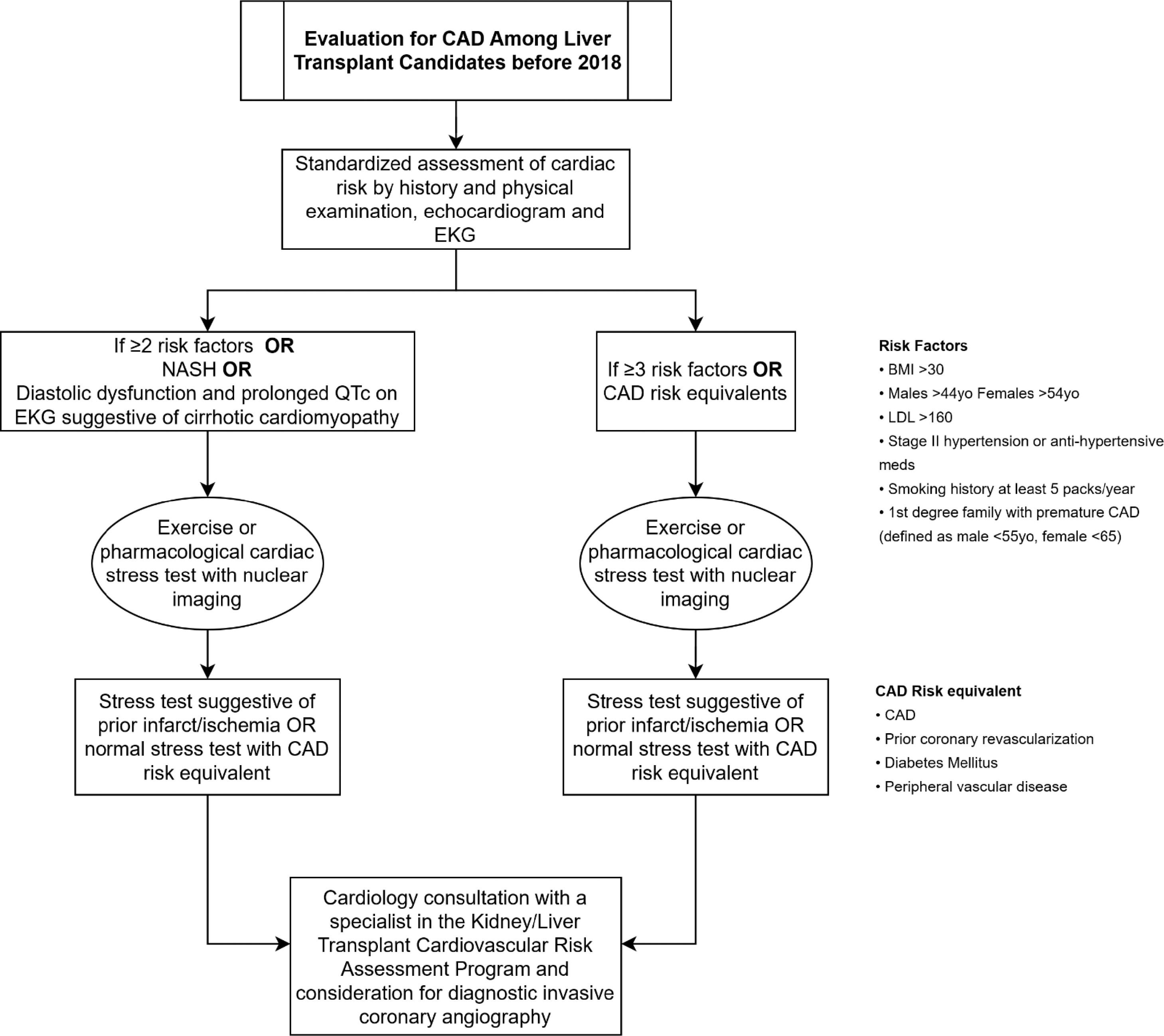
Recommendations on non-invasive imaging to assess pre-operative cardiac risk among liver transplant candidates vary amongst societal guidelines and individual institutional practices. In 2018, a standardized pre-transplant coronary evaluation protocol was established at Beth Israel Deaconess Medical Center, Boston MA, to ensure appropriate and consistent pre-operative testing was performed.
MethodsAll patients who underwent liver transplant evaluation between January 1st, 2016 and December 31st, 2019, were retrospectively analyzed and divided into three cohorts; before the introduction of the protocol (prior to 2018), initial protocol favoring invasive coronary angiography (ICA) (2018), and amended protocol favoring coronary computed tomography angiography (CCTA) (post-2018). We described clinical characteristics, candidacy for transplant, and cardiovascular complications during follow-up. As an unadjusted exploratory analysis, the Cochran-Armitage Exact Trend Test was used to examine univariate differences across time.
ResultsA total of 462 patients underwent liver transplant evaluation during the study period. Among these, 218 (47.2%) patients underwent stress test, 50 (10.8%) underwent CCTA, and 68 (14.8%) underwent ICA. Across the three time periods, there was an increase in the proportion of CCTAs performed (3%, 6.3%, and 26.3% respectively; p <0.001) and proportion of patients diagnosed with obstructive CAD using CCTA (0%, 30%, and 51.4% respectively; p = 0.04). There was no significant difference in post-transplant cardiac complications among patients evaluated before 2018, during 2018, and after 2018 (5.9% vs. 5.6 vs. 6.0%; p=1.0).
ConclusionOur findings suggest it is reasonable to shift practice to a less invasive approach utilizing CCTA or nuclear stress testing when assessing liver transplant candidates at increased cardiovascular risk.
Liver transplantation remains the only curative treatment for patients with advanced fibrosis and cirrhosis. However, due to high organ demand as manifested by rising Model for End-Stage Liver Disease (MELD) scores at the time of transplant, there remains a large number of patients awaiting same. By the end of 2018, 12,820 patients remained on the liver transplant waiting list despite 8,250 transplantations taking place that year [1,2]. With longer wait times, there has been a shift in the baseline characteristics of the typical liver transplant candidate, with patients having more comorbidities, including cardiovascular disease [3,4]. Further, more transplants are being performed in patients with non-alcoholic steatohepatitis (NASH) cirrhosis who are inherently at a higher cardiac risk due to its association with metabolic syndrome [5–9].
Cardiovascular disease remains the leading cause of morbidity and mortality both before and after non-cardiac organ transplantation. Therefore, preoperative risk stratification is paramount for those awaiting transplantation [10–13], in particular, assessment for obstructive coronary artery disease (CAD) which is associated with poorer outcomes post-transplantation [14]. While the ACC/AHA suggest consideration of non-invasive stress testing in liver transplantation candidates with multiple CAD risk factors regardless of functional status [15,16], some centers have advocated for routine invasive coronary angiography (ICA) to screen for CAD. [17,18] In the time since the publication of these guidelines, coronary computed tomography angiography (CCTA) has taken on a primary role in the evaluation of CAD [19]. The varying degree of accuracy for detecting of CAD among non-invasive testing balanced against the cost and risks associated with ICA, and the emerging role of CCTA has led to varying protocols for cardiac evaluation among transplant centers.
Prior to January 2018, pre-operative nuclear perfusion testing was performed on all patients with CAD, diabetes mellitus (DM), peripheral vascular disease (PVD), and NASH, as well as patients with 2 or more of the following: body mass index (BMI) > 30, male age ≥44 or female age ≥54, dyslipidemia, hypertension, >5 pack year smoking history, 1st degree relative with premature CAD. In the absence of a reversible or fixed defect on nuclear perfusion stress test, the decision to proceed with ICA was at the discretion of the cardiologist evaluating the patient based on the presence/absence of angina or an anginal equivalent, baseline functional capacity, and the number of cardiac risk factors. In January 2018, a task force committee formed by expert cardiologists on perioperative cardiovascular medicine and liver transplant specialists was instituted to update this protocol utilizing more frequent anatomic imaging with ICA and shifting to an “opt out” from ICA approach at the cardiologist's discretion. In 2019, based on increased institutional experience with CCTA, the protocol was amended to favor CCTA over ICA for patients at the highest risk [20,21]. This allowed the extent and location of CAD, along with the functional impact as assessed by CTFFR, to be included in patient risk assessment. We hypothesized that implementing the protocol would lead to more frequent cardiology consultation and diagnosis of CAD in the transplant screening period. The objective of this study was to describe patient characteristics undergoing evaluation and the effect of this protocol on downstream interventions, candidacy for listing, and clinical outcomes both before and after integration of CCTA.
2Methods2.1Study design and populationAll patients with end-stage liver disease referred for liver transplant evaluation at Beth Israel Deaconess Medical Center, Boston MA, between January 1st, 2016 and December 31st, 2019, were retrospectively analyzed. A study protocol was submitted and approved by the Institutional Review Board (IRB). Referral orders and demographic data were obtained using hospital administrative data, including both inpatient and outpatient consultations. All patient-specific data was obtained from a review of each patient's electronic medical record. Patients who did not complete transplant evaluation for any reason were excluded from the study. This encompassed patients who expired prior to evaluation, did not attend an initial clinic visit, did not complete the necessary imaging and laboratory workup, and did not attend multidisciplinary visits as part of the evaluation protocol (e.g., social work and nutrition). In an effort to standardize time of follow-up, the follow-up period for the “prior to 2018” cohort was defined as the date of initial transplant evaluation to December 31st 2018 or date of death for those patients who expired during this time period. Follow-up for the “protocol favoring ICA” cohort was defined as the date of initial transplant evaluation to December 31st 2019 or date of death for those patients who expired during this time period. Follow-up for the “protocol favoring CCTA” cohort was defined as the date of initial transplant evaluation to December 31st 2020 or date of death for those patient who expired during this time period. REDCap© was utilized to design a HIPAA-compliant data collection instrument to store and export de-identified data for statistical analysis [22]. Charts were accessed and systematically reviewed by three IRB-approved clinician investigators. These were randomly distributed to reviewers in equal proportions, and data extraction and synthesis were performed only after all charts were reviewed.
2.2Data characterizationVariables were operationally defined prior to data collection and included demographic characteristics, type of transplant, clinical characteristics of hepatic and cardiovascular co-morbidities, cardiovascular risk factors, and prescribed medications. Qualitative and quantitative results from electrocardiogram (ECG), transthoracic echocardiogram (TTE), ICA, and CCTA were collected. CCTAs were performed with a Revolution 256-detectors CT scanner (General Electric Medical Systems, Milwaukee, WI) or Aquilion One 320-detectors CT scanner (Toshiba America Medical Systems, Tustin, CA). CT-derived FFR was obtained using HeartFlow (Redwood City, CA).
Data was collected on the most recent investigation for patients with multiple studies prior to initial transplant evaluation. Echocardiographic data included left ventricular function, left ventricular size, presence/absence of wall motion abnormalities, presence/absence of valvular disease of at least moderate severity, and presence/absence of pulmonary artery systolic hypertension. Significant stenosis on CCTA was defined as a score ≥of 3 using the CAD-RADSTM scoring system [23]. The CAD-RADSTM scoring system grades coronary stenoses based on the maximum percentage diameter stenosis, ranging from CAD-RADS TM 0 representing complete absence of stenosis and plaque to CAD-RADS TM 5 representing the presence of at least one totally occluded coronary artery. CAD-RADS TM ≥3 correlates with a stenosis ≥50% in severity. Significant stenosis on ICA was defined as ≥50% stenosis involving the left main coronary artery (LMCA) or ≥70% stenosis involving all other epicardial vessels. When CT-derived FFR data was available, FFR ≤0.80 prevailed over CAD-RADSTM score to define obstructive disease. Data on whether or not a patient was referred for cardiology consultation and if further testing was recommended was collected to evaluate the implementation of the protocol. Clinical outcome data included listing status, transplant occurrence, and cardiovascular or transplant-related complications and mortality. For patients who had a change in listing status, the most recent listing status was recorded. Cardiovascular complications included myocardial infarction, decompensated heart failure requiring hospital admission, and new onset atrial fibrillation within 30 days of transplantation. Transplant complications included need for re-operation, acute cellular rejection, failure of donated liver, bile duct complications, infection, bleeding, and thromboembolism. Data was collected until December 31st 2018 for the “prior to protocol” cohort, December 31st 2019 for the “protocol favoring ICA” cohort, and December 31st 2020 for the “protocol favoring CCTA” cohort.
2.3Statistical analysisCategorical variables were described using counts and proportions. Continuous variables were described using the mean and standard deviation or median and interquartile range, depending on the symmetry and dispersion of the distribution. The cohort was divided into three sub-groups; prior to the introduction of the updated protocol (“pre-implementation period” prior to 2018), initial protocol favoring ICA (January 2018-December 2018), and amended protocol favoring CCTA (after January 2019). Statistical significance was denoted with a p-value <0.05. Cochran-Armitage Exact Trend Test was used to estimate the differences in proportions trends between intervention periods for testing modalities and clinical outcomes. Statistical analysis was executed using SAS/STAT® software.
3Results3.1Protocol descriptionThe protocol for evaluating coronary artery disease in liver transplant candidates with and without known CAD used from January-December 2018 is shown in Fig. 1a and Fig. 1b summarizes the updated protocol used from January 2019 on. After January 1st, 2019, the amended protocol recommended CCTA +/- CTFFR for those patients with a CAD risk equivalent or >=3 risk factors as long as eGFR was >50ml/min/1.73m2 instead of pharmacologic nuclear perfusion study. CAD risk equivalents included type II diabetes mellitus, peripheral vascular disease, and NASH cirrhosis. Risk factors included body mass index >30, age >44 years for males and >54 years for females, stage II hypertension (>140/90 mmHg) or use of an anti-hypertensive medication, five pack-year smoking history or greater, and 1st degree family relative with premature CAD. A result of CAD-RADSTM ≥3 necessitated referral to cardiology for consideration of ICA. Patients with known CAD continued to undergo pharmacologic nuclear perfusion evaluation for residual ischemia and were referred for cardiology evaluation and consideration of ICA. A multidisciplinary discussion was held between the interventional cardiologist, referring cardiologist, and liver transplant team if a patient was found to have significant obstructive coronary artery disease on an invasive coronary angiogram. If revascularization was deemed appropriate, drug-eluting stents (DES) were favored over bare-metal stents (BMS), with a minimum of 3 months of dual antiplatelet therapy (DAPT) recommended following DES implantation. Once DAPT has been completed, the patient was deemed to again be a candidate for liver transplant listing from a cardiovascular standpoint.
A. Initial protocol favoring ICA (2018) Fig. 1B. Amended protocol favoring coronary computed tomography angiography (post 2018).
Baseline characteristics of the liver transplant population are shown in Tables 1 and 2 summarizes the characteristics of the investigations performed. A total of 709 patients with end-stage liver disease were referred for liver transplant evaluation at Beth Israel Deaconess Medical Center, Boston MA, between January 1st, 2016 and, December 31st, 2019. Among these, 462 patients (65.2%) underwent liver transplant evaluation, 151 (32.7%) were female, the median age was 57 years, 313 (67.7%) were overweight or obese, 115 (24.9%) had diabetes mellitus, 171 (37%) had systemic hypertension, and 254 (55%) were current or former smokers. The three most common etiologies for cirrhosis were alcoholic liver disease (55.8%), hepatitis C (26.6%), and NASH (13.9%). The median MELD-Na score at the time of evaluation was 17. During the entire follow-up period, among patients who completed transplant evaluation, 376 patients (81.4%) had a baseline EKG and TTE. The remaining 86 patients did not proceed with transplant evaluation, including cardiac evaluation, for multiple reasons, most commonly due to transplant related candidacy concerns such as lack of social support or non-compliance with medical care. Median QTc was 447msecs (interquartile range [IQR] 424, 470). Among those who underwent an echocardiogram, 37 (9.8%) had moderate or severe valvular disease, and 19 (5.1%) had regional wall motion abnormalities. 218 (47.2%) patients underwent stress testing, with 12 (5.5%) having a reversible or partially reversible perfusion deficit. 50 patients (10.8%) underwent CCTA and 68 (14.7%) ICA, with significant obstruction being found in 21 (21/50; 42%) and 14 (14/68; 20.6%) patients, respectively. Of the 21 patients who were found to have obstructive CAD (CAD-RADSTM ≥3 or CTFFR ≤0.80) on CCTA, 11 would go on to obtain ICA, and of these 11 only 3 were found to have obstructive CAD and subsequently undergo percutaneous coronary intervention with DES. The remaining 10 did not undergo ICA for a variety of reasons including transplant list denial for non-cardiac reasons, patient loss to follow-up, patient death, and absence of obstructive disease in a high-risk vessel as determined by a cardiologist.
Baseline characteristics of liver transplant population.
| Characteristic | Total (N = 462) |
|---|---|
| Sex – no. (%) | |
| Female | 151 (32.7) |
| Age at time of evaluation – years | |
| Median | 57 |
| Range | [20, 72] |
| Ethnicity no. (%) | |
| Hispanic | 42 (9.1) |
| Unknown/Not specified | 47 (10.2) |
| Race no. (%) | |
| Caucasian | 316 (68.4) |
| Black | 30 (6.5) |
| Asian | 23 (5) |
| Native American | 8 (1.7) |
| Other | 34 (7.4) |
| Unknown | 51 (11) |
| Etiology of Liver Disease – no. (%) ! | |
| Alcohol | 258 (55.8) |
| Hepatitis C | 123 (26.6) |
| Non-alcoholic steatohepatitis | 64 (13.9) |
| Hepatitis B | 21 (4.5) |
| PSC/PBC*+ | 21 (4.5) |
| Autoimmune hepatitis | 12 (2.6) |
| Cryptogenic | 12 (2.6) |
| Other | 27 (5.8) |
| MELD-Na – median (IQR) | 17 (12, 23) |
| Smoking – no. (%) | |
| Current | 85 (18.4) |
| Former | 169 (36.6) |
| Never | 199 (43.1) |
| Unknown | 9 (1.9) |
| Body Mass Index – no. (%) | |
| Underweight | 4 (0.9) |
| Normal | 128 (27.7) |
| Overweight | 160 (34.6) |
| Obesity Class I | 84 (18.2) |
| Obesity Class II | 49 (10.6) |
| Obesity Class III | 20 (4.3) |
| Unknown | 17 (3.7) |
| Comorbidities – no. (%) | |
| Hypertension | 171 (37) |
| Diabetes mellitus | 115 (24.9) |
| History of angina/CAD | 52 (11.3) |
| History of heart failure | 17 (3.7) |
| History of atrial fibrillation | 21 (4.5) |
| History of PAD/CVD#^ | 13 (2.8) |
| Medications – no. (%) | |
| Statins | 51 (11) |
| ACE-inhibitors | 46 (10) |
| Beta-blockers | 179 (38.7) |
Characteristics and Results of Non-Invasive Testing
P–SP - Pulmonary artery systolic pressure.
CAD – coronary artery disease.
CABG – coronary artery bypass grafting.
CCTA – coronary computed tomography angiography.
IQR – interquartile range.
CAD-RADS™ - coronary artery disease reporting & data system.
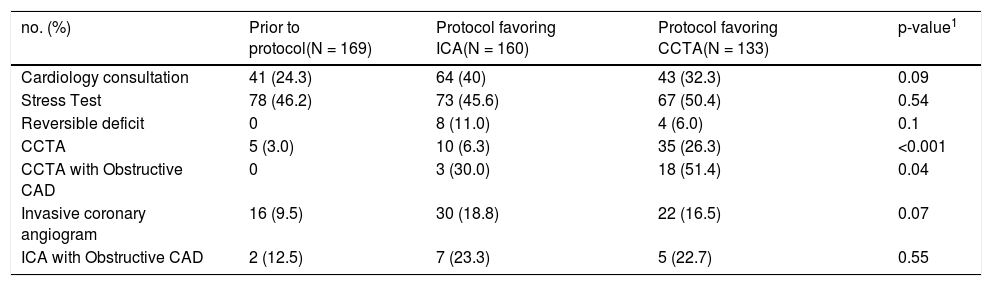
Changes in testing frequency and positivity across the three temporal periods are highlighted in Table 3. There were no statistically significant differences in the frequency of stress testing or ICA between January 2016 and December 2020; however, there was a higher proportion of CCTAs performed during 2018 (6.3%) and after 2018 (26.3%) when compared to the pre-implementation period (3%). There was also no statistically significant difference in the proportion of patients referred for ICA who had significant obstructive CAD across the three time periods (12.5% vs. 23.3% vs. 22.7%). There was a statistically significant difference in the proportion of patients referred for CCTA who had obstructive CAD over the three time periods (0% vs. 30% vs. 51.4%), likely reflecting the implementation of a formalized risk-based approach to proceeding with CCTA in the latter period.
Differences in cardiology testing across time.
CCTA – coronary computed tomography angiography.
CAD – coronary artery disease.
Changes in clinical outcomes across the three temporal periods are highlighted in Table 4. There was a trend towards significance in the proportion of patients listed for transplant evaluation across the study periods with an increase over time (39.6% in the pre-implementation period, 43.8% during protocol favoring ICA and 50.4% during protocol favoring CCTA, p=0.07). The study was not powered to compare complication rates across groups, however they were numerically similar across all three temporal periods.
Differences in Clinical Outcomes before and after implementation of protocol
| no. (%) | Before protocol(N = 169) | Protocol favoring ICA(N = 160) | Protocol Favoring CCTA(N = 133) | p-value 1 |
|---|---|---|---|---|
| Listed | 67 (39.6) | 70 (43.8) | 67 (50.4) | 0.07 |
| Listed and Transplanted | 30 (17.8) | 14 (8.8) | 25 (18.8) | 1.0 |
| Cardiac related complications | 10 (5.9) | 9 (5.6) | 8 (6.0) | 1.0 |
| • Decompensated heart failure• New onset atrial fibrillation• Myocardial infarction | 370 | 531 | 530 | |
| Death | 30 (17.8) | 26 (16.3) | 23 (17.3) | 0.94 |
Cardiovascular disease is the leading cause of 30-day mortality after liver transplant [11], however it is debatable whether chronic liver disease itself predisposes to obstructive CAD. This was highlighted in a study by An et al. which matched a registry of patients with cirrhosis undergoing CT coronary angiography to those without cirrhosis and identified a higher incidence of non-obstructive coronary disease (30.6% of cirrhotics versus 23.4% in non-cirrhotics, P=0.001) but not obstructive coronary disease (7.2% versus 7.9%, P=0.646) [24]. It is worth noting, this study included Korean patients evaluated between October 2007 and December 2012, with only 8% representing non-viral/non-alcohol-related liver disease. From a study by Patel et al. which identified obstructive CAD (defined as >70% stenosis using routine ICA) in 30.2% of patients with decompensated cirrhosis from NASH undergoing transplant evaluation at a US academic medical center [25], we know that NASH cirrhosis in particular may be correlated with CAD. With the increasing incidence of NASH as the etiology of patients with cirrhosis listed for liver transplant in the US (14% in our study population, 34% in a 2019 national sample), there is a growing concern for CAD in the contemporary liver transplant population [26] .
In an effort to address these concerns, we established a protocol that targeted patients with risk factors for CAD with normal stress testing and patients with abnormal testing to be referred to cardiology for consideration of ICA. Transition to routine use of ICA was favored because of the low positive (15-22%) and negative predictive values of vasodilator nuclear perfusion testing among liver transplant candidates, felt to be due to resting microvascular vasodilatory capacity in these patients, the basis on which nuclear perfusion imaging identifies CAD.[27] Moving to this protocol in 2018, we saw a doubling in the number of invasive coronary angiograms performed (18.8% versus 9.5%), with only five more patients being diagnosed with obstructive coronary artery disease by ICA. Of the seven patients with obstructive CAD diagnosed by ICA in 2018, only four underwent PCI while the others were medically managed. Of those medically managed, one is listed for liver/kidney transplant, one was denied on the basis of his cardiovascular disease, and one denied based on substance abuse relapse.
Evidence from the European Scottish Computed Tomography of the Heart (SCOTT-HEART) and the US-based Prospective Multicenter Imaging Study for the Evaluation of Chest Pain (PROMISE) trials firmly established the utility of CT coronary angiography in the evaluation of patients with suspected coronary artery disease [28,29]. Multiple groups have subsequently demonstrated the feasibility and safety of CCTA among liver transplant candidates [30–32]. With that said, although CCTA has been shown to have excellent sensitivity for the detection of obstructive CAD, the specificity can be quite variable for a variety of reasons including image quality and patient related factors. Jodocy et al. found that CCTA tends to overcall the severity of stenosis among LT candidates with only 3/24 patients with a high coronary calcium score (>300) and/or significant stenosis (>50% narrowing) on CCTA having confirmed obstructive coronary artery disease on ICA [33]. The 21 other patients with positive non-invasive tests were found to have diffuse CAD without significant stenosis. Our study also demonstrated the poor specificity of CCTA for obstructive CAD, likely further exacerbated by the low number of patients undergoing both CCTA and ICA in our cohort. Despite this, in the SCOTT-HEART trial, patients randomized to CT coronary angiography versus standard care saw a 41% reduction in nonfatal MI and death thought to be driven by a 40% increase in the use of preventive therapies among patients with obstructive and nonobstructive CAD. With an already high burden of metabolic complications in the post-LT period, identification of nonobstructive CAD by CCTA presents an opportunity to introduce statins early among these patients [34]. A randomized trial demonstrating the safety of pravastatin use in patients with chronic liver disease provides further support for this intervention. There is no data to the best of our knowledge as to the optimal time to restart or initiate statin therapy post liver transplant. In practice at our institution, once safe from a hepatology and transplant surgery perspective we start statin therapy.
Likely impacted by this data, and as the use of non-invasive anatomic coronary imaging increased across the world, there was non-protocol-driven growth in the use of CCTA during 2018 at our center (10 CCTA performed during 2018 versus 5 prior to 2018. With this experience, our protocol was modified to shift away from ICA towards CCTA for screening. This saw a non-significant decrease in the proportion of patients undergoing ICA (16.5% versus 18.8% in 2018) while the total number of patients diagnosed with obstructive CAD increased from 10/160 (6.25%) in 2018 to 23/133 (17.3%) after 2018. Despite identifying more patients with CAD after 2018, the proportion of LT candidates who went on to be listed increased.
Revascularization was rare in our population, with only 7/462 (1.5%) being treated with PCI or CABG. In comparison, Rachwan et al. report 14% of their LT candidates underwent revascularization as directed by their transplant protocol [35]. This group had previously reported an 8% one-year post-LT mortality and 0.7% MI rate [18]. For comparison, our center's post-LT one-year mortality and MI rate for patients transplanted in between 01/01/2018 and 12/31/2020, during which our protocols were in place, were 3.6% and 0%, respectively, with a similar proportion of patients listed for transplant after the institution of our transplant protocols (47% versus 52% in Rachwan et al.).
The low rates of revascularization were likely influenced by the consulting cardiologists’ extrapolation of data from the CARP trial, which failed to demonstrate a benefit of routine revascularization among patients with high-risk ischemia undergoing major vascular surgery.[36] Further, the COURAGE trial failed to demonstrate a benefit of upfront percutaneous revascularization among patients with stable CAD. [37] Favoring medical management of CAD is further supported by the ISCHEMIA trial published in 2019 after our study period, which failed to demonstrate an added benefit of performing revascularization among symptomatic patients with at least moderate burden ischemia. [38] Notably, the ischemia trial used CCTA to evaluate patients for obstructive left main coronary artery disease who were excluded from the trial. More frequent use of CCTA in the transplant population helps identify this highest risk subset of patients with CAD. Patients undergoing LT evaluation were not included in these trials. Recognizing gaps in the data, selected patients with high-risk coronary anatomy and/or symptoms related to their CAD should still be considered for revascularization.
4.1LimitationsThis descriptive retrospective study is limited as it focuses on changes in practice following the introduction of a standardized protocol at a single transplant center, thus reducing the external validity of our findings. In addition to this, due to the low number of clinical outcomes observed during our study follow-up period, we lack the statistical power to look for an association between protocol implementation and adverse clinical events.
5ConclusionFollowing the introduction of a standardized protocol for preoperative cardiac risk assessment, a significantly larger number of patients underwent preoperative cardiac risk assessment without a decline in the number of patients being listed for liver transplant. With a shift to the use of CCTA, there was an increasing trend in the proportion of patients with significant obstructive CAD diagnosed. While this study is not powered to test clinical outcomes among testing strategies, it does support prior studies supporting CCTA as a sensitive non-invasive screening tool for obstructive and non-obstructive CAD in pre-liver transplant patients. Future efforts should be aimed at further characterizing test performance of CCTA in the LT population, optimizing medical management for CAD in the LT population, and considering the institution of protocols identifying patients at the lowest risk for CAD who may not need any testing at all [35].
Authors' statementAll collaborators in this manuscript meet the definition for authorship based on ICMJE guidelines. All authors participated sufficiently in the work to take responsibility its content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Authors' contributionKillian J. McCarthy MB, BCh, BAO: Conceptualization, data curation, methodology, formal analysis, writing - original draft, writing - review and editing
Daniel Motta-Calderon MD, MPH: Conceptualization, data curation, methodology, formal analysis, writing - original draft, writing - review and editing .
Alisson Estrada-Roman, MD: Conceptualization, data curation
Karen M. Cajiao, MD: data curation
Michael P. Curry, MD: CStudy Designonceptualization, writing - review and editing .
Alan Bonder, MD: Study DesignConceptualization, writing - review and editing .
Anne-Marie Anagnostopoulos, MD: Conceptualization, writing - review and editing .
Michael Gavin, MD, MPH: St Conceptualization, data curation, methodology, formal analysis, writing - original draft, writing - review and editing .
FundingNone