This study was performed to investigate the serum level of interleukin (IL)-13, IL-4, and interferon (IFN)-γ in chronic rhinosinusitis with nasal polyps (CRSwNP) and subsequent inflammation pattern and comorbidities including asthma and aspirin intolerance.
MethodsA case–control study was conducted on 60 adult patients with CRSwNP with mean age of 37.7±12.7 (ranging from 18 to 70) years, and on 20 healthy controls. Serum levels of IL-13, IL-4, and IFN-γ were assessed, using enzyme-linked immunosorbent assay to be compared between case and control groups. Serum level of total immunoglobulin (Ig) E was also assessed in the patients with CRSwNP.
ResultsSerum level of IL-13 in the patients with CRSwNP was significantly higher than the controls (0.98±1.56 vs. 0.34±0.16 pg/ml, respectively, p=0.002). IL-4 and IFN-γ did not differ significantly between the two groups. Total IgE level was significantly increased in the patients with CRSwNP, compared to the normal values (301.43±516.54 IU/ml, p=0.033). Among the patients with CRSwNP, 12/60 (20%) had aspirin intolerance and 44/60 (73.3%) had asthma. IgE was also higher in asthmatics than non-asthmatics patients (364.9±586.6 vs. 126.7±135.7, respectively, p=0.015). Patients with aspirin intolerance had higher levels of IFN-γ (4.7±1.4 vs. 4.1±0.6, respectively, p=0.022).
ConclusionsIL-13 with high level of total IgE was observed in the patients with CRSwNP, which predisposes them to have concomitant asthma. IFN-γ seems to be down-regulated in the patients with CRSwNP, but could be over-expressed in the presence of aspirin intolerance.
Chronic inflammation of nasal cavities and paranasal sinuses defines chronic rhinosinusitis (CRS) with at least two distinct categories of CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).1 These categories have different underlying pathophysiology, response to treatment modalities and prognosis.2 CRSwNP affects approximately 1% of the population with decrease in quality of life and considerable economic burden.3 Patients with CRSwNP can be categorised into eosinophilic and non-eosinophilic, using nasal lavage cytology pattern.4 It is also reported that nasal polyps in patients with CRS can be discriminated from cystic fibrosis leading to nasal polyps.5
Cytokines play the central role in the pathophysiology of polyp formation in terms of recruitment of inflammatory cells, activation and promotion of their survival. Furthermore they can affect the behaviour of epithelial cells, smooth muscle cells and tissue remodelling.6–8 It seems that not only T helper (TH)-2, but also TH-1 cytokines are involved in the pathophysiology of CRSwNP; however, their effects are poorly understood9; and therefore a comprehensive insight into the underlying pathology of the disease is necessary to adopt the best treatment modality for these patients.10
Eosinophilia is believed to be the hallmark of disease establishment either in early or late phases, with a TH-2 biased cytokines.9 Previous studies by Zhang et al. in Asian populations showed that TH-1/TH-17 biased response and non-eosinophilic pattern in CRSwNP that denotes to the role of ethnic and maybe some uncovered aspects of disease immunopathology.11,12 Similarly, Coa et al. demonstrated high expression of TH-1 cytokines nearby TH-2 and TH-17 cytokines in CRSwNP, compared to controls.13 Cytokine expression in nasal mucus, but not plasma levels, were previously thoroughly investigated14; however, data upon plasma changes are very poor.
This study aimed to investigate the cytokine levels in the sera of patients with CRSwNP including interleukin (IL)-4, IL-13 and interferon (IFN)-γ, compared to healthy controls. We also investigated the association among cytokine levels, nasal smear cytology and total immunoglobulin (Ig) E in sera of patients with CRSwNP. Furthermore, cytokine and IgE levels were compared in asthmatics versus those without asthma and patients with or without aspirin intolerance.
Materials and methodsStudy populationA case-control study was conducted in a group of patients referred to a tertiary referral hospital from Tehran University of Medical Sciences. This study was approved by the local institutional review board of the university. Additionally, written informed consent forms were signed by all enrolled participants, while they were treated according to the Helsinki Declaration of the World Medical Association. A total of 60 adult patients (older than 18 years) with exact diagnosis of CRSwNP, confirmed by an expert clinical immunologist,15–17 were enrolled as cases, while 20 healthy adult participants without any evidence of atopy were also enrolled as controls of the study. All these cases had documents of presence of nasal polyps, using patient examination or endoscopic evaluations.2 Cases with incomplete medical records, cystic fibrosis and who received antibiotics or corticosteroids during last month prior to enrolment, or who were treated with specific immunotherapy (SIT) were excluded from the study. Controls of this study were free of any systemic disease, allergic symptoms, atopy or any category of rhinitis at time of enrolment. Indeed, controls were subjects without CRS, without NP and without evidence of atopy.
Data acquisitionA detailed history was taken from all the participants, including recurrent sneezing, runny nose, nasal congestion, symptoms indicative of conjunctivitis, reduced sense of smell, facial pain or pressure. Other allergic conditions such as atopic dermatitis, food allergy, drug allergy, aspirin intolerance and angio-oedema were also investigated and recorded. Onset of appearance of symptoms and time of diagnosis of CRSwNP were determined by asking the patients and extracting from medical records. Patient examination was done by expert physicians and the findings were recorded. Presence of asthma was considered according to patients’ symptoms and pulmonary function test (PFT), based on standard guidelines.18 If peak expiratory flow increased more than 12% following salbutamol administration, airway hyper-responsiveness was confirmed. To investigate the presence of atopy, skin prick test (SPT) was performed for all the participants with histamine (positive control) and normal saline dilution (negative control). The results were read after 20min and a diameter more than 3mm with respect to the negative control was considered as positive test.
Nasal smear cytologyNasal secretions of patients with CRSwNP were collected, using an appropriate swab. Afterward, specimens were stained by Haematoxylin & Eosin and a blinded pathologist reviewed the results, using a light microscope. A total of 10 fields of high power field (HPF) of microscope were investigated for infiltrated leukocytes and proportion of eosinophils to all inflammatory cells and proportion of neutrophils to all inflammatory cells were calculated. With respect to these two proportions predominance pattern of inflammation was determined.
Assessment of total IgE and cytokinesThe sera from all the patients with CRSwNP were collected after centrifuging the clotted blood, which were kept at −20°C until the time of assays. Total IgE levels were determined, using spectrophotometry according to international standards.19 IL-13, IL-4, and IFN-γ were assessed, using enzyme-linked immunosorbent assay (ELISA, Bender MED and Bioscience). All the procedures were done according to the manufacturer's recommendations. Levels of IgE, IL-13, IL-4, and IFN-γ were expressed as international units per millilitre (IU/mL) and picogeram per millilitre (pg/ml), respectively. The protocols employed to determine the levels of IgE and IL-13, IL-4, and IFN-γ were sensitive to detect minimum levels of 1IU/mL, 0.1pg/mL, 0.06pg/mL, and 0.9pg/mL, respectively.
Statistical analysisStatistical analysis was carried out using SPSS (Statistical Package for the Social Sciences, version 16.0, SPSS Inc., Chicago, IL, USA). Student t test was used to compare the serum levels of interleukins between patients with CRSwNP and controls. ANOVA was employed to compare cytokine levels among different atopic patterns of patients with CRSwNP. The reference value for comparison of total serum IgE with normal population was derived from a study by Carosso et al. as follows percent of females in case arm×148+percent male patients with CRSwNP×169.20 Afterwards, one sample t test was performed to compare the mean of total IgE in patients with CRSwNP with the calculated reference value for normal population with a similar gender distribution. Pearson correlation coefficient was used to assess the correlation between cytokine levels and symptoms. Data were expressed as mean±SD. All the tests were two sided with the significance level at probability below 0.05.
ResultsDemographicsA total of 80 participants (60 cases and 20 controls) with mean age of 37.7±12.7 (ranging from 18 to 70) years were enrolled. There were no differences between case and control groups with respect to the age (37.2±12.0 vs. 39.1±15.1, respectively, p=0.572) and gender distribution (female/male; 38/22 vs. 12/8, respectively, p=0.790).
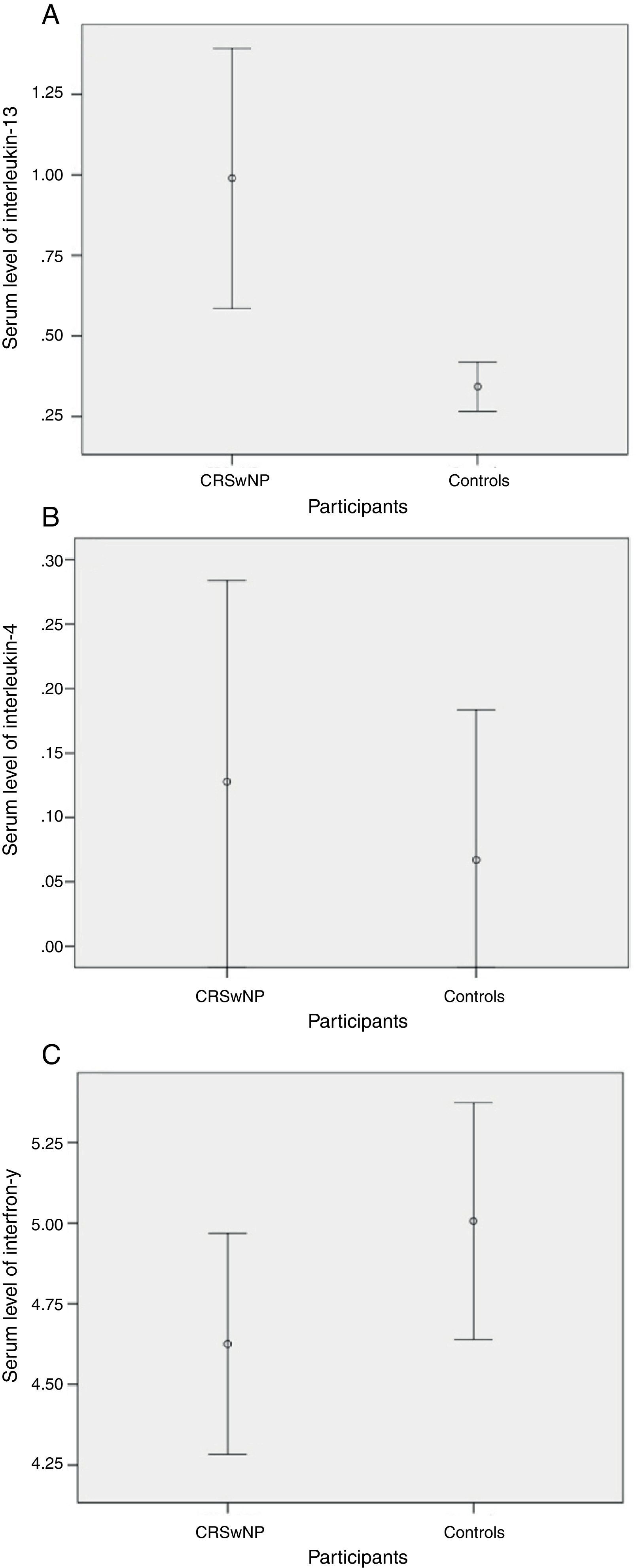
Cytokine profile of patients with CRSwNP versus controlsSerum level of IL-13 in the patients with CRSwNP was significantly higher than the controls (p=0.002, Fig. 1A). However, serum level of IL-4 did not differ (p=0.665, Fig. 1B) between the two groups. On the other hand, the serum level of IFN-γ was higher in non-atopic controls than the patients with CRSwNP, but this difference was not significant (p=0.230, Fig. 1C).
(A) IL-13 was significantly higher in patients with CRSwNP (0.98±1.56pg/mL vs. 0.34±0.16pg/mL, p=0.002), (B) IL-4 was higher in patients with CRSwNP, but did not attain significance (0.12±0.60pg/mL vs. 0.06±0.24pg/mL, p=0.665), (C) IFN-γ was non-significantly higher in controls (5±0.78pg/mL vs. 4.62±1.62pg/mL, p=0.230).
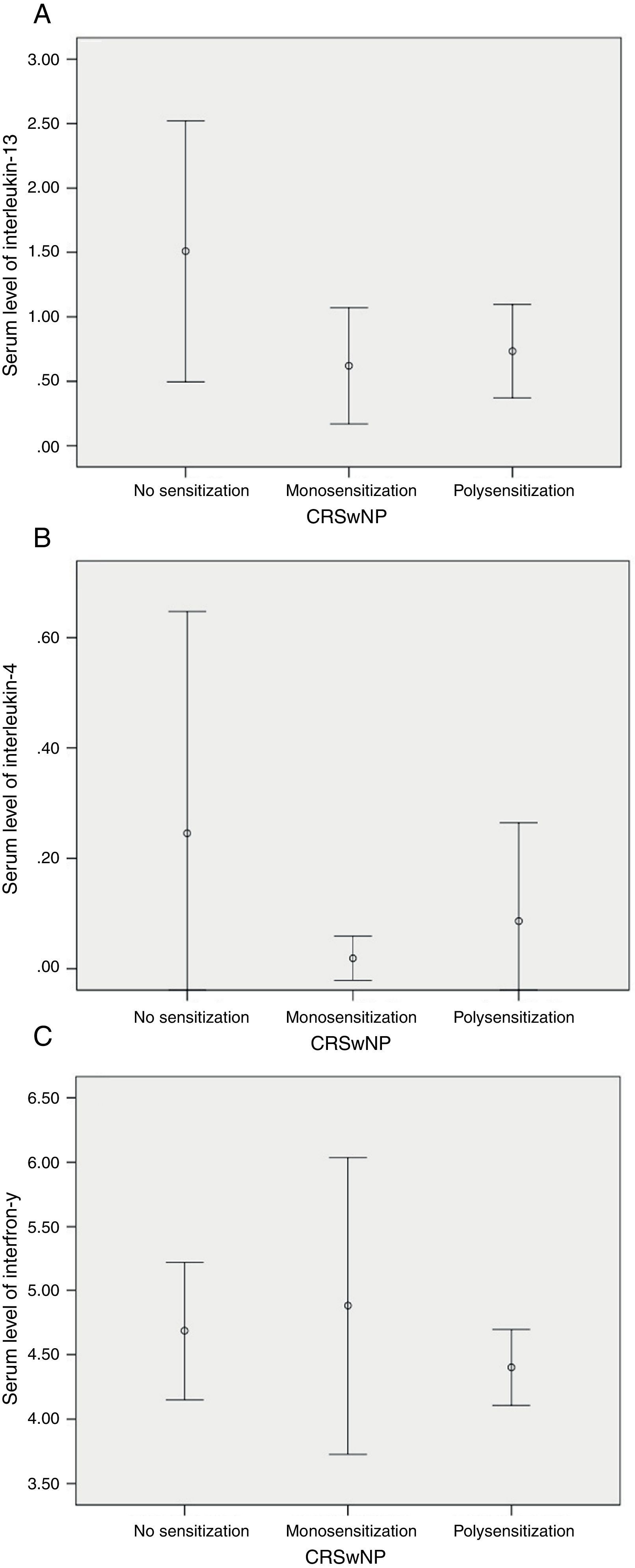
SPT revealed that 22/60 (36.7%) were not sensitised, while 15/60 (25%) were monosensitised and 23/60 (38.3%) were polysensitised. Neither IL-13/IL-4 nor IFN-γ differed significantly among these subgroups of patients with CRSwNP (Fig. 2).
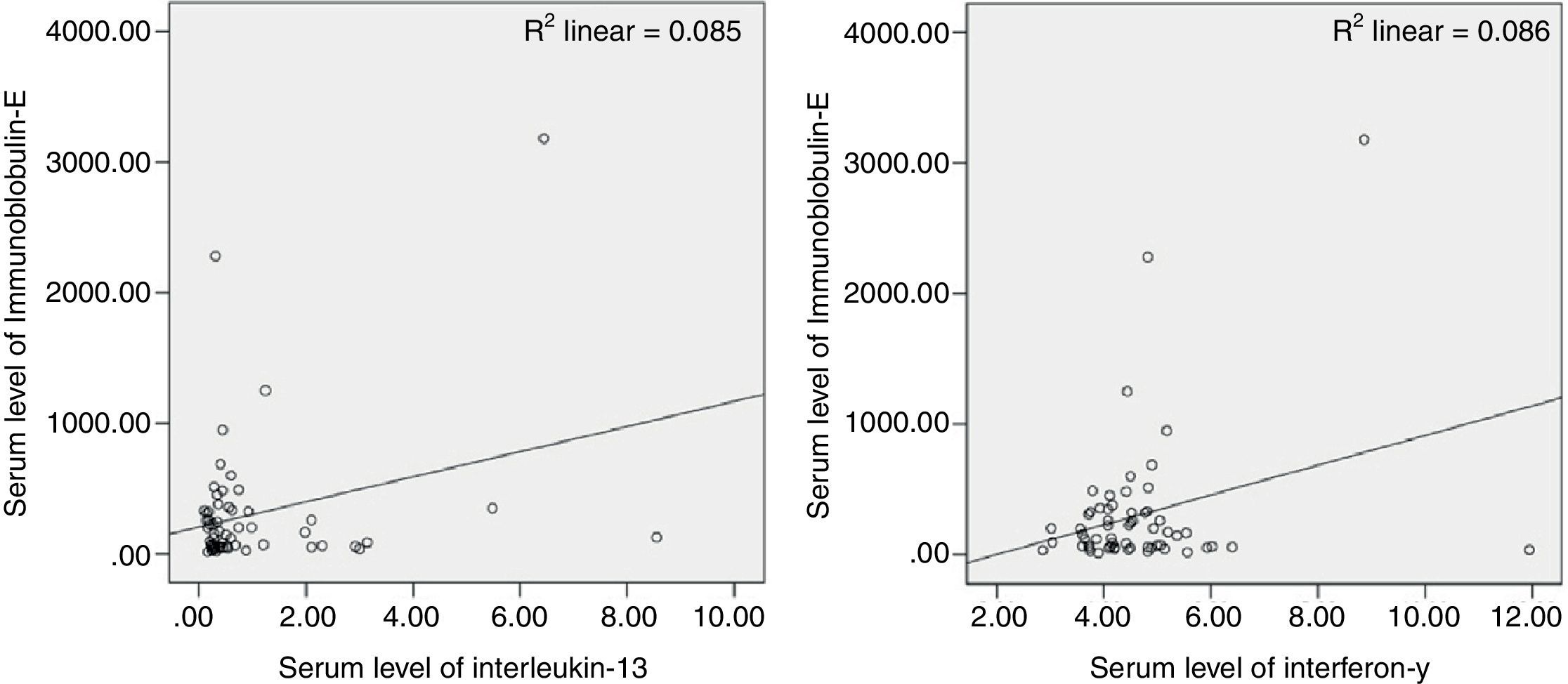
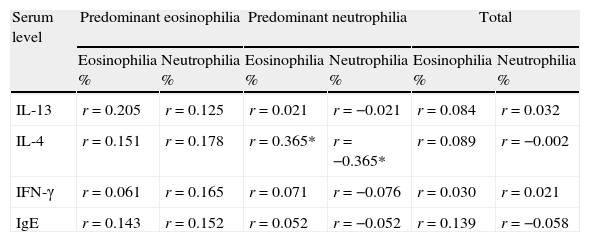
Nasal smear cytology and IgEPredominant pattern of infiltration was eosinophilia in 20/60 (33.3%) and neutrophilia in 40/60 (66.7%). Mean level of serum IgE was 301.4±516.5IU/mL with significant difference than the value (155.7) for normal population (p=0.033). Additionally, serum level of IgE was significantly correlated with IL-13 and IFN-γ, but not with IL-4 (Fig. 3). In patients with CRSwNP and predominant neutrophilia, the percentage of inflammatory cells in nasal secretions was significantly correlated with serum level of IL-4 (Table 1).
Correlation of percent of infiltration of inflammatory cells in nasal lavage with cytokine profile and total IgE.
| Serum level | Predominant eosinophilia | Predominant neutrophilia | Total | |||
| Eosinophilia % | Neutrophilia % | Eosinophilia % | Neutrophilia % | Eosinophilia % | Neutrophilia % | |
| IL-13 | r=0.205 | r=0.125 | r=0.021 | r=−0.021 | r=0.084 | r=0.032 |
| IL-4 | r=0.151 | r=0.178 | r=0.365* | r=−0.365* | r=0.089 | r=−0.002 |
| IFN-γ | r=0.061 | r=0.165 | r=0.071 | r=−0.076 | r=0.030 | r=0.021 |
| IgE | r=0.143 | r=0.152 | r=0.052 | r=−0.052 | r=0.139 | r=−0.058 |
Correlations that attain significance have been marked as *.
Of the patients with CRSwNP, 12/60 (20%) had ASA intolerance and 44/60 (73.3%) had asthma. From measured cytokines, none were differed significantly between asthmatics and who were free of asthma, while, IFN-γ was significantly higher in those with ASA intolerance (4.7±1.4pg/mL vs. 4.1±0.6pg/mL, p=0.022). IgE was significantly higher in asthmatics (364.9±586.6pg/mL vs. 126.7±135.7pg/mL, p=0.015), but not in patients with ASA intolerance.
DiscussionThis study revealed that patients with CRSwNP had higher levels of IL-13 in their sera compared to healthy controls. Interleukin-13 is believed to play a central role in orchestrating the airway inflammation more clearly in asthma21 and with a lesser extent in upper airway inflammation such as CRSwNP.22 Interleukin-13 is implicated in the production of IgE that binds to mast cells, basophils and inflammatory cells in exposed sites to aeroallergens like upper airways.23 Subsequent re-exposures will trigger inflammation and warrants its persistence to a chronic disease. We found patients with CRSwNP had higher serum level of IgE than that for the normal population corrected with respect to the age and gender. Interestingly, despite a decrease in levels of IFN-γ, it was still directly correlated with IgE levels. It could be justified with respect to the fact that whenever a balance is lost, the protective processes try to compensate it. Additionally, IFN-γ is increased in viral infections, and infections will be increased in patients with CRSwNP due to the abnormal anatomy of upper respiratory tract, hence levels of IFN-γ as important TH-1 cytokine would be correlated with IgE as a hallmark of atopic symptoms and changes.
Furthermore, SPT revealed sensitisation in 63.3% of our participants, which is considerable. Despite the predisposing effect of TH-2 cytokines such as interleukin-13 on atopy, we did not find any significant difference in serum cytokine levels of atopic versus non-atopic patients with CRSwNP. Scavuzzo et al.24 found no difference in IL-2/4/6 and IFN-γ of nasal mucus of patients with CRSwNP with or without atopy; that was similar to our findings in that there was no significant difference in serum level of IL-4/13 and IFN-γ among patients with different atopic status. Prevalence of atopy may be even higher in patients with CRSwNP as Tan et al. showed that in patients with CRSwNP who failed medical treatment, 85.5% had positive skin reaction to at least one allergen.25 The message would be the importance of immunomodulator therapies in refractory subjects to conventional treatments and necessitates deeper insight to the immune imbalance present in patients with CRSwNP.
More than IgE production and promotion of atopy, epithelial cell hypertrophy, goblet cell hyperplasia, mucus hyper-secretion, sub-epithelial fibrosis, eosinophilia, oedema and airway hyper-responsiveness; all were reported as subsequent changes that IL-13 promotes.26 However, all aforementioned changes are not seen in patients with CRSwNP due to interaction of other cytokines. In CRSwNP patients free of asthma, lower concentrations of transforming growth factor beta (TGF-β) leads to suppression of fibroblast proliferation and sub-epithelial collagen deposition, while in CRSsNP it is higher.27 Despite the significantly elevated levels of IL-13, we found predominant pattern of neutrophilia instead of eosinophilia in nasal specimens from patients with CRSwNP. This is interesting while IL-13 strongly supports the survival and activation of eosinophils and eosinophilia via induction of eotaxin production.28,29 Increased level of other cytokines such as IL-17 family could be assumed as the cause, but our study is limited to show it. Jiang et al. demonstrated that IL-17 is implicated in nasal polyp formation either in neutrophilic or eosinophilic CRSwNP but with a pronounced role in triggering the neutrophilic inflammation.30 An undetected imbalance in favour of cytokines promoting neutrophilia is supposed in the present study.
On the other hand, neutrophilic rather than eosinophilic inflammation could be due to polymorphisms of IL-13 or its receptors in Asian participants.31 In recent years, studies on Chinese adult population with CRSwNP demonstrated a pronounced neutrophilia rather eosinophilia. Cao et al. studied 151 patients with CRSwNP compared to 94 subjects with CRSsNP and 50 healthy controls. They found that TGF-β decreased and TH-1/2 and 17 all were increased in patients with CRS whether NP was present or absent. In their experience, TH-17 responses were higher in non-eosinophilic CRSwNP while eosinophilic CRSwNP showed skewed TH-2 response and CRSsNP was predominantly biased towards TH-1 cytokines.13 Zhang et al. investigated the cytokine profile of patients with CRSwNP in samples from both European and Asian people. Asian participants had neutrophilic inflammation with TH-1/TH-17 skewed response whereas Europeans had classic eosinophilic pattern with TH-2 biased response. Regulatory cytokines like TGF-β were significantly lesser in CRSwNP than controls.11,12 Whether the patients with CRSwNP are Asian or Europeans, regulatory cytokines will decrease significantly, approved by low levels of forkhead box protein 3 and others contributing to the remodelling process like TGF-β are highly preserved in such a way that significant local reduced level of TGF-β can discriminate CRSwNP from CRSsNP.27 The TH-17 family may be the key in elucidating the different inflammation pattern in nasal polyps of Asian and European patients and could be the target of future studies. However neutrophilia was predominant in our experience, participants had classic cytokine profile of TH-2 skewed response with increased amount of IL-13 and IL-4 and decrease in IFN-γ in comparison with controls. Additionally, we observed a weak, but significant correlation between IL-4 levels and amount of inflammatory cells in nasal lavage of patients with neutrophilic CRSwNP due to unknown reason.
Another question sought to be answered was whether cytokine levels have any association with the coexistence of lower respiratory involvement or other coexisting allergic conditions. In the current study, the presence of lower airway inflammation in asthmatics did not lead to higher serum levels of neither IL-13 nor IL-4 and IFN-γ, but IgE level was significantly higher in patients with CRSwNP that concomitantly suffered from asthma. This finding might be due to the locally increased levels of TH-2 cytokines like IL-13 or up-regulation of the receptors of IL-13 in effector cells of asthmatics. Moreover, other cytokines that were not measured here might be responsible for significant higher levels of immunoglobulin-E in non-significant elevation of IL-13. This is in agreement with the fact that immunoglobulin-E mediated sensitivity to aeroallergens is associated with asthma.32 The question remained to be answered is that whether in patients with CRSwNP who have concomitantly asthma, local level of cytokines differs from who are free of asthma or not? This idea arises from the concept of unified airway that justifies the coexistence of inflammation in both of upper and lower airways. It is interesting that there are observations that even surgical treatment of nasal polyps helps improvement of asthma but should be validated by strong methodological studies.33
Aspirin-exacerbated respiratory disease is defined by CRSwNP, asthma and aspirin hyper-sensitivity and was found to be 20% in this study. However, it is not common in a general population with maximum prevalence of 2.5% conferring the eight-fold risk of ASA intolerance in patients with CRSwNP. ASA intolerance jeopardises the course of CRSwNP with inappropriate response to conventional treatments but the knowledge regarding the underlying pathology is still unknown. Here, CRSwNP patients with ASA intolerance differed with others only with respect to the higher levels of interferon-y. Shome et al. studied only one case with ASA intolerance and found high expression of IFN-γ the expression of which increased by CD4+T cells and decreased by CD8+cells following aspirin desensitisation.34 Further studies on the cytokine profile of patients with Aspirin-exacerbated respiratory disease are still awaited.
One limitation of this study was its low power, which could be responsible for not detecting significant differences between patients and controls. In addition, serum level of cytokines may be affected by the level of other substances and blood carrier proteins and it may interrupt our results. On the other hand some cytokines may only be detectable locally.
In conclusion, IL-13 with high level of total IgE observed in patients with CRSwNP predisposes them to have concomitant asthma. IFN-γ was down-regulated in patients with CRSwNP as compared to controls but in the presence of ASA intolerance it would be over-expressed. In agreement with the studies on Asians, we found that the fruit of this imbalance was neutrophilia rather than eosinophilia that confers the implication of macrolide antibiotics in medical therapy. Macrolides reduce neutrophilia via inhibition of chemokine and adhesive molecule synthesis. Polymorphisms in cytokines and their receptors in addition to ethnic issues can be responsible for this observation. In neutrophilic CRSwNP, the amount of infiltrating inflammatory cells in nasal lavage is weak but significantly correlates with IL-4. Despite all efforts to elucidate changes in cytokine profile of patients with CRSwNP many questions still remain unanswered. Further studies with stronger methodologies are awaited to elucidate the cytokine profile changes of patients with CRSwNP and its effect on the course of disease.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
We would like to acknowledge the ENT-Head & Neck Research Center, Hazrat Rasoul-e-Akram Hospital, Tehran University of Medical Sciences, Tehran, Iran.