Background. Patient race and ethnicity have historically impacted HCV treatment response. This phase 3 study evaluated daclatasvir with peginterferon-alfa-2a/ribavirin (pegIFN alfa-2a/RBV) in treatment-naive black/African American (AA), Latino, and white non-Latino patients with chronic HCV genotype 1 infection.
Material and methods. In this single-arm, open-label study, 246 patients received daclatasvir plus pegIFN alfa-2a and weight-based RBV. Patients with an extended rapid virologic response (eRVR; undetectable HCV-RNA at treatment weeks 4 and 12) received 24 weeks of treatment; those without eRVR received an additional 24 weeks of treatment with pegIFN alfa-2a/RBV. The primary endpoint was sustained virologic response at post-treatment week 12 (SVR12; HCV-RNA < 25 IU/mL) compared with the cohort historical rate.
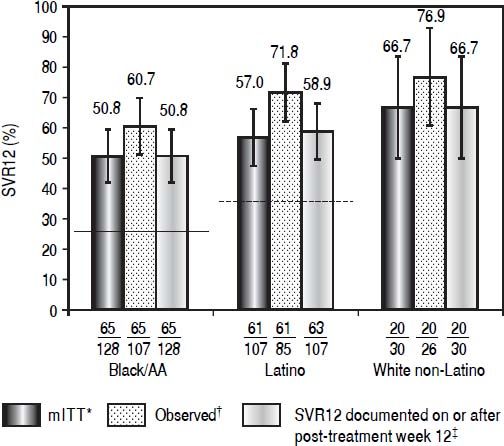
Results. Most patients were IL28B non-CC (84.4% black/AA; 77.6% Latino) genotype 1a-infected (72.7%; 81.3%), with HCV-RNA ≥ 800,000 IU/mL (81.3%; 64.5%). SVR12 rates were 50.8% (65/128; 95% confidence interval [CI], 42.1-59.4) for black/AA and 58.9% (63/107; 95% CI, 49.6-68.2) for Latino patients. The majority (55.5%; 58.9%) received 24 weeks treatment; rapid reductions (> 4-log10) in HCV-RNA levels were observed. Only 60.9% (78/128) of black/AA and 63.6% (68/107) of Latino patients completed treatment. On-treatment serious adverse events (SAEs) occurred in 21 patients. Discontinuations due to adverse events (aEs) occurred in 9 black/AA and 6 Latino patients.
Conclusion. SVR12 rates for black/AA (50.8%) and Latino (58.9%) cohorts treated with daclatasvir plus pegIFN alfa-2a/RBV and the lower bound of the 95% Cls were higher than the estimated historical control (black/AA, 26% SVR; Latino, 36% SVR) treated with pegIFN alfa-2a/RBV. These data support daclatasvir use in all-oral direct-acting antiviral combinations.
Up to 150 million people worldwide are chronically infected with hepatitis C virus (HCV), resulting in up to 500,000 deaths per year.1 HCV is a common cause of chronic progressive liver disease, leading to cirrhosis, hepatocellular carcinoma (HCC), liver transplant, and death.2 Seven major genotypes have been identified.3 Genotype 1 is the most prevalent worldwide, comprising 46.2% of all infections-subtype 1a is predominant in the United States; 1b is more prevalent in Europe and Asia.4 Sustained virologic response (SVR) rates have increased from 40%-50% with peginterferon-alfa and ribavirin (pegIFN/RBV) alone5,6 to up to 90% in combination with sofosbuvir7 in treatment-naive patients. Currently approved all-oral regimens provide SVR rates in treatment-naive patients of 90%-100%.8–11
Race and ethnicity have been shown to affect response to these interferon-based regimens; black/African American (AA) and Latino HCV-infected patients represent some of the more difficult-to-treat populations with historically low SVR rates. An SVR rate of 26% was observed among 200 black/AA patients treated with pegIFN alfa-2a/RBV in the IDEAL trial, which examined treatment response in major racial and ethnic groups.12 An averag SVR rate of36% was observed among non-black Latino patients treated with pegIFN alfa-2a/RBV across the IDEAL trial (66 patients) and a separate multicenter, prospective study (269 patients).12,13 Other factors common in these populations, including metabolic syndrome and insulin resistance, are thought to impact SVR with pegIFN/RBV treatment.14,15
Daclatasvir is a potent, pan-genotypic inhibitor of the HCV NS5A protein16,17 that has demonstrated efficacy and safety in combination with pegIFN/RBV18,19 and other direct-acting antivirals (DAAs).20 Daclatasvir has been well tolerated in more than 13,000 patients.9,20–24 Daclatasvir is currently approved in Europe and recommended for use in combination with other antivirals,25 and is approved in Japan with asunaprevir as part of an all-oral, RBV-free regimen for genotype 1 HCV infection. Daclatasvir is also approved in the US, indicated in combination with sofosbuvir, with or without ribavirin, for the treatment of chronic HCV genotype 1 or 3 infection.26 This phase 3 study was designed to evaluate the efficacy and safety of daclatasvir plus pegIFN alfa-2a/RBV in cohorts of black/AA, Latino, and white non-Latino HCV genotype 1-infected patients.
Material and MethodsStudy design and patientsThis was a phase 3, single-arm, open-label study (NCT01389323; COMMAND-US) evaluating daclatasvir plus pegIFN alfa-2a/RBV in black/AA, Latino, and white non-Latino patients with chronic HCV genotype 1 infection; the white non-Latino group served as a bridging cohort for comparison of primary results with other daclatasvir phase 3 studies. Patients were enrolled at 33 sites in the United States and Puerto Rico from September 2011 to April 2014 (Supplementary figure 1). All authors had access to the study data and reviewed and approved the final manuscript. Daclatasvir 60 mg was administered once daily in combination with pegIFN alfa-2a 180 μg once weekly and weight-based RBV 1,000 mg/day (< 75 kg) or 1,200 mg/day (≥ 75 kg) divided twice daily. Treatment duration was response guided: patients achieving extended rapid virologic response (eRVR; undetectable HCV-RNA at treatment weeks 4 and 12) received 24 weeks of treatment and were followed for 48 weeks posttreatment. Patients not achieving eRVR received an additional 24 weeks of treatment with pegIFN alfa-2a/RBV and were followed for 24 weeks post-treatment.
The study included men and women aged ≥ 18 years, with body mass index (BMI) ≥ 18 kg/m2 to ≤ 35 kg/m2, who were chronically infected with HCV genotype 1, with HCV-RNA ≥ 10,000 IU/mL. Patients self-reported non-mutually exclusive race and ethnicity into black/AA and Latino cohorts, as defined by the Food and Drug Administration Guidance for Industry.27 Compensated cirrhotics (liver biopsy) were eligible, capped at 25% of the study population. Women of child-bearing potential were required to use effective contraception. Key exclusion criteria included previous exposure to interferonbased regimens, RBV, or HCV DAAs; HBV/HIV coinfection; evidence of hepatic decompensation or HCC; alanine aminotransferase ≥ 5 x upper limit of normal or absolute neutrophil count ≤ 1.5 x 109 cells/L (≤ 1.2 x 109 cells/L for black/AA patients).
Patients discontinued study medication for virologic breakthrough (> 1-log10 increase in HCV-RNA levels over nadir or confirmed HCV-RNA levels at or higher than the lower limit of quantitation [LLOQ] after undetectable HCV-RNA levels while on-treatment, beginning at week 2), HCV-RNA > 1,000 IU/mL at treatment week 12, or HCV-RNA ≥ LLOQ at treatment week 24.
The protocol was approved by the Institutional Review Board/Independent Ethics Committee at each site. All patients provided informed written consent prior to study enrollment.
Assessments and endpointsHCV-RNA was quantified using the COBAS Taq-Man HCV test v2.0 (Roche Molecular Systems, Pleas-anton, CA) with an LLOQ of 25 IU/mL and a limit of detection of ≈10 IU/mL. HCV genotype/subtype was determined by the VERSANT HCV genotype 2.0 line probe assay (Siemens, Munich, Germany). IL28B genotype (rs12979860 single nucleotide polymorphism) was determined using the Applied BioSystems TaqMan assay (Life Technologies, Carlsbad, CA). Resistance analyses were performed using population-based sequencing of HCV NS5A on available plasma samples for all patients receiving daclatasvir with HCV-RNA ≥ 1,000 IU/mL. This included baseline samples and samples from patients who experienced virologic failure (on-treatment virologic breakthrough, HCV-RNA > 1,000 IU/mL at week 12, HCV-RNA ≥ LLOQ at week 24, detectable HCV-RNA levels at end of treatment [EOT], and relapse).
The primary endpoint was SVR12 (HCV-RNA < 25 IU/mL, detectable or undetectable at post-treatment week 12) rate compared with the cohort historical rate (black/AA, 26% SVR; Latino, 36% SVR) for all treated patients. Because no comparator group was used in this study due to clinical equipoise, response rates were compared with those historically reported in these cohorts receiving standard-of-care treatment at the time the trial commenced. Key secondary endpoints included the proportions of patients with undetectable HCV-RNA at weeks 1, 2, 4, 6, 8, and 12; at weeks 4 and 12; at EOT; and at follow-up week 24. Further endpoints included safety as measured by serious adverse events (SAEs), adverse events (AEs) leading to discontinuation, and the relationship of efficacy and IL28B genotype.
Statistical analysisThe statistical methods of this study were reviewed by the biometrics group at Bristol-Myers Squibb. For the primary analysis for each cohort, the SVR12 rate for daclatasvir plus pegIFN alfa-2a/RBV was inferred to be greater than the historical rate for pegIFN alfa-2a/RBV if the lower bound of the 2-sided 95% confidence interval (CI) was greater than the estimated historical rate for pegIFN alfa-2a/RBV. Based on 95% CI of a sample size of 100 patients in each cohort, if the true SVR12 rate was ≥ 36% for black/AA patients, the lower bound of the 95% CI would exceed 26%; if the true SVR12 rate was ≥ 47% for Latino patients, the lower bound of the 95% CI would exceed 36%. The study was not powered to compare the daclatasvir plus pegIFN alfa-2a/RBV rate of SVR12 with the historical rate. Binary antiviral efficacy endpoints and 2-sided 95% CI were calculated using a modified intent-to-treat (mITT; all treated subjects) analysis. For the primary endpoint, analyses based on observed values (available measurement at follow-up week 12) and SVR documented on or after post-treatment week 12 (missing data at post-treatment week 12 but SVR confirmed at subsequent visit) were also performed. Safety analyses included all patients who received ≥ 1 dose of study medication.
ResultsPatient dispositionOf 448 patients screened, 246 were treated; the main reason for not entering treatment was failure to meet study criteria (n = 156, [34.8%]). Treatment completion by cohort was 60.9% (78/128) black/AA, 63.6% (68/107) Latino, and 63.3% (19/30) white non-Latino. The majority of treatment discontinuations were due to lack of efficacy (n = 41, 16.6%) or AEs (n = 24, 9.8%). By cohort, 29 (22.7%) black/AA and 15 (14.0%) Latino patients discontinued due to lack of efficacy. Nine (7.0%) black/AA and 6 (5.6%) Latino patients discontinued due to AEs. Twelve (9.4%) black/AA and 18 (16.8%) Latino patients discontinued for other reasons, including patient request, withdrawal of consent, loss to follow-up, poor compliance/noncompliance, or no longer meeting study criteria. As a result of these discontinuations, 52.3% (67/128) of black/AA and 52.4% (56/107) of Latino patients completed > 90.0% of the planned treatment duration and received > 90.0% of the target daily/weekly dose.
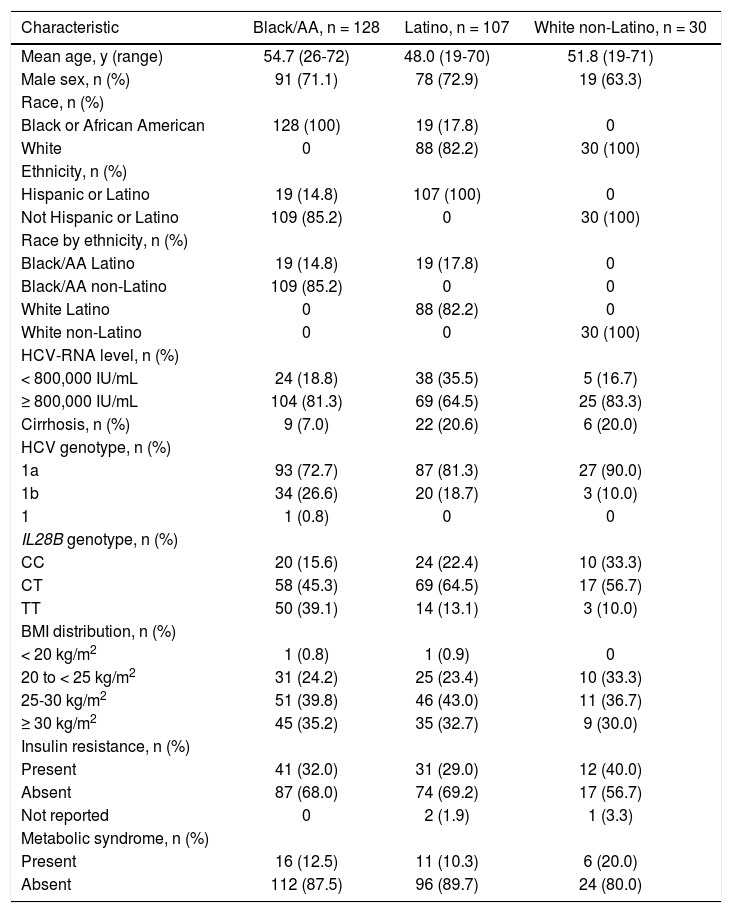
Baseline characteristicsBaseline characteristics are presented in table 1. A larger proportion of patients were infected with HCV genotype 1a (72.7% black/AA; 81.3% Latino; 90.0% white non-Latino) than genotype 1b (26.6%; 18.7%; 10.0%, respectively). A small proportion of patients were IL28B CC (15.6% black/AA; 22.4% Latino; 33.3% white non-Latino), IL28B TT was more common among black/AA patients (39.1%) than Latino patients (13.1%), and notably few black/AA patients had cirrhosis (n = 9, 7.0%). Overall, the black/AA and Latino cohorts included 19 patients who self-reported for both cohorts due to non-mutually exclusive race and ethnicity inclusion criteria.
Baseline demographics and disease characteristics.
| Characteristic | Black/AA, n = 128 | Latino, n = 107 | White non-Latino, n = 30 |
|---|---|---|---|
| Mean age, y (range) | 54.7 (26-72) | 48.0 (19-70) | 51.8 (19-71) |
| Male sex, n (%) | 91 (71.1) | 78 (72.9) | 19 (63.3) |
| Race, n (%) | |||
| Black or African American | 128 (100) | 19 (17.8) | 0 |
| White | 0 | 88 (82.2) | 30 (100) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 19 (14.8) | 107 (100) | 0 |
| Not Hispanic or Latino | 109 (85.2) | 0 | 30 (100) |
| Race by ethnicity, n (%) | |||
| Black/AA Latino | 19 (14.8) | 19 (17.8) | 0 |
| Black/AA non-Latino | 109 (85.2) | 0 | 0 |
| White Latino | 0 | 88 (82.2) | 0 |
| White non-Latino | 0 | 0 | 30 (100) |
| HCV-RNA level, n (%) | |||
| < 800,000 IU/mL | 24 (18.8) | 38 (35.5) | 5 (16.7) |
| ≥ 800,000 IU/mL | 104 (81.3) | 69 (64.5) | 25 (83.3) |
| Cirrhosis, n (%) | 9 (7.0) | 22 (20.6) | 6 (20.0) |
| HCV genotype, n (%) | |||
| 1a | 93 (72.7) | 87 (81.3) | 27 (90.0) |
| 1b | 34 (26.6) | 20 (18.7) | 3 (10.0) |
| 1 | 1 (0.8) | 0 | 0 |
| IL28B genotype, n (%) | |||
| CC | 20 (15.6) | 24 (22.4) | 10 (33.3) |
| CT | 58 (45.3) | 69 (64.5) | 17 (56.7) |
| TT | 50 (39.1) | 14 (13.1) | 3 (10.0) |
| BMI distribution, n (%) | |||
| < 20 kg/m2 | 1 (0.8) | 1 (0.9) | 0 |
| 20 to < 25 kg/m2 | 31 (24.2) | 25 (23.4) | 10 (33.3) |
| 25-30 kg/m2 | 51 (39.8) | 46 (43.0) | 11 (36.7) |
| ≥ 30 kg/m2 | 45 (35.2) | 35 (32.7) | 9 (30.0) |
| Insulin resistance, n (%) | |||
| Present | 41 (32.0) | 31 (29.0) | 12 (40.0) |
| Absent | 87 (68.0) | 74 (69.2) | 17 (56.7) |
| Not reported | 0 | 2 (1.9) | 1 (3.3) |
| Metabolic syndrome, n (%) | |||
| Present | 16 (12.5) | 11 (10.3) | 6 (20.0) |
| Absent | 112 (87.5) | 96 (89.7) | 24 (80.0) |
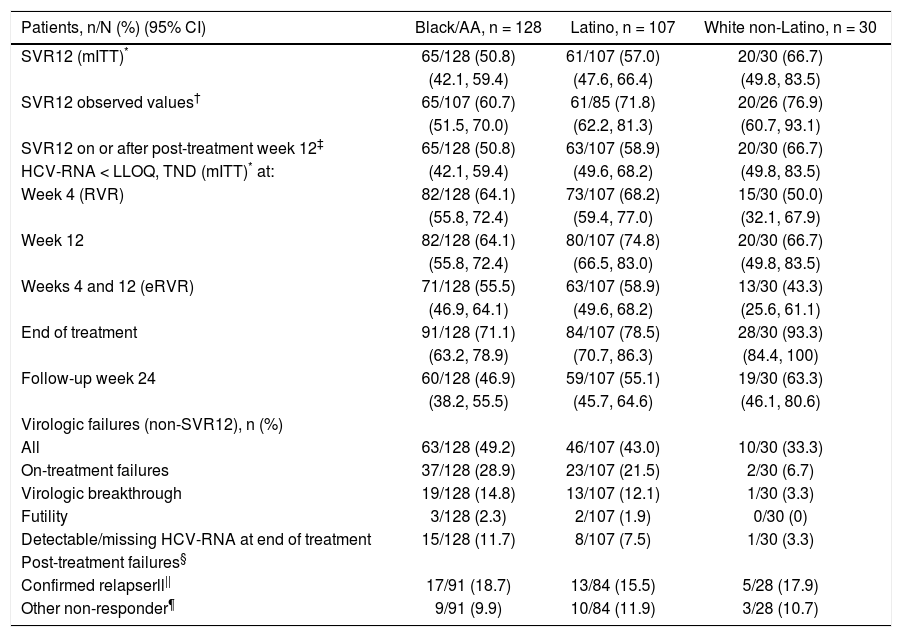
Daclatasvir plus pegIFN alfa-2a/RBV resulted in an SVR12 (mITT) rate of 50.8% (65/128; 95% CI: 42.1%-59.4%) among black/AA patients, 57.0% (61/107, 95% CI: 47.6%-66.4%) among Latino, and 66.7% (20/30, 95% CI: 49.8%-83.5%) among white non-Latino patients (Figure 1; Table 2). Observed SVR12 (available measurement at follow-up week 12) was achieved in 60.7% (65/107; 95% CI: 51.5%-70.0%) black/AA, 71.8% (61/85; 95% CI: 62.2%-81.3%) Latino, and 76.9% (20/26; 95% CI: 60.7%-93.1%) white non-Latino patients. SVR12 rates documented on or after post-treatment week 12 were identical to those in the mITT analysis for both black/AA and white non-Latino cohorts but increased in Latino patients to 58.9% (63/107; 95% CI: 49.6%-68.2%). There was a rapid and persistent reduction in HCV-RNA levels (> 4-log10) across the black/AA and Latino cohorts (Figure 2).
Patients with SVR12, according to cohort. Historical rate of SVR for the black/AA cohort (26% [solid line]) and Latino cohort (36% [dashed line]). * Patients with missing data at post-treatment week 12 were considered failures. † Patients with available data at post-treatment week 12. ‡ Patients with missing data at post-treatment week 12 were considered responders if the next available HCV-RNA value was < LLOQ.
Virologic response.
| Patients, n/N (%) (95% CI) | Black/AA, n = 128 | Latino, n = 107 | White non-Latino, n = 30 |
|---|---|---|---|
| SVR12 (mITT)* | 65/128 (50.8) | 61/107 (57.0) | 20/30 (66.7) |
| (42.1, 59.4) | (47.6, 66.4) | (49.8, 83.5) | |
| SVR12 observed values† | 65/107 (60.7) | 61/85 (71.8) | 20/26 (76.9) |
| (51.5, 70.0) | (62.2, 81.3) | (60.7, 93.1) | |
| SVR12 on or after post-treatment week 12‡ | 65/128 (50.8) | 63/107 (58.9) | 20/30 (66.7) |
| HCV-RNA < LLOQ, TND (mITT)* at: | (42.1, 59.4) | (49.6, 68.2) | (49.8, 83.5) |
| Week 4 (RVR) | 82/128 (64.1) | 73/107 (68.2) | 15/30 (50.0) |
| (55.8, 72.4) | (59.4, 77.0) | (32.1, 67.9) | |
| Week 12 | 82/128 (64.1) | 80/107 (74.8) | 20/30 (66.7) |
| (55.8, 72.4) | (66.5, 83.0) | (49.8, 83.5) | |
| Weeks 4 and 12 (eRVR) | 71/128 (55.5) | 63/107 (58.9) | 13/30 (43.3) |
| (46.9, 64.1) | (49.6, 68.2) | (25.6, 61.1) | |
| End of treatment | 91/128 (71.1) | 84/107 (78.5) | 28/30 (93.3) |
| (63.2, 78.9) | (70.7, 86.3) | (84.4, 100) | |
| Follow-up week 24 | 60/128 (46.9) | 59/107 (55.1) | 19/30 (63.3) |
| (38.2, 55.5) | (45.7, 64.6) | (46.1, 80.6) | |
| Virologic failures (non-SVR12), n (%) | |||
| All | 63/128 (49.2) | 46/107 (43.0) | 10/30 (33.3) |
| On-treatment failures | 37/128 (28.9) | 23/107 (21.5) | 2/30 (6.7) |
| Virologic breakthrough | 19/128 (14.8) | 13/107 (12.1) | 1/30 (3.3) |
| Futility | 3/128 (2.3) | 2/107 (1.9) | 0/30 (0) |
| Detectable/missing HCV-RNA at end of treatment | 15/128 (11.7) | 8/107 (7.5) | 1/30 (3.3) |
| Post-treatment failures§ | |||
| Confirmed relapserll|| | 17/91 (18.7) | 13/84 (15.5) | 5/28 (17.9) |
| Other non-responder¶ | 9/91 (9.9) | 10/84 (11.9) | 3/28 (10.7) |
TND: target not detected.
For patients with missing post-treatment week 12 HCV-RNA levels, the first available measurement after post-treatment week 12 was used.
Response rates (SVR12, mITT) categorized according to ethnicity for black/AA and Latino cohorts, respectively, were 52.6% (10/19, 95% CI: 30.2%-75.1%) of black/AA Latino patients, 50.5% (55/109, 95% CI: 41.1%-59.8%) of black/AA non-Latino patients, and 58.0% (51/88, 95% CI: 47.6%-68.3%) of white Latino patients.
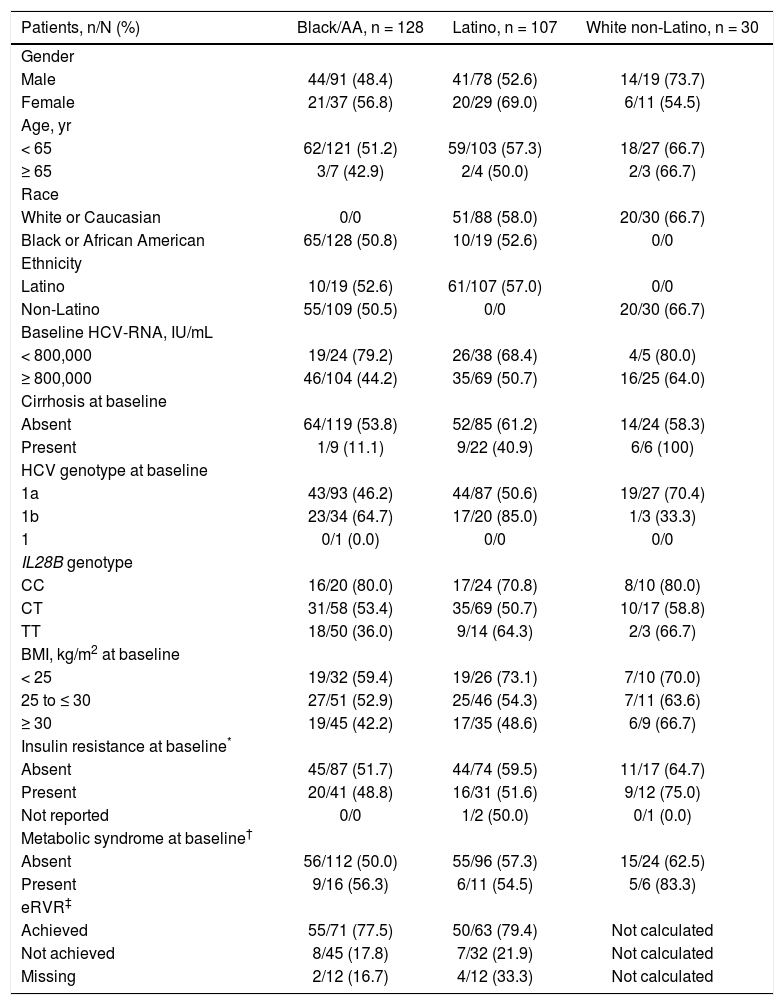
Patients with the IL28B CC genotype had higher SVR12 rates (80.0% black/AA, 70.8% Latino, and 80.0% white nonLatino) compared with CT (53.4%, 50.7%, and 58.8%, respectively) and TT patients (36.0%, 64.3%, and 66.7%, respectively) (Table 3). There was a higher rate of SVR12 in patients who achieved eRVR (24-week treatment duration) vs. those that did not in both the black/AA (77.5% [55/71] vs. 17.8% [8/45]) and Latino (79.4% [50/63] vs. 21.9% [7/32]) cohorts. Furthermore, HCV genotype 1a infection and high baseline HCV-RNA levels were associated with lower rates of SVR; in contrast, metabolic syndrome did not have a clinically significant impact on SVR. Lower SVR12 rates were also observed in patients with a BMI of 25 to ≤ 30 kg/m2 (52.9% black/AA, 54.3% Latino, and 63.6% white non-Latino) or ≥ 30 kg/m2 (42.2% black/AA, 48.6% Latino, and 66.7% white non-Latino) vs. patients with a BMI of < 25 kg/m2 (59.4%, 73.1%, and 70.0%, respectively); in contrast, clinically significant differences in SVR12 rates were not observed between patients with (48.8% black/AA, 51.6%, Latino, and 75.0% white non-Latino) or without (51.7% black/AA, 59.5% Latino, and 64.7% white non-Latino) insulin resistance at baseline (Table 3).
SVR12 (mITT) by subgroup.
| Patients, n/N (%) | Black/AA, n = 128 | Latino, n = 107 | White non-Latino, n = 30 |
|---|---|---|---|
| Gender | |||
| Male | 44/91 (48.4) | 41/78 (52.6) | 14/19 (73.7) |
| Female | 21/37 (56.8) | 20/29 (69.0) | 6/11 (54.5) |
| Age, yr | |||
| < 65 | 62/121 (51.2) | 59/103 (57.3) | 18/27 (66.7) |
| ≥ 65 | 3/7 (42.9) | 2/4 (50.0) | 2/3 (66.7) |
| Race | |||
| White or Caucasian | 0/0 | 51/88 (58.0) | 20/30 (66.7) |
| Black or African American | 65/128 (50.8) | 10/19 (52.6) | 0/0 |
| Ethnicity | |||
| Latino | 10/19 (52.6) | 61/107 (57.0) | 0/0 |
| Non-Latino | 55/109 (50.5) | 0/0 | 20/30 (66.7) |
| Baseline HCV-RNA, IU/mL | |||
| < 800,000 | 19/24 (79.2) | 26/38 (68.4) | 4/5 (80.0) |
| ≥ 800,000 | 46/104 (44.2) | 35/69 (50.7) | 16/25 (64.0) |
| Cirrhosis at baseline | |||
| Absent | 64/119 (53.8) | 52/85 (61.2) | 14/24 (58.3) |
| Present | 1/9 (11.1) | 9/22 (40.9) | 6/6 (100) |
| HCV genotype at baseline | |||
| 1a | 43/93 (46.2) | 44/87 (50.6) | 19/27 (70.4) |
| 1b | 23/34 (64.7) | 17/20 (85.0) | 1/3 (33.3) |
| 1 | 0/1 (0.0) | 0/0 | 0/0 |
| IL28B genotype | |||
| CC | 16/20 (80.0) | 17/24 (70.8) | 8/10 (80.0) |
| CT | 31/58 (53.4) | 35/69 (50.7) | 10/17 (58.8) |
| TT | 18/50 (36.0) | 9/14 (64.3) | 2/3 (66.7) |
| BMI, kg/m2 at baseline | |||
| < 25 | 19/32 (59.4) | 19/26 (73.1) | 7/10 (70.0) |
| 25 to ≤ 30 | 27/51 (52.9) | 25/46 (54.3) | 7/11 (63.6) |
| ≥ 30 | 19/45 (42.2) | 17/35 (48.6) | 6/9 (66.7) |
| Insulin resistance at baseline* | |||
| Absent | 45/87 (51.7) | 44/74 (59.5) | 11/17 (64.7) |
| Present | 20/41 (48.8) | 16/31 (51.6) | 9/12 (75.0) |
| Not reported | 0/0 | 1/2 (50.0) | 0/1 (0.0) |
| Metabolic syndrome at baseline† | |||
| Absent | 56/112 (50.0) | 55/96 (57.3) | 15/24 (62.5) |
| Present | 9/16 (56.3) | 6/11 (54.5) | 5/6 (83.3) |
| eRVR‡ | |||
| Achieved | 55/71 (77.5) | 50/63 (79.4) | Not calculated |
| Not achieved | 8/45 (17.8) | 7/32 (21.9) | Not calculated |
| Missing | 2/12 (16.7) | 4/12 (33.3) | Not calculated |
More than 3 of the following signs at baseline: systolic/diastolic blood pressure ≥ 130/85 mm Hg, fasting glucose ≥ 100 mg/dL, large waist circumference (men: ≥ 40 inches; women ≥ 35 inches), low fasting high-density lipoprotein cholesterol (men < 40 mg/dL; women < 50 mg/dL), fasting triglycerides ≥ 150 mg/dL.
SVR12 failure is summarized in table 2; the most common on-treatment failures were due to virologic breakthrough in 14.8% (19/128) of black/AA and 12.1% (13/107) of Latino patients. The majority of post-treatment failures were due to relapse in 18.7% (17/91) of black/AA and 15.5% (13/84) of Latino patients. A large proportion of failures (9.9% [9/91] black/AA; 11.9% [10/84] Latino) were due to missing data at post-treatment week 12.
ResistanceOf the 181 HCV genotype 1a-infected patients with available baseline NS5A sequences, 16 (8.8%) had NS5A polymorphisms at amino acid positions associated with daclatasvir resistance (M28V, Q30E/H/R, L31M, and/or Y93N); SVR12 was achieved in 25% (4/16) of these patients compared with 53.3% (88/165) of patients without polymorphisms. Among HCV genotype 1b-infected patients, 8.3% (4/48) had L31M, and/or Y93H polymorphisms; SVR12 was achieved in 50% (2/4) of these HCV genotype 1b-infected patients compared with 72.7% (32/44) patients without polymorphisms.
NS5A resistance-associated variants (RAVs) were generally detected at virologic failure. Emergent NS5A RAVs in HCV genotype 1a- and genotype 1b-infected patients were similar to those reported previously in clinical studies. Genotype 1a substitutions at Q30 emerged in the majority of patients experiencing virologic failure (74.6% [50/67]) and were frequently detected together with substitutions at M28 and/or substitutions at L31 (40.0% [20/50]) or 42.0% [21/50], respectively). Genotype 1b substitutions at L31 and/or Y93 emerged in all patients with failure (100% [9/9]); 77.8% (7/9) of virologic failures had both L31 and Y93 variants emerge together.
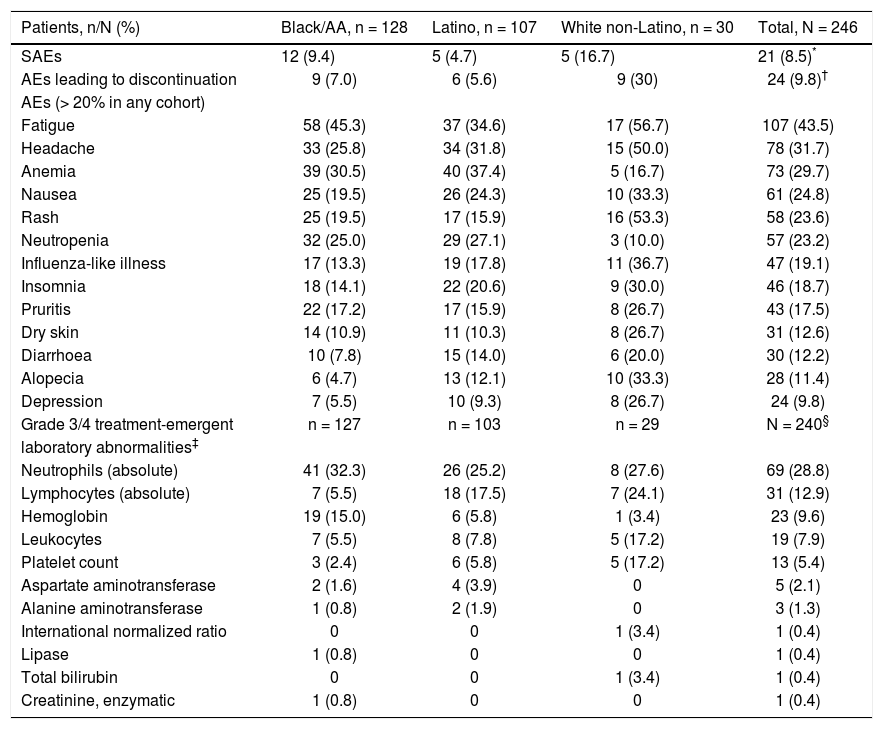
Safety and tolerabilityIn this study, daclatasvir plus pegIFN alfa-2a/RBV had a safety and tolerability profile consistent with pegIFN/ RBV-containing regimens. Twenty-one patients (8.5%) experienced on-treatment SAEs (Table 4), including 12 (9.4%) black/AA patients (including 3 treatment-related events of anemia), 5 (4.7%) Latino patients (4 treatment-related: 2 chest pain, 1 headache, and 1 syncope), and 5 (16.7%) white non-Latino patients (including 1 treatment-related opioid abuse). Treatment discontinuation due to AEs occurred in 24 patients (9.8%), which by cohort included 9/128 (7.0%) black/AA, 6/107 (5.6%) Latino, and 9/30 (30.0%) white non-Latino patients. The most frequently reported on-treatment AEs and grade 3/4 laboratory abnormalities were those typically associated with pegIFN/RBV therapy, including flu-like symptoms (fatigue and headache), anemia, and hematologic abnormalities (Table 4).28,29
Safety summary-treated subjects.
| Patients, n/N (%) | Black/AA, n = 128 | Latino, n = 107 | White non-Latino, n = 30 | Total, N = 246 |
|---|---|---|---|---|
| SAEs | 12 (9.4) | 5 (4.7) | 5 (16.7) | 21 (8.5)* |
| AEs leading to discontinuation | 9 (7.0) | 6 (5.6) | 9 (30) | 24 (9.8)† |
| AEs (> 20% in any cohort) | ||||
| Fatigue | 58 (45.3) | 37 (34.6) | 17 (56.7) | 107 (43.5) |
| Headache | 33 (25.8) | 34 (31.8) | 15 (50.0) | 78 (31.7) |
| Anemia | 39 (30.5) | 40 (37.4) | 5 (16.7) | 73 (29.7) |
| Nausea | 25 (19.5) | 26 (24.3) | 10 (33.3) | 61 (24.8) |
| Rash | 25 (19.5) | 17 (15.9) | 16 (53.3) | 58 (23.6) |
| Neutropenia | 32 (25.0) | 29 (27.1) | 3 (10.0) | 57 (23.2) |
| Influenza-like illness | 17 (13.3) | 19 (17.8) | 11 (36.7) | 47 (19.1) |
| Insomnia | 18 (14.1) | 22 (20.6) | 9 (30.0) | 46 (18.7) |
| Pruritis | 22 (17.2) | 17 (15.9) | 8 (26.7) | 43 (17.5) |
| Dry skin | 14 (10.9) | 11 (10.3) | 8 (26.7) | 31 (12.6) |
| Diarrhoea | 10 (7.8) | 15 (14.0) | 6 (20.0) | 30 (12.2) |
| Alopecia | 6 (4.7) | 13 (12.1) | 10 (33.3) | 28 (11.4) |
| Depression | 7 (5.5) | 10 (9.3) | 8 (26.7) | 24 (9.8) |
| Grade 3/4 treatment-emergent | n = 127 | n = 103 | n = 29 | N = 240§ |
| laboratory abnormalities‡ | ||||
| Neutrophils (absolute) | 41 (32.3) | 26 (25.2) | 8 (27.6) | 69 (28.8) |
| Lymphocytes (absolute) | 7 (5.5) | 18 (17.5) | 7 (24.1) | 31 (12.9) |
| Hemoglobin | 19 (15.0) | 6 (5.8) | 1 (3.4) | 23 (9.6) |
| Leukocytes | 7 (5.5) | 8 (7.8) | 5 (17.2) | 19 (7.9) |
| Platelet count | 3 (2.4) | 6 (5.8) | 5 (17.2) | 13 (5.4) |
| Aspartate aminotransferase | 2 (1.6) | 4 (3.9) | 0 | 5 (2.1) |
| Alanine aminotransferase | 1 (0.8) | 2 (1.9) | 0 | 3 (1.3) |
| International normalized ratio | 0 | 0 | 1 (3.4) | 1 (0.4) |
| Lipase | 1 (0.8) | 0 | 0 | 1 (0.4) |
| Total bilirubin | 0 | 0 | 1 (3.4) | 1 (0.4) |
| Creatinine, enzymatic | 1 (0.8) | 0 | 0 | 1 (0.4) |
Abdominal pain (1), alopecia (1), anemia (2), arthralgia (1), cerebral infarction (1), cerebrovascular accident (1), chest pain (1), depression (2), diarrhea (1), fatigue (2), headache (2), homicidal ideation (1), influenza-like illness (2), insomnia (1), intracranial aneurysm (1), lymphadenopathy (1), malaise (1), muscular weakness (2), nausea (2), neutropenia (1), pain in extremity (1), palpitations (1), paresthesia (1), pneumonia (1), pruritus (1), pyrexia (1), rash (3), maculopapular rash (1), pruritic rash (1), weight decrease (1).
This phase 3 study evaluated the efficacy and safety of daclatasvir plus pegIFN alfa-2a/RBV in black/AA and Latino patients. These cohorts represent a patient population less likely to achieve SVR with historical standard-of-care pegIFN/RBV therapies. These results demonstrated that the addition of daclatasvir to pegIFN alfa-2a/RBV therapy resulted in higher SVR12 rates (50.8% [95% CI: 42.1%-59.4%] in black/AA patients and 58.9% [95% CI: 49.6%-68.2% in Latino patients) compared with the respective estimated historical rates of 26% and 36% with pegIFN alfa-2a/RBV. These conservative historical control SVR rates are equivalent to or higher than those of other studies for HCV genotype 1-infected black/AA or Hispanic cohorts treated with pegIFN/RBV30–35 and allowed for a matched comparison of interferon type (pegIFN alfa-2a) and dosing; the use of pegIFN alfa-2b and varied dosing strategies often complicate like-for-like race and ethnicity historical comparisons. Daclatasvir plus pegIFN alfa-2a/RBV was relatively well tolerated, with low rates of SAEs and discontinuations due to AEs; the safety profile was consistent with that of historical pegIFN/RBV treatment, and no unique safety signals were observed with daclatasvir.
The rapid reductions in HCV-RNA levels (> 4-log10) observed in this study highlight the potency of daclatasvir. The majority of patients achieved eRVR and so were eligible for a treatment duration of 24 weeks. The rate of SVR12 (mITT) in such patients (black/AA: 77.5%; Latino: 79.4%) compare to a SVR12 rate of 88% for patients treated with simeprevir plus pegIFN alfa-2a/pegIFN alfa-2b plus RBV—also for 24 weeks.36 In contrast to the present study, the SVR12 data from the pooled analysis for the simeprevir plus pegIFN/RBV trials included only 7% black/AA and 17% Hispanic patients and comprised 29% IL28B CC patients compared with 15.6% black/AA IL28B CC and 22.4% Latino IL28B CC patients presented here. These factors combined could contribute to a between-study difference in SVR.
All-oral DAA regimens have shown high response rates irrespective of race or ethnicity in treatment-naive HCV genotype 1-infected patients. Black and Hispanic patients treated with ombitasvir/paritaprevir/ritonavir and dasabuvir for 12 weeks achieved SVR12 rates of 96.4% and 96.2%, respectively.11 SVR12 rates in ledipasvir/sofosbuvir-treated black (93.5%-100%) and Hispanic/Latino patients (100%) were equally high following 12 or 24 weeks of treatment; however, response rates did not consider patients lost to follow-up.10 Similarly, SVR12 rates achieved in black patients (93%) following 12 weeks of treatment with a fixed-dose combination of daclatasvir, asunaprevir, and beclabuvir were comparable to white patients (91%).37 Of note, these populations are small (< 15% patients) in the context of phase 3 studies, highlighting the need for further studies evaluating safety and efficacy in these patient groups.
The lower response rates observed in this study, relative to the current context of HCV therapies, reflect in part the high study attrition rate, unrelated to lack of efficacy or safety, and the predominance of unfavorable baseline factors that still may impact single DAAs combined with pegIFN/RBV-based therapies, including HCV genotype 1a, high baseline HCV-RNA levels, and IL28B nonCC genotypes. Thus, in the observed analysis, the SVR12 rates of 60.7% in black/AA and 71.8% in Latino patients who completed therapy may be better indicators of regimen efficacy.
Baseline factors (HCV genotype 1a, high baseline HCV-RNA levels, IL28B non-CC genotype) appeared to affect response rates more than the pre-existence of NS5A polymorphisms at amino acid positions associated with daclatasvir resistance. The frequency of virologic failure was high in patients with IL28B non-CC genotypes, irrespective of the presence or absence of noted NS5A polymorphisms. Although SVR rates in patients without NS5A polymorphisms at L28, R30, L31, and/or Y93 were higher than in patients with these NS5A polymorphisms, their prevalence was low (≤ 10%). Though IL28B genotype appeared to impact efficacy despite the addition of da-clatasvir to pegIFN alfa-2a/RBV therapy, the presence of metabolic syndrome and fasting glucose levels ≥ 100 mg/dL at baseline had no clinically significant impact on SVR12. In contrast, higher SVR12 rates were observed in patients with a BMI of < 25 kg/m2 versus patients with a BMI of 25 to < 30 kg/m2 or ≥ 30 kg/m2; these results are in agreement with a previous study of genotype 1-infected patients treated with pegIFN alfa-2b/RBV therapy.38
Study limitations include the low percentage (7%) of black/AA cirrhotic patients. This may have increased the rate of SVR observed for this population, although the low proportion may represent the historically slower progression of this cohort toward cirrhosis.39 Despite this, the higher SVR12 rates in this study compared with response rates for other pegIFN/RBV studies not included in the historical analysis demonstrates the added benefit of daclatasvir for HCV genotype 1-infected patients. Moreover, patients used for the Latino historical control data were exclusively non-black/AA.12,13 Thus, SVR rates in this study may be under-representative vs. a cohort of Latinos containing black/AA patients, known for less favorable responses to pegIFN/RBV therapy.
In summary, the SVR12 rates for the black/AA (50.8%) and Latino (58.9%) cohorts treated with daclatasvir plus pegIFN alfa-2a/RBV were higher than the estimated historical control treated with pegIFN alfa-2a/RBV. Furthermore, patients from both cohorts who achieved eRVR showed higher SVR12 rates than those who did not achieve eRVR (black/AA: 77.5% vs. 17.8%; Latino: 79.4% vs. 21.9%). These data support the use of daclatasvir-contain-ing regimens for the treatment of chronic HCV genotype 1 infection in patient groups of varied ethnicities and with challenging-to-treat baseline characteristics.
Abbreviations- •
AA: African American.
- •
AE: adverse event.
- •
BMI: body mass index.
- •
CI: confidence interval.
- •
DAA: direct-acting antiviral.
- •
EOT: end of treatment.
- •
eRVR: extended rapid virologic response.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
LLOQ: lower limit of quantitation.
- •
mITT: modified intent to treat.
- •
pegIFN: peginterferon-alfa.
- •
RAVs: resistance associated variants.
- •
RBV: ribavirin.
- •
SAE: serious adverse event.
- •
SVR: sustained virologic response.
The study was sponsored by Bristol-Myers Squibb.
Financial DisclosuresM. Rodriguez-Torres has served as a consultant for Akros Pharmaceutical, Bristol-Myers Squibb, Genentech, F. Hoffmann-La Roche, Inhibitex, Janssen R&D Ireland, Merck, Pharmasset, Santaris Pharma A/S, and Vertex Pharmaceutical Inc; and reports grants or research support from Inhibitex, Johnson & Johnson, Merck, Mochida Pharmaceutical, Novartis, Pfizer, Pharmasset, Santaris Pharma A/S, Scynexis, Inc, Siemens Healthcare Diagnostics, Vertex Pharmaceutical Inc., Zymogenetics, Abbott Laboratories, Akros Pharmaceutical, Anadys Pharmaceutical, Beckman Coulter, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Gilead Pharmaceuticals, Glaxo SmithKline, F. Hoffmann-La Roche, Human Genome Sciences, Idenix Pharmaceutical, and Idera Pharmaceutical. E Lawitz reports grants from AbbVie, Achillion Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Idenix Pharmaceuticals, Janssen, Merck, Novartis, Presidio, Roche, Salix, Santaris Pharmaceuticals, Theravance, and Vertex Pharmaceuticals; and reports consulting for AbbVie, Achillion Pharmaceuticals, BioCryst, Biotica, Bristol-Myers Squibb, Enanta, Gilead Sciences, Idenix Pharmaceuticals, Janssen, Merck, Novartis, Santaris Pharmaceuticals, Regulus, Theravance, and Vertex Pharmaceuticals. V Rustgi reports grants from AbbVie, Gilead, and BMS; served as a speaker for Genetech, Gilead, and Janssen; served on advisory boards for AbbVie, Gilead, Janssen, and Merck; and served as a consultant for Vertex. S. Harrison is an Associate Editor for Hepatology, and has served as a consultant/advisor for AbbVie and Gilead. R Ghalib reports grants from AbbVie, Bristol-Myers Squibb, Evoke, Gilead, Idenix, Janssen, Merck, Pfizer, Salix, Takeda, Vertex, and Virochem. JM Vierling reports grants from AbbVie, Conatus, Genentech, Gilead, Idenix-Novartis, Merck, Novartis, and Roche; has received personal fees from Merck; and personal fees for advisory boards for AbbVie, Gilead, Janssen, Merck, Novartis, Roche, and Sundise. PJ Zamor reports research support from AbbVie, Bristol-Myers Squibb, Gilead, and Merck; and has served on a speakers bureau and advisory board for AbbVie and Janssen. TR Morgan reports grants from AbbVie, Bristol-Myers Squibb, Gilead, F. Hoffmann-La Roche, Merck, and Vertex. B Pearlman reports non-financial support from BMS, outside the submitted work. C O’Brien reports grants from AbbVie, Bristol-Myers Squibb, Gilead, and Janssen. N Pyrsopoulos reports research grants from AbbVie, Gilead, and Merck; and has participated in advisory boards for AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck. G Kong, F McPhee, PD Yin, E Hughes, and M Treitel are all employees of Bristol-Myers Squibb. SH Han, PJ Thuluvath, and N Ravendhran all report research grants from Bristol-Myers Squibb. B Yangco, L Jeffers, V Luketic, and H Khallafi have no conflicts of interest to disclose.
AcknowledgementsWith the sad news of the passing of Dr. Maribel Rodriguez-Torres in December 2015, we wish to thank and acknowledge her guidance and leadership in the development of this manuscript, and the tremendous contribution she made to the HCV community as a whole.
The study was designed and conducted by the sponsor (Bristol-Myers Squibb) in collaboration with the principal investigators. The sponsor collected the data, monitored study conduct, and performed statistical analyses. All authors had access to the study data and have reviewed and approved the final manuscript. This material is the result of work supported, in part, with resources and the use of facilities at the McGuire VA Medical Center, Richmond, VA. Technical appendix, statistical code, and dataset are available from Michelle Treitel (michelle.treitel@bms.com). Patients gave informed consent regarding the relevant use and sharing of key-coded data. Editorial support was provided by Ori Bowen of Articulate Science and funded by Bristol-Myers Squibb.








![Patients with SVR12, according to cohort. Historical rate of SVR for the black/AA cohort (26% [solid line]) and Latino cohort (36% [dashed line]). * Patients with missing data at post-treatment week 12 were considered failures. † Patients with available data at post-treatment week 12. ‡ Patients with missing data at post-treatment week 12 were considered responders if the next available HCV-RNA value was < LLOQ. Patients with SVR12, according to cohort. Historical rate of SVR for the black/AA cohort (26% [solid line]) and Latino cohort (36% [dashed line]). * Patients with missing data at post-treatment week 12 were considered failures. † Patients with available data at post-treatment week 12. ‡ Patients with missing data at post-treatment week 12 were considered responders if the next available HCV-RNA value was < LLOQ.](https://static.elsevier.es/multimedia/16652681/0000001500000006/v1_201906150956/S1665268119311093/v1_201906150956/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





