To evaluate the efficiency for the recovery of spermatozoa using the standard technique and a technique based on ultrasound.
Materials and methodsAn experimental study was carried out to compare two techniques for spermatozoa recovery; (i) the standard technique, based on elution of the evidence in distilled water with a buffer and, (ii) a modified technique that included concentration by centrifugation and ultrasound. The efficiency was based on manual counting of spermatozoa using dilutions 1:1 to 1:500.
ResultsThe modified technique substantially improved the recovery of spermatozoa. While the standard technique recovered 3.1%, the modified one resulted in a recovery of 17.5% using the 1:10 dilution, which represents a six-fold increase. The modified technique was the only one able to recover spermatozoa in dilutions higher than 1:250.
ConclusionThe implementation of the ultrasonic technique improves the recovery of spermatozoa.
Evaluar la eficiencia para la recuperación de espermatozoides utilizando una técnica estándar y una técnica basada en ultrasonido.
Materiales y métodosSe realizó un estudio experimental para comparar 2 técnicas de recuperación de espermatozoides: 1) la técnica estándar, basada en la elución de la evidencia en agua destilada con un solución tampón, y 2) una técnica modificada, que incluyó la concentración por centrifugado y el uso de ultrasonido. La eficacia se basó en el recuento manual de espermatozoides utilizando diluciones de 1:1 a 1:500.
ResultadosLa técnica modificada mejoró sustancialmente la recuperación de los espermatozoides. En la dilución 1:10, la técnica estándar recuperó el 3,1%, la modificada recuperó un 17,5%; representando un aumento de 6 veces más en la recuperación. La técnica modificada fue la única capaz de recuperar espermatozoides en diluciones superiores a 1:250.
ConclusiónLa implementación de la técnica ultrasónica mejora la recuperación de espermatozoides.
Different disciplines are involved in the study of sexual offenses within the penal context. They attempt to reconstruct a criminal act by analysing physical elements gathered at the scene of the crime and the body of possible victims and/or aggressors. The stains and samples collected are studied by forensic biology, which by using biological methods and techniques applied to a penal context determine whether a sexual offense has been committed. These methods help to confirm or refute versions, identify possible aggressors, clarify certain circumstances which may have favoured the occurrence of an act, correlating the victim with the aggressor and/or with the scene.1
Evaluating an alleged victim of a sexual offense includes searching for semen or seminal fluid among the evidence in the alleged victim. The most important cells here are the sperm cells, which are detected using staining techniques such as the “Christmas tree”, which concentrates extracts from garments and applicator samples taken from the alleged victims.2 This involves an initial dilution of the evidence in distilled water with a buffer3 so that the spermatozoa are freed into the medium. This has been the conventional technique used in laboratories, but it requires using the technique in such a way that ensures these cells are freed into the medium without suffering any alteration to their morphology.4 Due to the previous consideration the aim is to improve the efficiency of spermatozoa recovery in the investigation of alleged sexual offences in forensic laboratories by means of a variation in the method, to permit the increased freeing of sperm cells and thereby increase positive evidence.
Materials and methodsStudy typeAn experimental study was conducted in which 2 techniques for sperm cell recovery were compared.
Description of the experimentCotton swabs were impregnated with a known sample of semen to perform the procedure. The semen sample was supplied by a healthy volunteer and collected in a sterile plastic tube. Dilutions of 1:1, 1:10, 1:100, 1:250 and 1:500 were prepared, and sperm counts were carried out using a Neubauer camera.
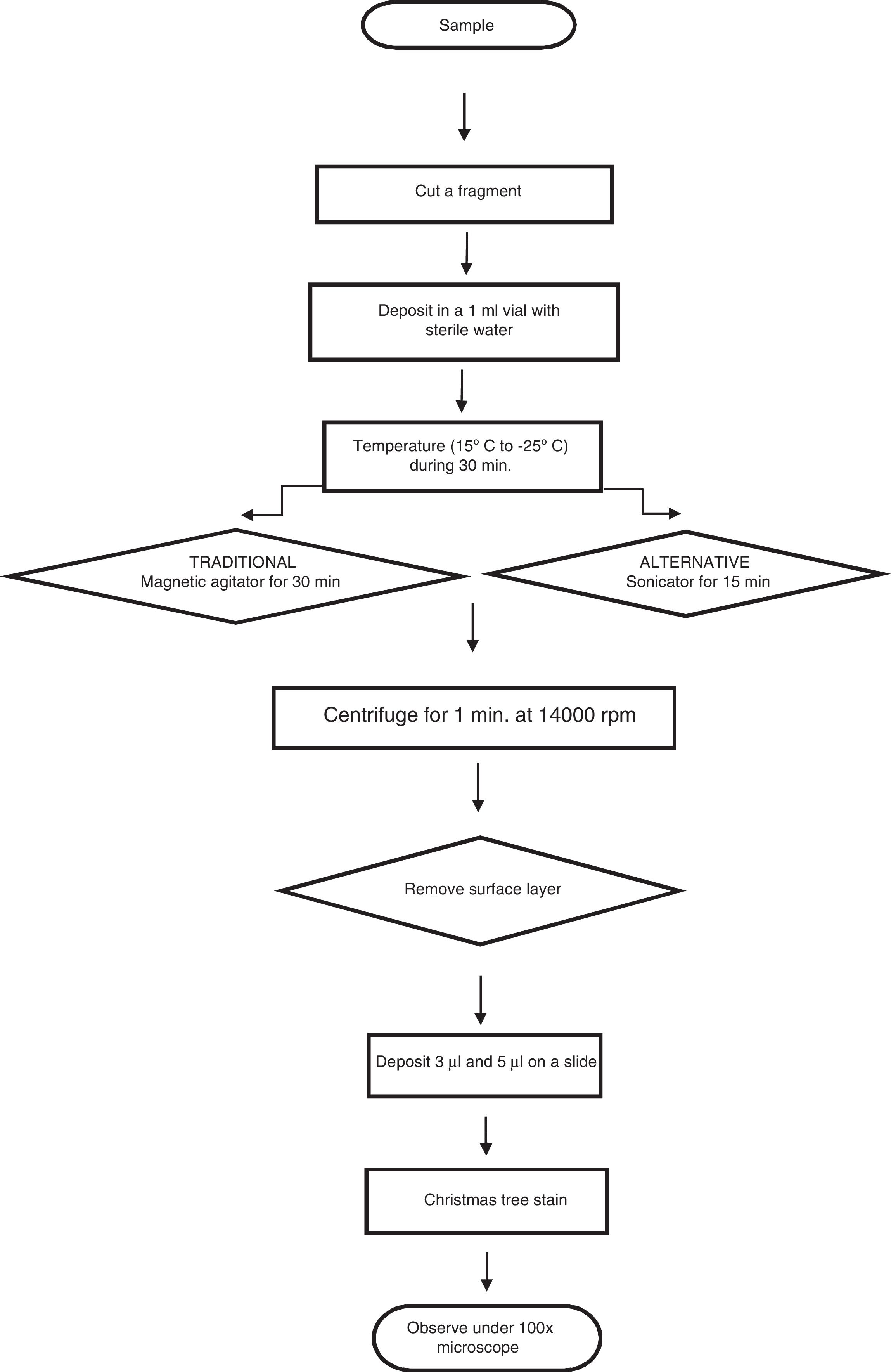
Twenty microliter were added by dilution to the swabs using a micropipette in triplicate for both methods, while preventing crossed contamination; a portion of cotton was removed from the swabs using dissection scissors, and these portions were deposited in vials with 1ml of sterile distilled water, and they were left for 30min in a cold chain process.
Of the total of 30 samples, 15 were treated using magnetic agitation for 5min, while the other 15 samples were subjected to ultrasound using a sonicator (35kHz) continuously during 15min. The cotton fragment was then extracted and centrifuged at 14,000rpm during 1min, after which the floating layer and pellet were placed on slides in 2 volumes (3μl and 5μl).
The samples were fixed at 60°C during 30min, and they were coloured using “Christmas Tree” stain.5,6 A sperm count was then performed using the strip method of searching, and the results of this were recorded (Fig. 1).
Finally the average, number and percentage of recovered spermatozoa were calculated for each one of the dilutions in the volumes analysed. The proportions of sperm cells recovered using the ultrasound and traditional tests were compared for the 3μl and 5μl volumes by using the Chi-squared test.
ResultsA count of 23,837 spermatozoa per cubic millimetre was observed in the initial sample of pure semen. In the different dilutions the counts were a total of 359 sperm cells in the 1:10 dilution, 225 in the 1:100 dilution, 81 in the 1:250 dilution and 25 in the 1:500 dilution, with a significant reduction in the number of cells in each increasing dilution.
The results obtained in microscopic observation once the swab extracts were read after the agitation-only technique in 3μl and 5μl samples in triplicate are described in Table 1.
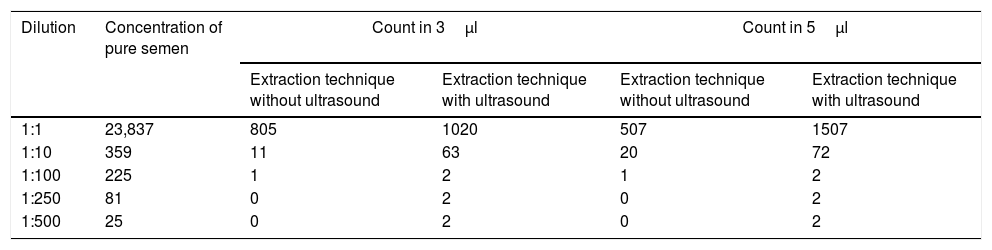
Results obtained in sperm cell counts in 3μl and 5μl volumes for 1:10, 1:100, 1:250 and 1:500 dilutions and total semen, using samples subjected to ultrasound and samples not subjected to ultrasound.
| Dilution | Concentration of pure semen | Count in 3μl | Count in 5μl | ||
|---|---|---|---|---|---|
| Extraction technique without ultrasound | Extraction technique with ultrasound | Extraction technique without ultrasound | Extraction technique with ultrasound | ||
| 1:1 | 23,837 | 805 | 1020 | 507 | 1507 |
| 1:10 | 359 | 11 | 63 | 20 | 72 |
| 1:100 | 225 | 1 | 2 | 1 | 2 |
| 1:250 | 81 | 0 | 2 | 0 | 2 |
| 1:500 | 25 | 0 | 2 | 0 | 2 |
A significantly higher count was obtained for all of the dilutions and different sediments (3μl and 5μl) after ultrasound treatment in comparison with the traditional dilution technique (Table 1). Even at the higher dilutions a small number of sperm cells were recovered. This made it possible to consider the rate as positive.
In the 3μl volume the total sample of semen recovered amounted to 805 cells using the agitation technique and 1020 were recovered using the ultrasound technique. 11 sperm cells were extracted from the 1:10 dilution and 63 were extracted using ultrasound. No sperm cells were recovered from the agitated 1:100 dilution, while 2 were recovered using the ultrasound technique from the other dilutions.
In the count using the 5μl volume of total semen without exposure to ultrasound 507 cells were counted, while in the sample exposed to ultrasound 1507 spermatozoa were counted, a difference of 29.2%. In the same way, after the 1:250 dilution no sperm cells were recovered from the samples not exposed to ultrasound, while in the samples that had been exposed to ultrasound at least 2 spermatozoa were counted.
According to the initial comparison and the findings obtained in the samples subjected to ultrasound and those which were not, 3.1% was recovered from the 3μl 1:10 dilution, while for the ultrasound sample the corresponding figure was 17.5%; this gives a statistically significant difference e in the recovery percentage (P<.001). This shows a recovery of 6 times more spermatozoa using the ultrasound technique.
In the 5μl volume the standard technique recovered 5.6%, while 20% was recovered using the ultrasound technique, giving a statistically significant different in the percentage recovered (P=.000) with a recovery of 4 times more sperm cells using ultrasound. For the 1:100 dilution the samples subjected to ultrasound recovered 0.88% in comparison with the initial count, and this was twice the figure for the samples that were not subjected to ultrasound. From the 1:250 onwards only the ultrasound technique recovered any spermatozoa.
DiscussionBiological evidence in cases of sexual offenses may identify a victim or exclude a suspect; when studying these crimes it is necessary to collect biological evidence in the body or garments7,8: this collection takes place in the victim by taking a smear with a swab.9 The swab then contains a sample for the extraction of sperm cells in microscopic study,10,11 and this is why cotton swabs are used to hold samples.
The use of ultrasound during the process of extracting biological fluid aids the liberation of cells into the diluting medium, as the sound waves moving through an aqueous medium free the spermatozoa held in the cotton. This is shown in the present study by the higher proportion of sperm cells recovered in different dilutions. It is even possible to recover spermatozoa in negative samples at high dilutions, as Hueske11 too observed in garments.
The relationship between the method of extraction and the volume extracted did not make it possible to establish significant differences, as in both methods of extraction the results were the same. New studies with larger sample sizes are suggested to confirm this finding.
This study found that the use of ultrasound may be an effective technique for the recovery of spermatozoa. This experiment only used pure semen in a cotton support, so that it will have to be repeated under real conditions of working, using other supports and with the presence of cells from the victim as well as sperm cells.
This study shows the need for laboratories to evaluate the efficiency of the different methods used to recover sperm cells, given that a suitable standardisation and validation of the different methods of analysis would improve the differences which may exist between different laboratories.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thanks the Instituto Nacional de Medicina Legal y Ciencias Forenses and the Laboratorio de Genética, Medellín, Colombia.
Please cite this article as: Lezcano Martinez AN, Giraldo Vasquez LE, Vanegas Londoño E, Espinosa Montoya T, Zuluaga Barrios JD, Molina Castaño CF. Eficacia del tratamiento ultrasónico en la recuperación de espermatozoides en la investigación de delitos sexuales. Rev Esp Med Legal. 2019;45:18–22.