presence of fat necrosis can mimic cancer recurrence so imaging has a relevant role and sometimes findings will need to be biopsied due to difficult differentiation from tumor recurrence
Fat necrosis has a wide range of mammographic features including radiolucent masses, focal asymmetries, calcifications (usually seen 6–12 months after reconstruction) and spiculated masses.61 In the reconstructed breast peripheral location of the lesion along with central fat content is an important clue.
The US appearance is variable, as it may manifest as a cyst simple or complex, a mixed mass with poorly defined margins. The use of special fat-saturated sequence can be diriment in diagnosis fat necrosis on MRI.
Despite all the effort for a non-invasive diagnosis a biopsy may be needed of characteristic appearance.
Epidermal inclusion cyst are formed by proliferation of squamous cells implanted in the dermis, and they have been reported secondary to trauma such as reduction mammoplasty62,65 or following autologous myo-cutaneous flap surgery. The sonographic appearance is varied as they may appears as cystic, complex or hyperechoic masses, but the close contiguity with the skin and the absence of vascularity suggests the diagnosis. Careful evaluation of these superficial masses is necessary since the most common sites of recurrence in the reconstructed breast are the skin and subcutaneous tissue.62,66
Fibrosis develops gradually over 1 to 1 ½ years in the region of the scar, it may manifest at imaging as irregular mass, it lacks enhancement on MRI so performing MRI may be useful to differentiated fibrosis from local recurrence.
Capsular contractions is usually a clinical diagnosis although it presence may be interfered by imaging. Implant rupture can be evaluated with US but the most sensitive examination is MRI that does not require the use of contrast media for this purpose but specific sequences.
Surveillance of local recurrenceLoco-regional recurrences and new ipsilateral or contralateral BCs during 15–20 years after the first diagnosis of breast cancers are expected with an annual rate of 1.0–1.5%,4,5 the risk of recurrence is influenced by age at diagnosis, primary tumor subtype and size, presence of local or regional node disease, surgical treatment and the use of radiation therapy adjuvant hormonal therapy and chemotherapy.62
With the increasing number and variability of oncoplastic procedures, there are many potential ways in which local recurrence can appear and be detected on imaging.
Mammographic surveillance has shown utility in the detection in the recurrent disease, and in screening of the contralateral breast. It detects recurrence before it clinically detectable improving prognosis overall.
As results mammographic follow-up has become the norm for women who have been treated for breast cancer. The mammographic manifestation of recurrence may resemble those of the primary tumor and may include a spiculate mass, distortion, pleomorphic microcalcifications and skin thickening. In presence of abnormal findings on mammography, they are investigated with US; US recurrences are often seen as irregular, vascular masses with speculated margins. However recurrent breast cancers can demonstrate benign appearance and even simulate post-operative collection therefore high level of suspicion regarding any solid mass is recommended.
Although it is an effective technique, mammography is not enough for some women for instance young patient with dense breast or with an originally mammographic occult primary BC. This finding is demonstrated by the fact that some recurrences are diagnosed clinically between two surveillance screening mammography.
Although there is agreement that surveillance of patient who are post BCT is necessary there is lack of consensus on how frequently and for how long we should provide routine surveillance after treatment.67
In a UK review68 surveillance mammography sensitivity ranged from 64 to 67% and specificity from 85 to 97%. The UK review found that MRI sensitivity range from 86 to 100% with a specificity of 93%.
Supplemental technique as MRI or CEM may be considered for patient with high risk of recurrence, even in patient with mastectomy if they are particularly high risk.
Furthermore, mammography of the treated breast is seldom performed routinely for patients who have had a mastectomy and reconstruction as in presence of mastectomy recurrence are rare and most are clinically palpable being commonly located in the skin or subcutaneous tissue. The second most common site of recurrence is deep adjacent to the pectoralis muscle where the autologous tissue or the implant may camouflage the recurrence, for these cases imaging would be helpful in anticipating the diagnosis.
Although there has been increased diffusion of oncoplastic surgery the reconstructed breast has not been subject of imaging surveillance until recently and the use of mammography has been controversial. No specific guidelines are present and advices from the literature are inconclusive. In fact some advice the use of mammographic surveillance of autologous reconstruction that can be performed just like any other surveillance mammogram, while others advocate to select only women with a high risk- or recurrence score.67,69
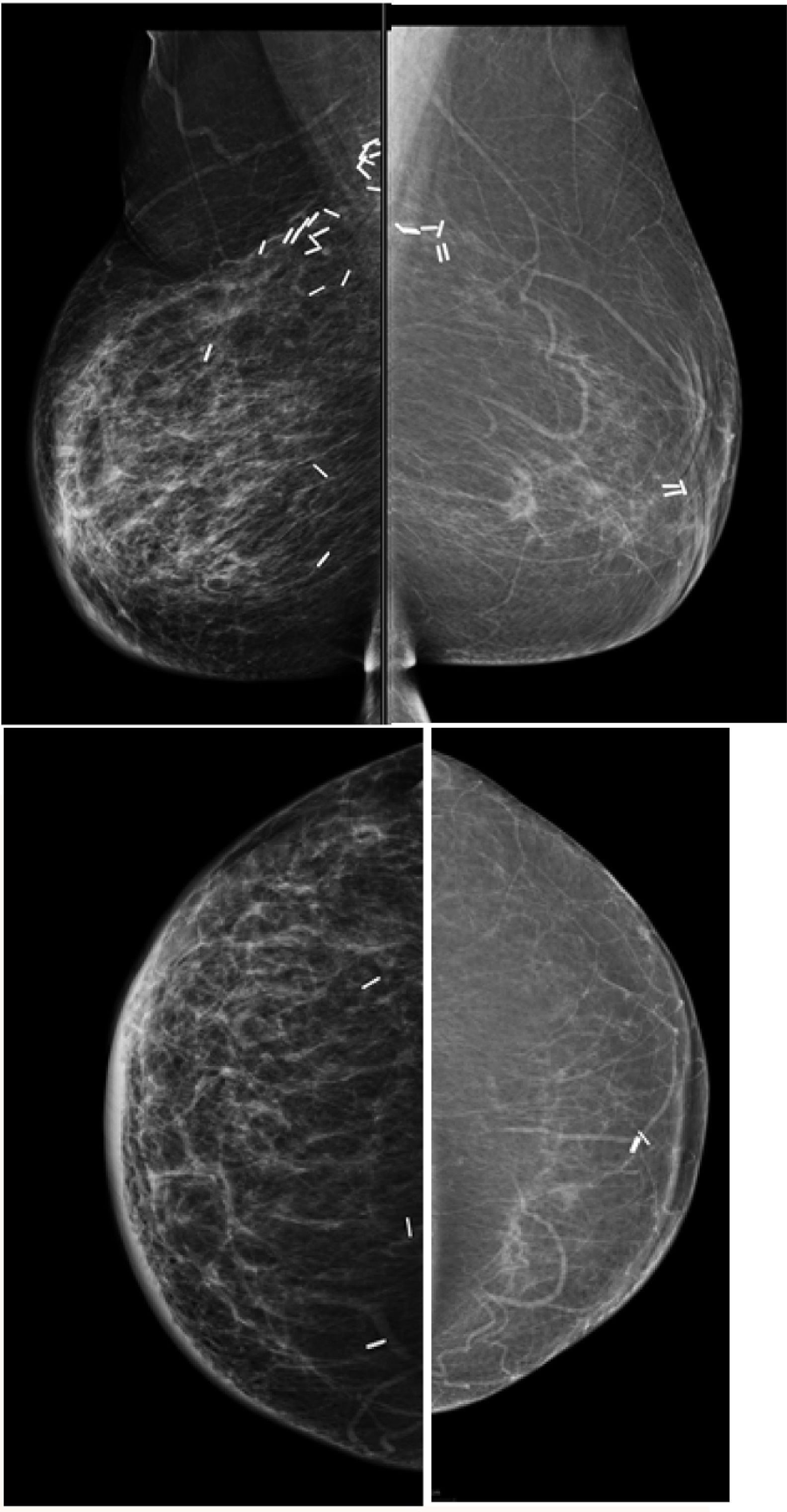
In patient that have had an autologous reconstruction the tissue appears fatty leading to the possibility of easily detecting changes and possible deeper recurrence that are not palpable, mammography could be easily integrated when the contralateral breast is performed. This is not recommended in presence of implant reconstruction (Fig. 4).
The most sensitive test for the reconstructed breast is MRI, but it is an expensive test not widely available. Improved access to dedicated breast MRI is making evaluation of the reconstructed breast more affordable and accessible, it might want to be considered in patient with implants reconstruction and high risk of recurrence (due to young age and biologically aggressive tumor) particularly in the first 3 years after diagnosis.
A more personalized approach should be developed in the future, tailored for every patient according with risk of recurrence, patient characteristics and initial tumor imaging.
Supplemental images that can be consider included DBT, US, CEM and MRI. Recent studies shows that DBT does not increase cancer detection of recurrent breast cancer but reduce false positive rate70 in evaluating palpable abnormality and guiding interventional procedures, but it is not very effective as screening tool. In fact, in surveillance screening increase the number of false positive and even combined with mammography US may not be sufficient for surveillance after curative therapy.71
The use of imaging technique that offer a morphological and functional information as MRI and CEM should be consider for their added value. MRI has been shown to reduce the number of interval cancer in a screening population with dense breast, demonstrating that MRI can detect biologically relevant tumor. CEM has shown similar sensitivity and specificity of MRI. They seem promising technique that should be offer for patient with higher risk of developing recurrent disease.
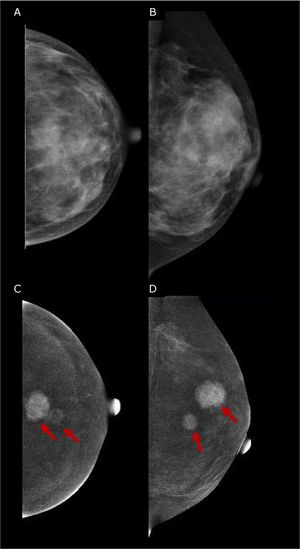
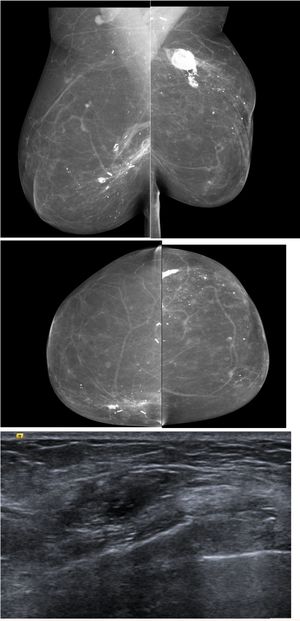
In presence of palpable finding in the reconstructed breast s ultrasound is the first image of choice, but should be used as problem-solving tools in detecting calcified fat necrosis avoiding the need of further biopsy (Fig. 5). MRI should be considered when DM and US are inconclusive.
67 yo patient previous bilateral mastectomy with autologous reconstruction, presented with bilateral palpable lumps, ultrasound was performed and shows findings in keeping with calcified fat necrosis, bilateral mammography was performed to confirm the presence of fat necrosis and avoid the biopsy.
Radiology has a key role in diagnosis, planning oncoplastic surgery and during the follow up for early diagnosis of recurrence. Communication between radiologists and surgeons is fundamental to guarantee the best personalized treatment for the patient, further guidelines for surveillance screening particularly for patient that had oncoplastic surgery will need to be developed in the near future.
FundingNo funding has been received for this paper.
Confidentiality of dataConfidentiality of data has been maintained.
Conflict of interestNo interest to declare.