Studies indicate that exposure to polycyclic aromatic hydrocarbons (PAH) is associated with adverse respiratory and allergy outcomes. Exposure to PAH may impair the immune function of the foetus and, subsequently, be responsible for an increased susceptibility of children to allergic diseases.
ObjectivesThe aim of the present study was to assess the association between mother's exposure to PAH during pregnancy and allergy diseases in their infants. We also assessed the above associations using measured PAH exposure in children's urine during the first two years of life.
MethodsThe current analysis was restricted to 455 mothers and their children from Lodz district. The women were interviewed three times during the pregnancy in order to collect demographic, socio-economic and medical history data. Children's health status was assessed at the age of 10–18 months and repeated at two years of age. The associations between dependent dichotomous variables and urine concentrations of 1-hydroxypyrene (1-HP) were analysed using logistic regression.
ResultsWe showed that higher urine concentrations of 1-HP in mothers at 20–24 weeks of pregnancy increased the risk of more frequent respiratory infections (p=0.02) in children during their first year of life. Higher 1-HP concentrations in children's urine increased the risk of food allergy (p=0.002) in children during their first two years of life.
ConclusionsThis study suggests awareness of environmental factors, which may affect children's health since PAH showed to be a risk factor for airway infections and food allergy in children after adjustment for other risk factors.
Polycyclic aromatic hydrocarbons (PAH) are a well-known class of environmental pollutants that usually occur as complex mixtures of more than 300 compounds composed of fused aromatic rings. The source of PAH includes emissions from car fumes, coal-fired power plants, residential heating (e.g. coal or wood stoves, fireplaces) unvented gas appliances, environmental tobacco smoke and domestic cooking procedures such as grilling and roasting.
Exposure to PAH may impair the immune function of the foetus and, subsequently, be responsible for an increased susceptibility of children to respiratory diseases.1–3 For some symptoms the effect of PAH exposure is partly modified by exposure to environmental tobacco smoke (ETS). The study by Miller et al. has shown that prenatal PAH exposure in the presence of postnatal ETS exposure was associated with increased cough and wheeze at 12 months of age and breathing problems and reports of probable asthma at two years of age.1 Another study has shown an increased risk of various respiratory symptoms (including barking cough, wheezing, sore throat) and ear infections associated with PAH exposure. In addition, in this study the impact of PAH exposure on duration of respiratory symptoms has been observed.2 It has also been demonstrated that combined prenatal exposure to PAH and ETS was associated with asthma but not atopy at age 5–6.3 It is important to remember that many PAH are powerful carcinogens and that prenatal exposure to many air pollutants besides PAH is linked to allergic diseases in children such as asthma.4,5
The measurement of urinary PAH metabolites provides a valuable tool to assess the individual level of internal PAH exposure.6,7
Levels of 1-hydroxypyrene (1-HP) – the main pyrene metabolite, have been used as representative indicators of PAH exposure. Our previous analysis based on Polish Mother and Child Cohort (REPRO_PL) indicated that non-smoking pregnant women suffer from a higher PAH exposure (based on nine PAH metabolites) than those from other western countries.8 1-HP was highly correlated with other PAH metabolites.
In our previous report, associations between different factors affecting pregnant women and young children and children's health status were found.9–12 In the present study, we hypothesised that maternal prenatal urine metabolite concentration of PAH would be associated with wheezing, food allergy, early eczema and infections among city children. The aim of the present study was to assess the association between mother's exposure to PAH during pregnancy and respiratory infections and allergic diseases in their infants. We also assessed the above associations using measured PAH exposure in children's urine during the first two years of life.
Materials and methodsStudy areaLodz voivodeship (highest-level administrative subdivision of Poland corresponding to a district or province) is situated in Central Poland. At the end of 2013 Lodz voivodeship had a population of 2,607,380 with 622,290 inhabitants below 19 years (<5 years: 139,613; 6–13 years: 246,861; 14–19 years: 235,816). Lodz district is the centre of the textile and chemical industry with many power stations. Air dust pollution in Lodz district is one of the highest in Poland. In 2013 mean dust concentration in the city centre of Lodz was 38mcg/m2 and emission of sulphur dioxide per 1km2 in total was 23.1.13 Data presented by the WHO indicate that cities and towns in Poland are the most polluted in the EU (based on the annual average PM2.5 level mcg/m3; https://www.reddit.com/r/europe/comments/4j82zs/most_polluted_cities_and_towns_in_the_eu/?). Of 154 cities in Poland, Lodz was located on the 69th position of the highest PM2.5 levels (with the annual PM2.5 level in 2013 equal 27mcg/m3; comparing to the highest 43mcg/m3 in Zywiec and the lowest 8mcg/m3 in Inowroclaw) (http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/). Our previous analysis performed on the population of pregnant women from Lodz district has also confirmed high levels of PAH metabolites.8
There are some major sources of PAH in Lodz district such as: industry, motor vehicles and trucks, coal burning, and burning of biomass such as trash. However, there is limited data on airborne levels of PAH in the Lodz district. The epidemiological situation in the city centre of Lodz is far worse with a percentage of adult asthma of 13.2 and children asthma of 18.4.14,15
Study design and populationThe present study was based on the data from the Polish Mother and Child Cohort (REPRO_PL) – a multicentre prospective cohort study established in 2007.9–12 The mothers’ recruitment and follow-up procedures and complete description of the methodological assumptions has been published elsewhere.16,17 The women were recruited from the general population during the first trimester of pregnancy at maternity units or clinics if they fulfilled the following inclusion criteria: single pregnancy up to 12 weeks of gestation, no assisted conception, no pregnancy complications (including possible spontaneous abortion or child malformations), and no chronic diseases as specified in the study protocol (including pre-pregnancy diabetes, hypertension, cancer, heart diseases).16 Maternal asthma and allergy did not constitute an exclusion criterion for the study. The current analysis was restricted to 455 mothers and their children from Lodz district.
The study was approved by the Ethical Committee of the Nofer Institute of Occupational Medicine, Łódź, Poland (Decision Nos. 7/2007 and 3/2008) and a written consent was obtained from all the subjects (pregnant women and patents of the children) before the study.
Mother health assessmentThe women were interviewed three times during the pregnancy (once in each trimester) in order to collect and update demographic and socio-economic data, medical and reproductive history, and information about environmental and occupational exposures.
Child health assessmentChildren's health status was assessed at around one (range, 10–18 months) and repeated at two years of age (range, 23–30 months). For the appropriate recognition of children's health status, an interview was performed with mothers and supplemented (verified) with information from the medical chart of each child.9–12,17 In addition, current child health status assessment was performed by a paediatrician/allergist. At that time, information concerning socio-demographic, environmental and lifestyle factors has also been updated by conducting interviews with mothers.
The first part of the questionnaire covers certain sociodemographic information (i.e., family size, material status of the family, and parental educational level). Material status of the family has been measured during pregnancy and after child birth based on the following question: ‘What is the financial status of your family?’ Women who declared that they have sufficient money for current expenses and that it was possible for them to put a substantial sum aside were allocated into the high income category. Those who indicated sufficient money for current expenses, with possibility to put aside some money were allocated into the medium category, and those who declared insufficient money for current expenses were defined as those with the worst financial situation. The occurrence of allergy among family members was noted. The second part of the questionnaire (developed by an allergist, based on recommendations from the International Study of Asthma and Allergies in Childhood) investigated the child's health and condition. The respiratory outcomes were analysed for the following symptoms: wheezing, cough, dyspnoea. The incidence of upper and lower respiratory tract infections (RTIs) were counted, wheezing, dyspnoea or cough were noted if child ever wheezed or had dyspnoea or cough off infection, and any symptoms of allergy to food and inhalant allergens were noted. The duration and course of each infection and disease, medications taken, and hospitalisations, if any, were identified. Patients were defined as having food allergy or atopic dermatitis if they had ever been diagnosed by a physician. In addition, the occurrence of allergy among family members was defined by questionnaire.
Assessment of mother's and children's exposure to PAHThe urine samples were collected from the pregnant women at the time of their second (between 20th and 24th weeks of gestation) and third (between 30th and 34th weeks of gestation) prenatal clinic visit scheduled within the study and from their children at 1 or 2 years of age into polypropylene cups. The child urine collection was performed by the mother at home. Taking into account the child's age, in the situation when it was not possible to collect the urine into the cups, the urine sample collection bags appropriate for child age (U-bag 100ml) were used. The mothers were trained how to collect the urine from their children. The samples were stored at −20°C before the analysis. Assessment of prenatal and postnatal exposure to PAH was based on measurement of 1-hydroxypyrene (1-HP) in urine using high performance liquid chromatography (HPLC). The analysis was performed at NIOM laboratory based on the method described elsewhere.18,19
ETS exposure assessmentPrenatal exposure to tobacco constituents was assessed based on questionnaire data. Full description of assessment of children's exposure to tobacco smoke was published elsewhere.17
Statistical analysisThe associations between dependent dichotomous variables and urine 1-HP concentrations were analysed using logistic regression. Histograms of continuous variables revealed that all 1-HP concentrations were positively skewed and, therefore, were log10-transformed before analysis. Next, for all outcomes, we fitted logistic regression models with 1-HP levels as continuous variables and we adjusted each model for the effect of a different set of cofounders (given in the footnotes of Table 2) defined in a previous study from the same cohort. The Wald testing was done to determine the final model. We modelled each of the individual 1-HP concentrations separately. Spearman's test was used to assess correlations between different measurements of 1-HP. All of the statistical analyses were performed using STATISTICA v8.0 (StatSoft, Inc., Tulsa, OK, USA). The null hypothesis was rejected if p<0.05.
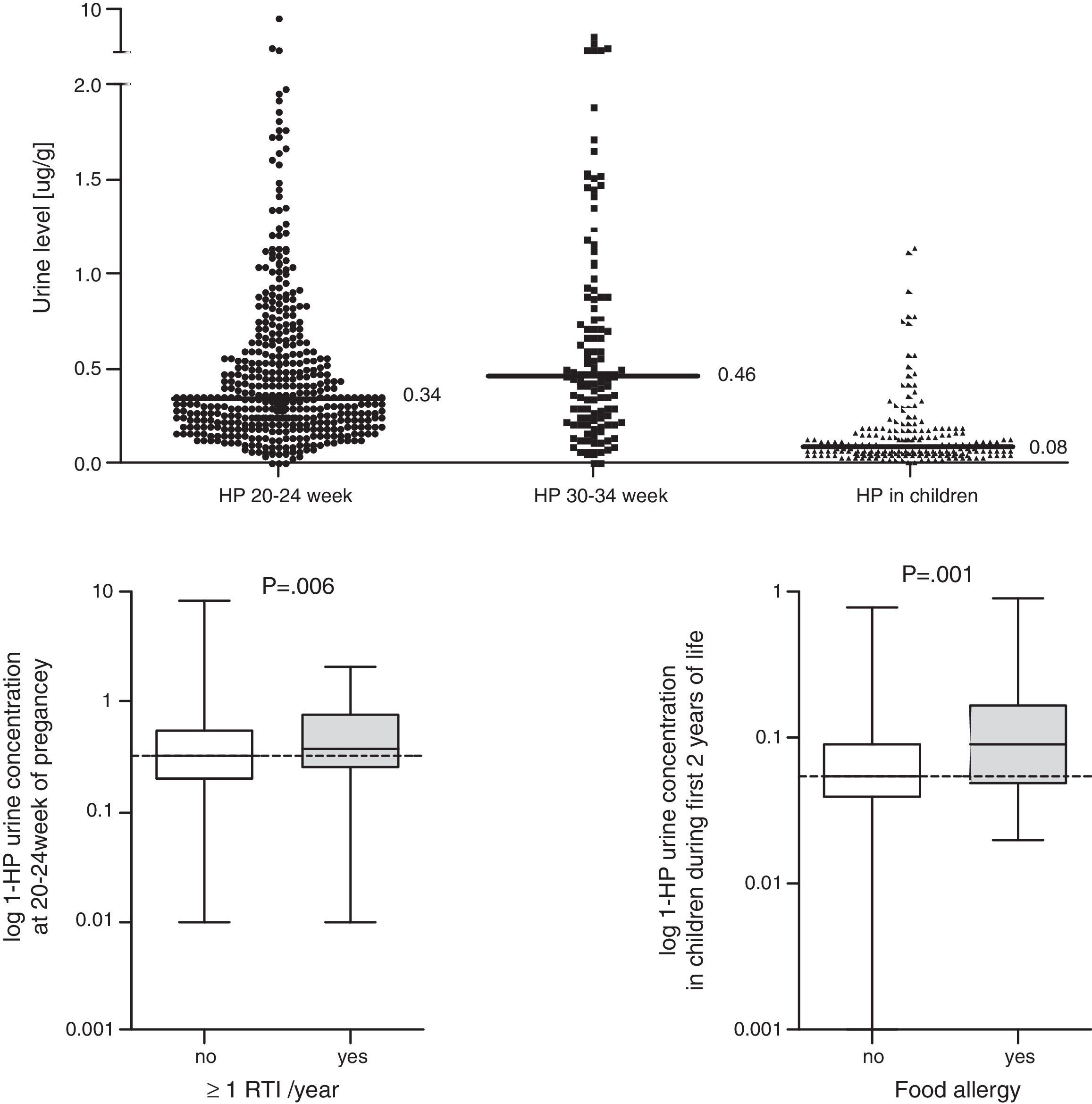
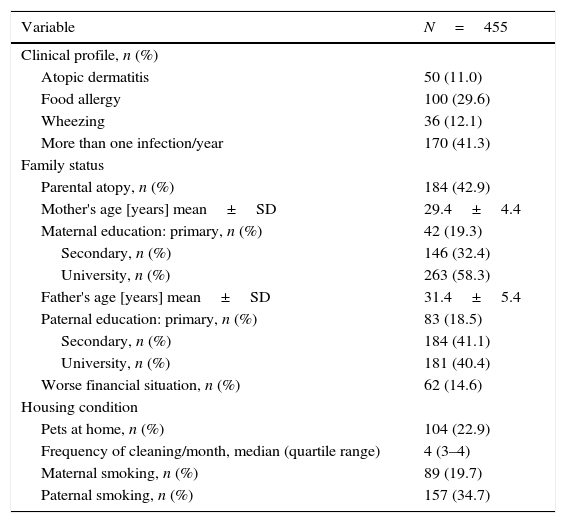
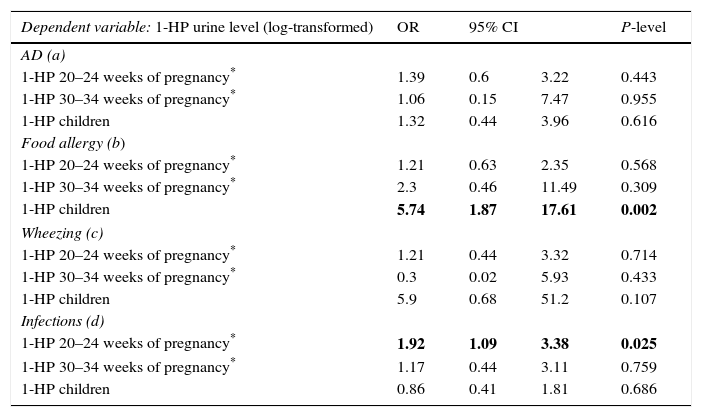
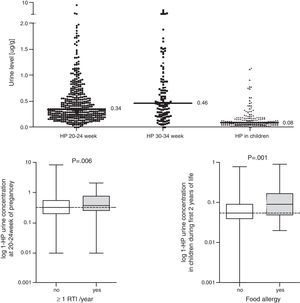
ResultsData from 455 participants were included in the analysis. A description of the study cohort is provided in Table 1. Median values and the distribution of the concentration in urine (mother and child) of 1-HP are shown in Fig. 1. In our study, we showed that higher urine concentrations of 1-HP in mothers at 20–24 weeks of pregnancy increased the risk of more frequent (>median: >1/year) respiratory infections in children during their first year of life (Table 2). Higher 1-HP concentrations in children's urine increased the risk of food allergy in children during their first two years of life (Table 2). The above associations were adjusted for the effect of previously defined (in the same cohort) statistical predictors of study outcomes.
Clinical characteristics of the participants.
| Variable | N=455 |
|---|---|
| Clinical profile, n (%) | |
| Atopic dermatitis | 50 (11.0) |
| Food allergy | 100 (29.6) |
| Wheezing | 36 (12.1) |
| More than one infection/year | 170 (41.3) |
| Family status | |
| Parental atopy, n (%) | 184 (42.9) |
| Mother's age [years] mean±SD | 29.4±4.4 |
| Maternal education: primary, n (%) | 42 (19.3) |
| Secondary, n (%) | 146 (32.4) |
| University, n (%) | 263 (58.3) |
| Father's age [years] mean±SD | 31.4±5.4 |
| Paternal education: primary, n (%) | 83 (18.5) |
| Secondary, n (%) | 184 (41.1) |
| University, n (%) | 181 (40.4) |
| Worse financial situation, n (%) | 62 (14.6) |
| Housing condition | |
| Pets at home, n (%) | 104 (22.9) |
| Frequency of cleaning/month, median (quartile range) | 4 (3–4) |
| Maternal smoking, n (%) | 89 (19.7) |
| Paternal smoking, n (%) | 157 (34.7) |
Concentration of 1-hydroxypyrene (1-HP (mcg/g)) in urine (mother and child); comparison of 1-HP concentration in urine between children with frequent (>1/last year) vs. infrequent (≤1/last year) infection and between children with vs. without food allergy; Mann–Whitney test was used.
Associations between presence of: (i) atopic dermatitis (AD), (ii) food allergy, (iii) at least 1 episode of wheezing (wheezing), (iv) >1 episode of respiratory infections/last year and 1-HP urine level in mother during pregnancy and in children (in the first or second year of life) (included in the model as log-transformed continues variables).
| Dependent variable: 1-HP urine level (log-transformed) | OR | 95% CI | P-level | |
|---|---|---|---|---|
| AD (a) | ||||
| 1-HP 20–24 weeks of pregnancy* | 1.39 | 0.6 | 3.22 | 0.443 |
| 1-HP 30–34 weeks of pregnancy* | 1.06 | 0.15 | 7.47 | 0.955 |
| 1-HP children | 1.32 | 0.44 | 3.96 | 0.616 |
| Food allergy (b) | ||||
| 1-HP 20–24 weeks of pregnancy* | 1.21 | 0.63 | 2.35 | 0.568 |
| 1-HP 30–34 weeks of pregnancy* | 2.3 | 0.46 | 11.49 | 0.309 |
| 1-HP children | 5.74 | 1.87 | 17.61 | 0.002 |
| Wheezing (c) | ||||
| 1-HP 20–24 weeks of pregnancy* | 1.21 | 0.44 | 3.32 | 0.714 |
| 1-HP 30–34 weeks of pregnancy* | 0.3 | 0.02 | 5.93 | 0.433 |
| 1-HP children | 5.9 | 0.68 | 51.2 | 0.107 |
| Infections (d) | ||||
| 1-HP 20–24 weeks of pregnancy* | 1.92 | 1.09 | 3.38 | 0.025 |
| 1-HP 30–34 weeks of pregnancy* | 1.17 | 0.44 | 3.11 | 0.759 |
| 1-HP children | 0.86 | 0.41 | 1.81 | 0.686 |
a Associations are corrected for other independent risk factors of atopic dermatitis in this cohort: atopy in family, father's education, frequency of cleaning, breastfeeding.
b Associations are corrected for other independent risk factors of food allergy in this cohort: pets at home during pregnancy, breastfeeding.
c Associations are corrected for other independent risk factors of wheezing in this cohort: number of infections and paternal smoking.
d Associations are corrected for other independent risk factors of more frequent infection in this cohort: paternal smoking.
We did not find any other associations, also when assessing 1-HP concentrations at 30–34 weeks of pregnancy.
We followed the correlation analysis between different measurements of 1-HP concentration. We showed significant correlation between concentrations of 1-HP in mothers at 20–24 weeks of pregnancy and 1-HP concentrations in children's urine (Spearman's correlation coefficient: 0.418; p=0.017). There were no other linear or non-linear correlations between 1-HP concentration measurements.
DiscussionIn the literature there is little evidence for the association between exposure to polycyclic aromatic hydrocarbons during pregnancy and prevalence of respiratory symptoms during the first year of life. In our study we found a significant association between mothers’ exposure to PAH during pregnancy and an increased risk of recurrent respiratory tract infections in infants. This association was especially strong for women exposed to PAH in the second trimester of pregnancy with a 70% increase in the risk of respiratory tract infections. The second interesting finding in our study was the association between high PAH metabolites concentrations measurement in infant's urine (by the age of 1 or 2 years) and an increased risk of food allergy.
Jedrychowski et al.2 have shown that in Poland the mean concentration of PAH exposure is 26.1ng/m3, which is much higher than respective data coming from other urban populations.2,20 They have shown an increased risk of various respiratory symptoms (including barking cough, wheezing, sore throat) and ear infections associated with PAH exposure. They have also shown a significant association between prenatal PAH exposure and the occurrence of respiratory outcomes observed in infants. The highest risk of respiratory episodes related to PAH exposure in that study were noted for barking cough (RR=4.8; 95% CI: 2.7–8.4) and wheezing without cold (RR=3.8; 95% CI: 1.2– 12.4).2 Our findings are consistent with Jedrychowski et al., the recurrent respiratory tract infections in infants were more frequent in the infants with higher prenatal PAH exposure, compared to those with a lower urinary PAH concentration. It might be interesting to correlate airborne levels of PAH to urinary PAH levels in the pregnant women. Unfortunately, there are no data on airborne PAH levels in the Lodz area.
Moreover, in a study by Miller et al., a significant association between PAH prenatal exposure and increased cough and wheezing at age of 12 months, and breathing problems and reports of probable asthma at age of 24 months have been shown.1 In a recently published study by Jedrychowski et al.21 the association between prenatal and postnatal exposure to airborne PAH and severity of wheeze and recurrent wheeze has been found. The authors conclude that prenatal PAH may precipitate and intensify an early onset of wheezing symptoms in childhood, resulting from the postnatal exposure and suggest that success in reducing the incidence of respiratory diseases in children would depend on reducing both foetal and childhood exposure.21 However, in different research by Miller et al.22 the authors have not found an association between PAH metabolite levels and asthma or respiratory symptoms, but they have found a positive association between PAH concentrations and higher anti-mouse IgE and anti-cat IgE. This may be due to several ethnic differences in the levels of PAH concentrations in that research.
Multiple epidemiological studies have suggested that asthma risk is determined by early life exposure to pollutants and allergens, which can later modify lung function and T helper cells to allergic phenotype. A study performed in adults has demonstrated an association between PAH concentrations and decreased FEV1, increased asthma severity and suppression of T-cell function.23 What is more, the suppression of T-cells was also significantly associated with enhancement of the Th-2 phenotype that is linked to allergic asthma. Recurrent respiratory tract infections, especially associated with wheezing in young children, are often classified as asthma symptoms. Asthma is the most common chronic disease and its risk may be strongly influenced by prenatal events.24,25 High levels of PAH are thought to cause aberrant DNA methylation changes, leading to dysregulation of gene expression and perhaps asthma.24 In a follow-up FACES study performed in children 6–11 years of age the authors have also found a significant correlation between PAH exposure and increased wheeze among asthmatics.26 In our study, we did not assess asthma symptoms; however, we found higher prevalence of respiratory tract infections in the children exposed to PAH, which can be a predictor of later asthma.
The second interesting finding in our study is the association between high PAH metabolites concentrations measured in infant's urine (by the age of 1 or 2 years) and an increased risk of food allergy. There is no evidence in the literature about this association. Brauer et al.27 observed in their study a positive association between other traffic-related air pollutants and sensitisation to food allergy at the age of two years. However, the assessments were not performed separately for PAH exposure and there are no data about mothers’ exposure to PAH during pregnancy. In our study, we showed that higher 1-HP concentrations in children's urine increased the risk of food allergy in children during the two first years of life. Studies have shown that for some respiratory symptoms (such as barking cough, wheezing, sore throat, ear infections) the effect of PAH exposure is partly modified by simultaneous exposure to ETS. The study by Miller et al. indicated that prenatal PAH exposure in the presence of postnatal environmental tobacco smoke (ETS) exposure was associated with increased cough and wheeze at 12 months of age (PAH x ETS interaction OR=1.4; p<0.01 and OR=1.3; p<0.05, respectively) and breathing problems, and reports of probable asthma at two years of age (PAH×ETS interaction OR=1.5 and OR=1.6; p<0.05, respectively) (1). A study by Rosa et al. has demonstrated that combined prenatal exposure to PAH and ETS was associated with asthma but not atopy at the age 5–6.3 The National Health and Nutrition Examination Survey (NHANES) has shown that exposure to polycyclic aromatic hydrocarbons has been associated with allergic sensitisation and asthma in children.28
In our study we did not find a significant association between PAH levels in maternal urine and wheezing and atopic dermatitis in children.
The main limitation of the present study is the fact that we were not able to clearly distinguish the effect of prenatal from postnatal PAH and whether prenatal exposure to PAHs would explain the findings versus increased PAH exposure postnatally. Taking into account the child's age (1 or 2 years), neither skin prick testing nor pulmonary function testing were performed. However, the children from REPRO_PL cohort are currently followed up at the age of seven with these tests included in the child health status examination. This will allow for a better (more objective) outcome evaluation in future assessments. Another limitation is that the current analysis was restricted to 455 mothers recruited from the general population, and they were not compared with mothers not in the study to assess the level of bias in the sample.
Our data support the hypothesis that early childhood exposure to air pollutants such as polycyclic aromatic hydrocarbons determines respiratory conditions, and is linked to allergic diseases. However, the underlying mechanisms still need further exploration.
The strength of our study is the use of a urinary biomarker of PAH exposure in mothers and their offspring, as this method has been shown to have a good reproducibility. In our study, we showed that higher urine concentrations of 1-HP in mothers at 20–24 weeks of pregnancy increased the risk of more frequent respiratory infections and food allergy in children during their first year of life. Our study suggests awareness of environmental factors, which may affect children's health since PAH were shown to be risk factors for airway infections and food allergy in children after adjustment for other risk factors. For pregnant women living in highly developed environments, like ours, with very high exposure to PAH, controlling the environment may be necessary. Also, other studies are needed to assign a critical value in urine for harmful PAH effect.
Authors’ contributionsDaniela Podlecka – Literature search; Kinga Polańska – Data collection; Iwona Stelmach – Study design; Wlodzimierz Stelmach – Analysis of data; Joanna Jerzynska – Manuscript preparation; Review of manuscript – Wojciech Hanke.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestAll authors state no conflict of interest.
The study was supported by the DEC-2014/15/B/NZ7/00998 grant from the National Science Centre.