Hepatitis C virus-related end-stage liver disease, alone or in combination with alcohol, has become the leading indication for liver transplantation in most transplant programs accounting for approximately half of transplants performed in European centers. The aim of this review is to analyze the factors involved in the results in different groups of patients with HCV underwent to liver transplantation. The groups involved those pretransplantation, post-transplant HCV infection, preventive early post-transplantation and with of recurrent hepatitis C.
HCV-related end-stage liver disease, alone or in combination with alcohol, has become the leading indication for liver transplantation in most transplant programs accounting for approximately half of transplants performed in European centers.1 There is real concern that the number in need of transplantation will increase in coming years given the prevalence of infection in the general population (1.8%), the three-fold greater prevalence in those who are 30-50 years old as compared with older age groups, the eventual progression to cirrhosis in approximately one fifth of these infected, and the lack of consistently effective antiviral therapy.2
Indication for liver transplantationLiver transplantation should be considered a therapeutic option when the course of the disease is sufficiently advanced that median-term survival is unlikely without this intervention. While this assumption has clearly been demonstrated for decompensated HCV-related end-stage liver disease, data confirming the benefits of transplantation over traditional management are less conclusive for compensated HCV cirrhotic patients.3 Indeed, the 4-5 year survival of compensated HCV-cirrhotic patients has been shown to range between 84% to 91%, with rates of hepatic decompensation and hepatocellular carcinoma of 18%- 20% and 7%-11.5%, respectively.3 Thus, in the absence of controlled trials assessing the efficacy of liver transplantation, the 5-year outcome of compensated HCV cirrhotic patients favors observation in absence of transplantation. Given the long-term implications of recurrent hepatitis C, mainly among those transplanted in recent years (see later), decisions should be made very carefully, and these patients should not be offered transplantation just because they meet the minimal listing criteria. Patients should be aggressively treated with traditional medical therapies, including antivirals, particularly those infected with HCV genotype 2 and 3, in whom sustained virological response rates of up to 80% may be achieved (see later). In addition, in patients with small hepatocellular carcinomas (HCC) and preserved hepatic function, transplantation could be delayed to a second alternative, and other treatments, including transarterial chemoembolization, percutaneous ethanol injection or ablation through radio-frequency could be used first in an attempt to ablate the tumor. These patients, typically with good hepatic function, are usually excellent candidates to receive interferon therapy, which may have not only acted as an antiviral but also preventing the recurrence of the HCC.4 The need for transplantation could be thus obviated in those with a successful tumoral ablation who achieves a sustained virological response.
There is a growing discrepancy between the number of donor organs and number of potential recipients, further limiting the assessment of candidacy for liver transplantation. Inclusion of donors who are anti-HCV positive could become a means to enlarge the pool of potential donors. The reported prevalence of anti-HCV (EIA2) and of HCV RNA (PCR) in cadaver organ donors in the U.S. are 4.2% (range 2.3%-8.3%) and 2.4% (range: 0.8%-4.2%), respectively.5 Whether the use of these organs is suitable, largely depends on the prognosis of recipients receiving HCVinfected grafts. Documentation of progressive liver disease in a substantial proportion of patients with recurrent HCV infection (see later) raises serious concerns about the suitability of anti-HCV positive organs for uninfected liver transplant candidates. An alternative option is the use of anti-HCV positive organs for candidates who are already HCV infected. Superinfection in that setting has been described,6 but medium-term outcome of anti-HCV positive recipients receiving HCV-infected grafts seems to be similar to that of anti-HCV positive recipients receiving anti-HCV negative grafts.6,7
HCV infection and liver transplantation Source of infectionSource of infectionWhen the patient is infected at the time of transplantation, recurrent infection, defined as the presence of virus in serum, is universal.8 In these circumstances, a rapid and sharp decline in viral load occurs immediately after removal of the infected liver followed by a progressive increase in serum HCV RNA starting 72 hours after liver transplantation to reach levels 10 to 20 fold higher than those detected prior to transplantation.9 Interestingly, a lack of correlation has been demonstrated between serum RNA levels and intrahepatic viral replication rate in the post-transplant period10 suggesting that the elevated levels of serum HCV RNA typically observed post-transplantation are not a result of increased replication but rather of decreased clearance in the setting of immune suppression.
The virus may be acquired in those without evidence of viral infection prior to transplantation from contaminated blood and organ donors, and by nosocomial acquisition of virus during the transplant hospitalization.11 Routine donor screening with specific serologic tests for HCV and improvement in surgical techniques are likely the reasons for the drop in de novo acquisition of HCV infection following transplantation which currently ranges from 0 to 5%.11
Natural historyRecurrent infection leads to the development of chronic hepatitis in the majority of patients if they are followed for at least 5 years.12-18 The natural history of this hepatitis is characterized by the progression to cirrhosis in a percentage of patients that varies between 6% and 23% at a median of 3-4 years post-transplantation12-18, with a cumulative probability of reaching the stage of cirrhosis of 30% and 51% at 5 and 7 years of follow-up, respectively.13,19 This wide range in percentages between studies is likely related to the use of different case definitions (biochemical vs virological vs histological criteria). Indeed, liver function tests are not correlated with either viremia or with histologic disease severity,3,13,18 and protocol liver biopsies are generally needed to identify progression to severe forms of chronic hepatitis.18 This course of the disease is clearly more aggressive than that reported in immunocompetent patients, in whom progression to cirrhosis occurs in only one fifth of those infected after decades of follow-up. Indeed, in a recent multicenter study based on annual protocol liver biopsies, disease progression was found to be accelerated in transplant recipients compared to the pre-transplant evolution with an estimated time to graft cirrhosis of only 9-12 years.17 This agressivity of recurrent hepatitis C is present not only prior to the development of cirrhosis but also afterwards. In a recent study aimed at defining the natural history of HCV-related compensated graft cirrhosis, the oneyear risk of clinical decompensation was 42%,20 a percentage significantly higher than that described in the literature in non-transplanted cirrhotic patients infected with HCV.1 Finally, a recent study has shown that HCV-related disease progression is increasing in recent years17,19 with patients transplanted recently doing worse than those transplanted years ago. The clinical impact of HCV infection is beginning to be reported in several studies.19,21 Two recent studies have shown that HCV infection significantly impairs patient and allograft survival after liver transplantation19,21(Figure 1). In addition, the worse histological outcome seen in recent years is beginning to translate in some centers into a reduced survival among those transplanted in recent years as compared to those transplanted in earlier cohorts, with recurrence of the original disease, that is HCV-cirrhosis, being the main cause of death.19
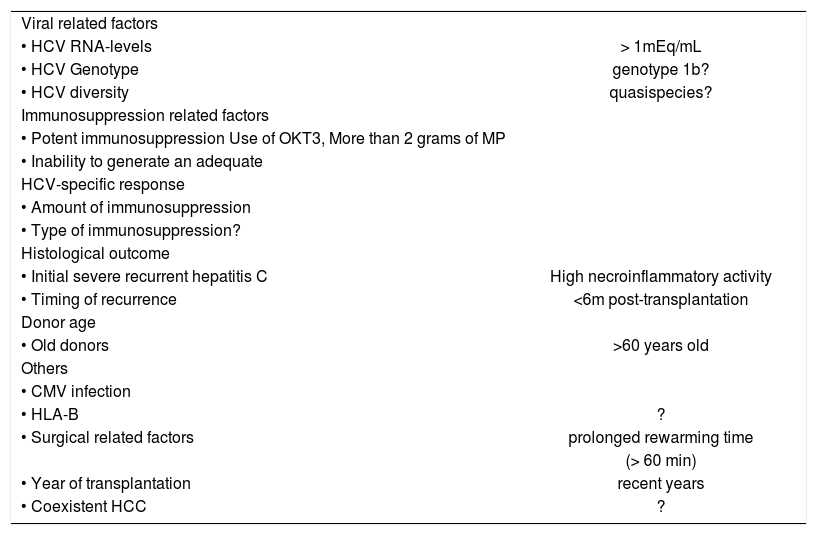
The natural history of recurrent HCV infection is however highly variable, and while there are patients developing post-transplantation viremia with minimal liver injury, there are others who progress to severe hepatitis and graft failure within very short periods of time.12-19 In one study based on yearly protocol liver biopsies, 12% of the patients were shown to reach a fibrosis stage 4 at one year post-transplantation. In contrast, 32% of the patients continued to have fibrosis 0 in the 5th year protocol biopsy,18 thus emphasising the high variability in outcome. Reasons that explain this variability are many and likely related to the virus, the host and the environment (Table I).
Factors influencing the natural history of HCV infection after liver transplantation.
| Viral related factors | |
| • HCV RNA-levels | > 1mEq/mL |
| • HCV Genotype | genotype 1b? |
| • HCV diversity | quasispecies? |
| Immunosuppression related factors | |
| • Potent immunosuppression Use of OKT3, More than 2 grams of MP | |
| • Inability to generate an adequate | |
| HCV-specific response | |
| • Amount of immunosuppression | |
| • Type of immunosuppression? | |
| Histological outcome | |
| • Initial severe recurrent hepatitis C | High necroinflammatory activity |
| • Timing of recurrence | <6m post-transplantation |
| Donor age | |
| • Old donors | >60 years old |
| Others | |
| • CMV infection | |
| • HLA-B | ? |
| • Surgical related factors | prolonged rewarming time |
| (> 60 min) | |
| • Year of transplantation | recent years |
| • Coexistent HCC | ? |
- •
HCV genotypes: Studies evaluating the relationship between severity of liver disease posttransplantation and infecting genotypes are conflicting.22 Some, but not all studies have implicated genotype 1, and in particular subtype 1b, in a more aggressive post-transplantation disease compared to non-1 genotype. Factors that could account for discrepant results include differences in genotype distribution in the study population, differences in genotyping methods, presence of unmeasured confounding variables such as type and amount of administered immunosuppression, length of histological follow-up, and differences in case definitions (histological disease severity vs patient or graft survival). In addition, different strains within genotype 1b may be implicated23 since most studies demonstrating an adverse effect of HCV genotype 1b come from European Centers while those showing no association come from the US.
- •
HCV-RNA levels: Results of studies evaluating the association between levels of viremia and disease severity have been discrepant.22 The majority of cross-sectional studies have documented a lack of correlation between HCV-RNA levels and disease severity, suggesting an immune-mediated mechanism of chronic liver injury. High levels of viremia however, have been described in the setting of fibrosing cholestatic hepatitis24 and during the acute phase of recurrent hepatitis C,25 suggesting that in these situations, liver damage may be due to the direct cytopathic effect of HCV.
Hepatitis C viremia levels prior to,17,26 and/or early following transplantation27 have been shown to be associated with both the clinical and histological outcome.
- •
HCV diversity: With an individual infected with a certain genotype, further heterogeneity exists. The closely related yet significantly different viral genomes produced over time in response to host pressures in an individual known as quasispecies are characterized by extensive genetic mutation within the second envelope gene (E2) hypervariable region (HVR).2,22 Data suggest that HCV quasispecies mutation may play a role in pathogenesis of progressive HCV infection. Results from published studies are however inconclusive and somewhat discrepant, and may be related to the small number of patients included, the different methodologies applied to assess HCV heterogeneity (singlestrand conformation polymorphism, heteroduplex mobility assay, sequencing), the type of end-point chosen (viral complexity vs viral diversity vs viral divergence), the region of the genome evaluated, and the definition of disease severity.22
Immunosuppression-related factors: These are probably the single most important factors in influencing the outcome. Most studies have found a positive correlation between a high rate of cirrhosis-development and potent immune suppression high number of boluses of methylprednisolone, 22 use of OKT3 or anti-lymphocytic preparations, 22 use of high total cumulative doses of steroids.22 In one study, 40% of the patients with mild HCV recurrence demonstrated a proliferative response of peripheral Thelper cells against some of HCV antigens. In contrast, none of those with severe recurrence demonstrated this type of response28 thus suggesting that the inability to generate an adequate HCV-specific T-cell response plays a major role in the pathogenesis of chronic hepatitis C of the graft. Results on the association between the type of administered immunosuppression and disease severity are less clear,22 and warrant prospective studies comparing different types of immunosuppression-based regimens in HCV-infected recipients. In particular, conflicting data exist as to whether cyclosporine-neoral based regimens are better or worse than tacrolimus-based regimens,22 and whether mycophenolate mophetil (MMF) is detrimental. 22,29 A recent study suggested a deleterious effect of the combination MMF with IL-2 receptor antibodies.30 Finally, indirect data point towards an adverse effect of an early and abrupt withdrawal of second-line immunosuppressive drugs, particularly prednisone.17,19,31 In fact, this policy, adopted by several centers in recent years, may have contributed to the worse histological outcome observed recently.17,19
Early histological findings: An initial severe recurrent hepatitis C is predictive of subsequent progression to cirrhosis. In two studies,12,13 only 3 to 10% of those with mild necroinflammatory activity in the first-year liver biopsy progressed subsequently to cirrhosis. In contrast, up to 66% of those with moderate to severe activity in the first-year biopsy developed cirrhosis. The timing of recurrence is also important with early recurrence (less than 6 months post-transplantation) associated with worse outcome.15
Donor age: Recent data suggest that recipients of organs from old donors are at increased risk to develop progressive hepatitis C post-transplantation.19 This may be one reason for the worse histological outcome seen in recent years.17,19
Others: Some but not all studies, have suggested that while HLA-B sharing between the donor and the recipient reduces the incidence of acute cellular rejection,22 it also promotes the recurrence of viral hepatitis in the liver transplant recipients. Patients who develop CMV viremia may be at increased risk of severe HCV recurrence.22 The effect of HBV coinfection on HCV recurrence is unclear. 22 In contrast, coinfection with other viruses such as HGV22 does not influence the post transplantation course of HCV disease. Race has recently been found to influence outcome in patients with recurrent HCV infection, with non-Caucasians doing worse than Caucasians.17,22 A prolonged rewarming time was found to be predictive of a bad outcome in one study.22 The rate of fibrosis progression prior to transplantation was shown to be unrelated to that observed post-transplantation,17 although the data evaluating this issue are incomplete.
Treatment of post-transplant HCV infection and diseaseThere are three possible strategies for prevention and/or treatment of HCV infection in the setting of liver transplantation. The first involves the use of prophylactic therapy at the time of transplantation in an attempt to prevent recurrence. The second is to treat patients early in the post-transplant period before histologic liver injury has occurred. The third is to wait until post-transplantation hepatitis has been demonstrated histologically before instituting treatment. Unlike HBV-infected patients undergoing liver transplantation who can be treated successfully with prophylactic therapy, HCV-infected patients lack this option. Treatment options for HCV infection are still severely limited. Interferon and ribavirina are the only two drugs available to treat this infection, and both their efficacy and tolerability appear to be low in the transplant setting.
Pre-transplantation treatmentPreventive therapy may be initiated while awaiting liver transplantation in order to (i) stabilize and/or improve the hepatic function so that the need for liver transplantation may be delayed or even obviated; and (ii) suppress viral replication so that the risk of post-transplantation HCV recurrence and/or aggressive recurrent HCV disease is reduced. There have been anecdotal case reports of the use of interferon in decompensated HCV-cirrhotic patients, since these patients have typically been excluded from randomized trials. Interferon is poorly tolerated in this setting and could potentially precipitate worsening hepatic function. Modified regimens or combinations with ribavirin may be considered in these patients. Early experience with combination therapy in this setting has shown to be dangerous since it induces severe cytopenias and frequent infections.32 However, in the few cases where HCV clearance was achieved, recurrent HCV infection was prevented.33 More data are needed regarding this approach before it can be recommended in patients with clinically decompensated HCV disease.
Preventive early post-transplantation treatmentTherapy early following transplantation has been studied in a few controlled studies with two agents, interferon and ribavirin. Two studies have evaluated the effect of early interferon treatment initiated within the first 2 weeks post-transplantation.34,35 In none of these studies was either patient/graft survival or HCV persistence affected by therapy. While histologic disease recurrence was observed less frequently in interferontreated patients (8 of 30 evaluable at one year) than in those untreated (22 of 41; p = 0.01) in one study,34 in the second,35 treatment only delayed the development of histologic hepatitis (at a median of 408 days after transplantation in the treated group versus 193 days in the untreated group). Results with combination therapy appear more promising. In a case series,36 36 recipients (30 of whom were infected with HCV genotype 1b) were treated with IFN-alpha2b and ribavirin starting the third post-transplant week and continued for 1 year. After a median follow-up of 52 months, the actuarial 5-year survival was excellent (87.5%). At 36 months post-discontinuation of therapy, a sustained virological and biochemical response was achieved in 12 patients (33%), 20% in those infected with HCV genotype 1 and 100% in those with genotype 2. Liver biopsies were normal in these patients. In contrast, progression to severe hepatitis C was observed in 4 of the non responders (11% of the overall series). Common side effects included hemolytic anemia and asthenia which were well controlled with dose reduction.36 Well-designed controlled randomized studies are needed to confirm these encouraging findings.
Treatment of recurrent hepatitis CTreatment of recurrent HCV disease is the last alternative. Experience with interferon or ribavirin in monotherapy has thus far been disappointing.37-39 Interferon at doses of 3MU, thrice weekly for 6 months, has failed to clear serum HCV RNA, despite normalization of ALT values in a subset of patients treated (0%-28%).37-39 Relapse after discontinuing treatment is almost the rule, and post-treatment improvement in liver damage is uncommon. In turn, ribavirin monotherapy is associated with biochemical improvement in many patients but virological clearance in none.39 Biochemical relapse is universal after cessation of therapy and no histological improvement is generally observed. The main side effect is hemolysis which typically resolves after the cessation of therapy.
Initial results from combination therapy with interferon and ribavirin are encouraging.40 There have been several studies now using this combination in liver transplant recipients with response rates following discontinuation of therapy ranging between 8% and 33%.40-45 Therapy is however limited by low tolerance and need for frequent dose reductions and discontinuations (Table II). Early intervention with combined therapy at a stage when patients have not progressed to severe forms of liver injury may explain the better results obtained in some studies than in others. The need for maintenance therapy with ribavirin is currently unknown. Sustained HCV clearance is likely associated with cure of the infection. Indeed, a recent report on the 12-months follow up of 11 treated patients who cleared HCV RNA from serum and liver after 12 months of ribavirin monotherapy showed that 90% (10/11) of patients maintained a sustained biochemical and virological response, without significant histological changes compared to the end-of-treatment biopsies.46 These preliminary data suggest that maintenance therapy may be discontinued in patients who have responded virologically.
Recurrent hepatitis C: therapy with interferon and ribavirin.
| Author, year (N°) | Treatment | ETBR/ETVR (%) | SBR/VSR (%) | Histological improvement | DC (%) |
|---|---|---|---|---|---|
| Bizollon, 1997, (21) | 6 mo IFN + Rbv + 6 m Rbv | 100/48 | 86/24 | Yes | 14 |
| Alberti, 2001 (18) | 12 m IFN + Rbv + long-term Rbv | 83/44 | 78/33 | Yes | 22 |
| Ahmad, 2001 (60) | 6 mo IFN (n = 40) vs 12 mo combination (n=20) | 20 vs 25 /15 vs 40 | NA vs NA/2.5 vs 20 | No | 25 |
| De Vera, 2001 (32) | IFN + Rbv ≥ 12 m | 77/9 | 71/9 | No | 40 |
| Gopal 2001 (12) | IFN + Rbv indefinitely | NA/50 | NA/8 | NA | 0 |
DC = discontinuation; ETBR = end-of-treatment biochemical response; ETVR = end-of-treatment virological response; SBR = sustained biochemical response; SVR = sustained virological response; IFN = interferon; Rbv = Ribavirin; NA = Not available
There are preliminary data on peg-interferon used both as a post-transplantation preventive drug and as therapy of established disease. The tolerance appears adequate with a 7% discontinuation rate due to side effects.
Given the relatively poor efficacy of current therapies, it is unclear which is the best strategy. Some authors advocate the use of early preemptive therapy in all transplant recipients. However, this approach is limited by the high cost and interventions with attendant side effects in patients who might progress only slowly to significant liver disease. An alternative is to treat at early time points patients at high risk of severe recurrence since treatment initiated in the early phases of disease recurrence appears to have a better efficacy than treatment in advanced stages of disease. Based on published data, the profile of a patient at high risk of severe recurrence would be the following: a patient transplanted recently, infected with HCV genotype 1b, with high levels of HCV RNA prior to ((1 Meq/mL) and/or early following transplantation, whose induction immunosuppression regimen is potent with an early and abrupt withdrawal of second-line immunosuppressive drugs, treated with a high number of boluses of methylprednisolone ((2 g) and/or with anti-lymphocytic preparations, and who develops a severe (with cholestasis, abundant steatosis and hepatocyte ballooning) and early hepatitis ((6 months).
RetransplantationBecause of the progressive nature of recurrent HCV disease, it is likely that in the next decade there will be a marked increase in the number of HCV-infected recipients in need of retransplantation. It is thus imperative to determine whether all patients with graft failure due to recurrent HCV disease are candidates for further transplantation. This is a difficult issue to resolve currently since very few studies have focused on this problem. Data that do exist suggest poor outcome with liver retransplantation.47,48 The debate is complicated further by increasing shortage of organ donors, and by concerns about the severity of recurrent HCV disease in the second graft. Initial data have suggested that survival following retransplantation is particularly poor in patients with recurrent HCV.47,48 More recent data have suggested improved outcome when retransplantation is performed before severe hyperbilirubinemia and development of renal complications.49 The combination of an increasing shortage of organ donors and a growing number of patients in need of first transplantation will likely determine the candidacy of patients being considered for retransplantation. Until a consensus is reached, each center will likely develop its own policy.
ConclusionRecurrent HCV infection is universal leading to liver failure in a significant proportion of patients. The time course over which this progression occurs is shorter than in the immunocompetent population. As the disease process moves into its second decade, an impact on patient and graft survival is being shown. Strategies to prevent or to reduce the effect of HCV infection after liver transplantation are therefore essential. Our ability to intervene in this disease is however currently limited. The main obstacles are the difficulty in predicting the outcome in the individual patient and the lack of effective therapy. Neither interferon nor ribavirin, when administered as single agents result in sustained viral clearance. Administration of both drugs given in combination either to prevent disease or to treat recurrence is a relatively better approach. There is a great need for more efficacious antivirals. Retransplantation is the last option and is generally associated with a poorer outcome.