Background & aims. Studies about the natural history of hepatitis C virus (HCV) infection report variable progression to cirrhosis depending on study design. Retrospective cross-sectional liver clinic studies overestimate the rate of fibrosis progression due to inclusion of patients with more severe disease leaving mild and asymptomatic patients underrepresented. We evaluated fibrosis progression in a group of “healthy” asymptomatic subjects, attending to a voluntary campaign for the detection of HCV infection.
Material and methods. A detection campaign was launched on subjects transfused before 1993. Of 1699 volunteers, 61(3.6%) had HCV infection. A liver biopsy was performed in 40 (65%). Assessed risk factors for liver fibrosis were: sex, body mass index, alcohol consumption (> 20 g/d♀ - >40g/d♂), genotype, HLA-DRB1 alleles, present age, age at infection and duration of infection.
Results. 25 (62.5%) were women with a median age of 52.5 years. The median duration of infection was 21.5 years with a median age at infection of 27 years. As regards fibrosis, 25 (62.5%) had a Low Stage (F0-F1), 8 patients, 20%, had severe fibrosis, one patient (2.5%) had cirrhosis. Alcohol consumption was the only risk factor associated with fibrosis progression.
Conclusions. The low progression to cirrhosis may be explained by the clinical characteristics of ourpopulation: asymptomatic middle-aged “healthy” subjects infected at young age. The progression to severe fibrosis was noticeable; hence a longer follow-up might demonstrate changes in this outcome. Significant alcohol consumption clearly worsens the natural history of HCV infection; this is no so evident for occasional or mild alcohol consumers.
The natural history of chronic hepatitis C virus (HCV) infection is benign in most patients, principally due to a slow fibrosis progression.1 Nevertheless some patients have a poor outcome progressing to cirrhosis and related complications. The high global incidence of HCV infection in the world makes HCV associated cirrhosis the leading indication of transplantation;2-3 in addition to accounting for the increasing incidence of hepatocellular carcinoma in many regions.4 The proportion of patients with benign or poor outcome reported in the different studies is variable. The risk of developing cirrhosis ranges from 1% to 50% over periods of 25 to 30 years.5 Factors associated with fibrosis progression and the impact of these factors on the natural history is also variable.6 This variation depends on the study design; study setting and study population.7 The best outcome is reported from prospective and community-based cohort studies, with poor results being recorded in retrospective studies and cross-sectional liver clinic series.8-9 While an ideal study would be a prospective one with a long follow-up, such undertaken sounds difficult given the relatively short term by which the HCV infection is certainly diagnosed. Also, the study population has to be a large community-or population-based one avoiding the referral bias. Furthermore, different population representing the whole spectrum of the disease should be included. For instance, the outcome seems to be very different between men transfused at middle age10 and young pregnant women.11-12
Given this constraints, studies employing other designs should be taken into account when attempting to expand our knowledge on the natural history of chronic HCV infection.
Our study is a retrospective-prospective cohort study where subjects with a recognized source of infection in the past were followed up prospectively. The population consists of a group of "healthy" subjects, attending to a voluntary campaign for detecting HCV infection. Participants were transfused before 1993, had no antecedents of hemodialysis or intravenous drug use.
In these subjects, we sought to evaluate the hepatic fibrosis progression and some factors associated with the development of this event.
Material and MethodsPatientsA voluntary detection campaign was launched in Rosario, a 1,000,000 inhabitant city of Argentina, between August and October 2005. Subjects transfused before 1993 were called through a TV and radio advertisement campaign to which 1,699 subjects attended. Year 1993 was chosen as a cut off because the ministerial resolution that regulates the screening for HCV in blood banks in Argentina was promulgated in December 1992. The oldest date of transfusion in our population was in 1960.
In the first visit patients were informed of the study purposes, completed an epidemiological and clinical form and a blood sample was collected to asses HCV infection. Informed consent was obtained from each subject. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s Human Research Committee.
Subjects with antecedents of hemodialysis or intravenous drug use were excluded at the time of filling an epidemiological/clinical form that was administered by trained personnel.
HCV antibodies (anti-HCV) were evaluated by third generation enzyme immunoassay (ELISA-Wienner) and HCV-RNA were detected using Amplicor Monitor HCV assay Roche (detection limit = 50 IU/ mL). HCV genotype was determined by using restriction fragment length polymorphism (RFLP).
All anti-HCV(+)/HCV-RNA(+) patients where offered to continue their follow up at the Gastroenterology and Hepatology Department, Hospital Provincial del Centenario, Rosario, Argentina.
Assessments during follow-up included routine haematological, biochemical tests, and liver function tests together with clinical, physical and ultrasound examination.
Hepatitis B surface antigen (HBsAg), antibodies against hepatitis B core antigen (anti-HBc) and Human Immunodeficiency Virus (HIV) antibodies where also evaluated in all cases.
A liver biopsy was offered to all patients and HLA-DRB1 (Human Leukocyte Antigen DRB1) alleles where evaluated in biopsied patients. The alleles were tested using polymerase chain reaction and sequence-specific primers technique (PCR-SSP).
Among the 1,699 attendees, 85 (5.1%) were anti-HCV(+), of these, 61 (71%) were anti-HCV(+)/HCV-RNA(+) and were included in the study. The anti-HCV(+)/HCV-RNA (-) subjects, were regarded as having a resolved infection and not included. All Anti-HCV(+) (ELISA) patients were confirmed by Line Immunoassay (LIA-Innogenetics) so false positives were discarded.
A liver biopsy could be performed in 40 of them (65 %). Liver biopsy was not performed in 21 remaining patients for the following reasons: 7 patients never attended after the first visit, 8 patients had an incomplete follow-up (4 of them refused to have a liver biopsy performed). Three patients under a regular follow up, with no evidence of severe liver disease, have conditions precluding liver biopsy: abnormal fibrinolysis, non Hodgkin lymphoma, and anticoagulant treatment. Another patient had a prolonged prothrombin time related to his hepatic disease. Two patients received HCV treatment without liver biopsy.
HistopathologyLiver biopsy was performed with a 14 gauge trucut needle, and a specimen of at least 1.5 cm was obtained for each patient. All specimens were stained with H&E (Hematoxylin and Eosin) and Masson trichrome stains and evaluated by an expert liver pathologist. Fibrosis was staged according to METAVIR as follows: F0-no fibrosis; F1-portal fibrosis without septa; F2-portal fibrosis and few septa; F3-numerous septa without cirrhosis; and F4-cirrhosis.13
Risk factors for fibrosis progressionThe following risk factors for progression of liver fibrosis were evaluated: sex, body mass index (BMI): (< 25 kg/m2 or > 25 kg/m2), alcohol consumption (> 20 g/d $ -> 40 g/dôó, genotype 1 and no 1 HLA-DRB1 alleles, present age, age at infection and duration of infection. Duration of infection was analyzed as a continue variable and also categorized in < 20 years or > 20 years of infection. Date of transfusion was considered the date of infection. Duration of infection was considered the time between date of infection (transfusion) and liver biopsy.
Alcohol consumption: Data were thoroughly recorded during the follow up by the same researcher. Consumption was graded according to a scale taking multiples from a basal 20 g/day value.
BMI was collected at the first visit of the follow up and at the time of the liver biopsy. There was no significant difference between these values. We consider BMI at the time of liver biopsy for the analysis.
Alcohol consumption and BMI could not be evaluated in all the subjects in whom liver biopsy was not performed. Smoking habit was not assessed.
Viral coinfection (HIV and HVB) were evaluated in all. The patients included never received treatment before liver biopsy.
Evaluation of fibrosis progressionFibrosis progression was evaluated in two ways. First, all biopsied patients where classified into two groups: low stage (F0-F1) and intermediate/high stage (F2-F3-F4). Second, the rate of fibrosis progression (Fibrosis Stage in METAVIR units/Duration of infection in years) was calculated according transfusion date. In both cases the association with different factors for fibrosis progression was analyzed.
Statistical analysisDescriptive analysis was carried out for both continuous and qualitative variables. Categorical variables were analyzed by the Pearson's x2 and Mantel Haenzsel tests. For analysis of quantitative variables the Mann Whitney U test was employed. The exact logistic regression was also applied to evaluate whether significant variables were able to predict hepatic fibrosis. Differences in the distribution of HLADRB1 alleles between groups were analyzed by Pearson's x2 test with Yates correction, if applicable. Odds Ratios (OR) were calculated; with p values being corrected according to the number of comparisons (a/n). The significance was established at p < 0.05.
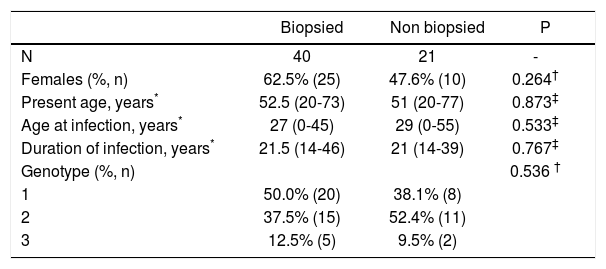
ResultsLiver biopsy was performed in 40 patients, representing 65% of the anti-HCV(+)/HCV-RNA(+) 61 patients detected in this voluntary campaign. Cases had a median present age of 52.5 years, median age at infection of 27 years and median duration of infection of 21.5 years; 25 of them (62.5%) were women (Table 1). Of the 1699 subjects who attended 1326 (78%) were females and 373 (22%) were males. No significant difference was found between all the attendees and infected subjects regarding age. There was a predominance of females either among attendees or those that finally were recruited into the study, more evident in the former group.
Patient characteristics.
| Biopsied | Non biopsied | P | |
|---|---|---|---|
| N | 40 | 21 | - |
| Females (%, n) | 62.5% (25) | 47.6% (10) | 0.264† |
| Present age, years* | 52.5 (20-73) | 51 (20-77) | 0.873‡ |
| Age at infection, years* | 27 (0-45) | 29 (0-55) | 0.533‡ |
| Duration of infection, years* | 21.5 (14-46) | 21 (14-39) | 0.767‡ |
| Genotype (%, n) | 0.536 † | ||
| 1 | 50.0% (20) | 38.1% (8) | |
| 2 | 37.5% (15) | 52.4% (11) | |
| 3 | 12.5% (5) | 9.5% (2) |
Data about genotype, alcohol consumption and BMI are shown in table 1 and 3. No significant difference was found between biopsied and non biopsied patients regarding sex, present age, age at infection, duration of infection and genotype (Table 1). All patients had undetectable HIV antibodies and HBsAg. Two patients were positive for anti-HBc.
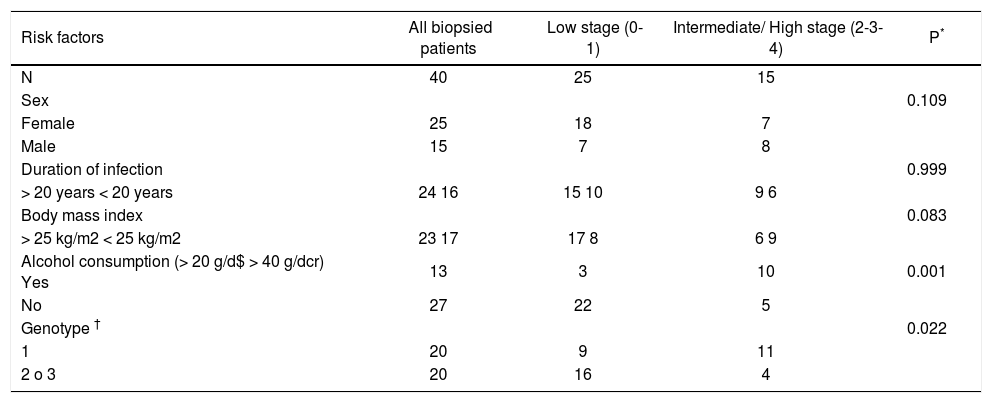
Presence of risk factors for fibrosis progression according to histological stage.
Presence of risk factors for fibrosis progression according to histological stage.
| Risk factors | All biopsied patients | Low stage (0-1) | Intermediate/ High stage (2-3-4) | P* |
|---|---|---|---|---|
| N | 40 | 25 | 15 | |
| Sex | 0.109 | |||
| Female | 25 | 18 | 7 | |
| Male | 15 | 7 | 8 | |
| Duration of infection | 0.999 | |||
| > 20 years < 20 years | 24 16 | 15 10 | 9 6 | |
| Body mass index | 0.083 | |||
| > 25 kg/m2 < 25 kg/m2 | 23 17 | 17 8 | 6 9 | |
| Alcohol consumption (> 20 g/d$ > 40 g/dcr) Yes | 13 | 3 | 10 | 0.001 |
| No | 27 | 22 | 5 | |
| Genotype † | 0.022 | |||
| 1 | 20 | 9 | 11 | |
| 2 o 3 | 20 | 16 | 4 |
Fibrosis stage was as follows:
- •
Low stage 25 (62.5%) F0 = 8, F1 = 17.
- •
Intermediate/High stage 15 (37.5%) F2 = 6; F3 = 8; F4 = 1.
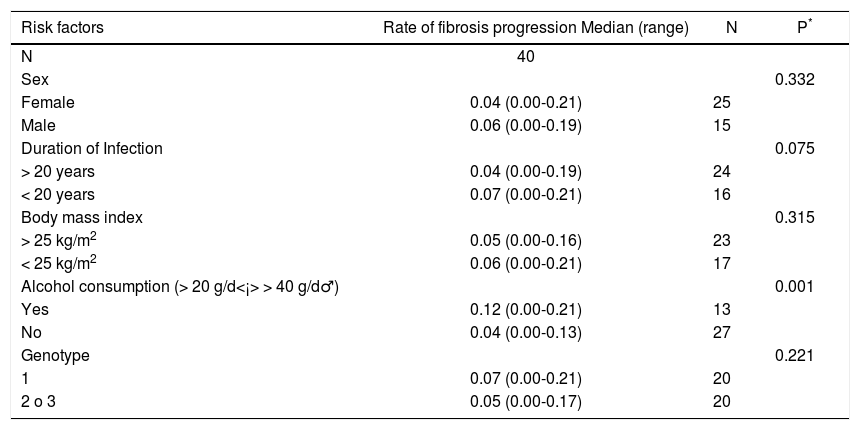
The median rate of fibrosis progression was 0.0520 units of fibrosis/year (range: 0.000-0.214).
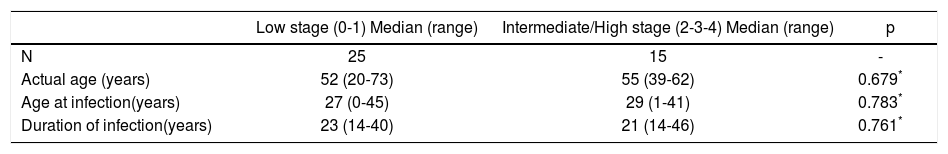
No association between severity of fibrosis and, sex, BMI, present age, age at infection and duration of infection was found (Tables 2, 3 and 4). The same was true when analyzing HLA-DRB1 alleles (data not shown). As regards the viral genotype distribution, the initial statistical difference seen in the univariate analysis became insignificant when adjusting for alcohol consumption (Table 3). Analysis by employing the exact logistic regression showed that alcohol was the only factor being significantly associated with fibrosis [OR = 13.38; 95% CI 2.38-105.64; intercept estimation-1.4816 ± 0.494 (SE), p = 0.0015; regression coefficient 2.5942 ± 0.8027, 0 = 0.0012]. The median rate of fibrosis progression was significantly higher in patients with alcohol consumption (0.12 units of fibrosis/year) respect to patients without it (0.04, p = 0.001) (Table 4).
Rate of fibrosis progression according to risk factors.
| Risk factors | Rate of fibrosis progression Median (range) | N | P* |
|---|---|---|---|
| N | 40 | ||
| Sex | 0.332 | ||
| Female | 0.04 (0.00-0.21) | 25 | |
| Male | 0.06 (0.00-0.19) | 15 | |
| Duration of Infection | 0.075 | ||
| > 20 years | 0.04 (0.00-0.19) | 24 | |
| < 20 years | 0.07 (0.00-0.21) | 16 | |
| Body mass index | 0.315 | ||
| > 25 kg/m2 | 0.05 (0.00-0.16) | 23 | |
| < 25 kg/m2 | 0.06 (0.00-0.21) | 17 | |
| Alcohol consumption (> 20 g/d<¡> > 40 g/d♂) | 0.001 | ||
| Yes | 0.12 (0.00-0.21) | 13 | |
| No | 0.04 (0.00-0.13) | 27 | |
| Genotype | 0.221 | ||
| 1 | 0.07 (0.00-0.21) | 20 | |
| 2 o 3 | 0.05 (0.00-0.17) | 20 |
Mean follow up after the first visit (time between diagnosis and liver biopsy) was 9.9 months, median: 7 months (range: 1-60 months). Most of the subjects were biopsied during the first year of the diagnosis and more than a half received HCV treatment after that.
DiscussionOur findings show a low occurrence of cirrhosis in an asymptomatic community-based population. While our results are in line with previous reports showing that significant alcohol consumption is associated with more severe fibrosis, mild alcohol intake did not worsen the natural history of HCV infection in this series patients.
The risk of developing cirrhosis is quite variable among studies analyzing the natural history of chronic HCV infection.7
Retrospective-prospective studies in women infected at a young age report the lowest rates of cirrhosis, 1% to 2%,11,12 whereas higher rates, 20% to 50%, were seen in cross-sectional retrospective studies from referral centres.14-19 In the latter studies, individuals attend for better diagnosis and treatment, requiring complementary studies and specific therapeutic procedures. Hence, subjects with more severe disease are overrepresented for which fibrosis progression could be overestimated (referral bias). On the other hand, in community-based studies in nonclinical settings, asymptomatic subjects with mild disease (too "healthy" to be recognized in former designs) are overrepresented. It follows that some grade of ascertainment bias (systematic failure to represent equally all classes of subjects supposed to be represented in a sample) is almost unavoidable due to the heterogeneity of the population included in different studies with quite variable rates of fibrosis progression. A global estimation of progression to cirrhosis is very difficult given the wide variation of studies including subjects with quite different rates of fibrosis progression.
One of the limitations of our study is that enough and reliable data about all transfused patients in our city, is in this period is lacking. Patients with more severe disease or faster progression attended to referral centers for which they have been missed or underrepresented in our study. On the other hand, we think that people with mild disease could be detected mainly in population, blood donors or screening studies. Probably a large proportion of our subjects would never seek attention provided they never heard about the campaign. Therefore, although our study may have excluded patients with more severe disease or faster progression, it took into consideration subjects “too healthy” to be recognized in other designs (e.g. cross-sectional retrospective liver clinic studies) and whose evolution is also part of the wide variable scenario of the natural history of HCV infection.
Our sample derives from a large population of healthy persons that voluntarily attended to a detection campaign, composed of well-diagnosed asymptomatic individuals, unlikely to be included in cross-sectional liver clinic studies. Furthermore, people included in our study ignored that had been infected with HCV for many years until the time they where included. This is particularly important regarding alcohol consumption, keeping in mind that in other large retrospective-prospective cohorts including young women,11,12 alcohol abstinence was advised and probably the natural history of the disease could have been modified with this sole action.
It is worth commenting that present study deals with a retrospective-prospective cohort where subjects with a recognized source of infection in the past were followed-up prospectively. Previous retrospective-prospective studies report a lower prevalence of cirrhosis than retrospective ones.10-12,20-24
In our cohort, the follow up after the first visit (time between diagnosis and liver biopsy) was short: mean: 9.9 months, median: 7 months (range: 1-60 months). Many patients received treatment after liver biopsy was performed. Hence, although the evaluation and staging of all the patients were prospective, the prospective follow-up was short compared with the retrospective follow-up, for ethical reasons.
The patients were categorized in two groups according to stage of fibrosis, the decision to consider F2 Stage as the limit to intermediate/high stage was based on the fact that the presence of fibrous septa in a liver biopsy is a marker of progressive fibrosis. It is also well known, that persons with no or minimal fibrosis (METAVIR stage 0-1) have a low risk for liver-related complications and liver-related death, over the next 10 to 20 years.25 Although only 65% of the subjects could be biopsied, we consider that the histological findings represent the entire series, as no significant difference was found between biopsied and non biopsied patients (Table 1).
For transfusion-acquired infection, the date of transfusion is conventionally used as the time of HCV infection. This allows calculating the rate of fibrosis progression (Fibrosis Stage in METAVIR units/Duration of infection). In previous studies the estimated fibrosis progression calculated indirectly according the date of infection and stage of fibrosis in a single biopsy was similar to the estimated observed in paired biopsy samples.1,26
This approach assumed that progression rates are constant and linear across fibrosis stages. However it has been strongly suggested that fibrosis progression is non linear and that the rate of fibrosis varies between stages,7,27 and accelerates with patient ageing. For these reasons, using fibrosis progression ratio for estimating de velocity of fibrosis has clear limitations; but it is useful when comparing with another series.
Our sample was a homogeneous population of transfused people, of whom 60% had minimal or no fibrosis after a reasonably long follow-up (median 21.5 years). The median global rate of fibrosis progression was 0.0520 units of fibrosis/year, the half of published in another series,1,28 and coincident with the low incidence of cirrhosis (2.5%), although the occurrence of severe fibrosis (F3) was 20%. We considered that liver biopsy was enough to evaluate disease severity in biopsied patients. Nevertheless other lab/clinical data which may reflect disease severity might have been useful in subjects without liver biopsy but could not be assessed in them.
Age, age at infection, duration of infection and sex are recognized factors associated with fibrosis progression but no relationship was found in our sample between these factors and fibrosis. Previous studies showed that age of infection was the main risk factor for fibrosis even after controlling for duration of infection.1,28 In our series median age at infection was 27 years (0-45); only two patients where over 40 years, approximately the age of infection at which the fibrosis progression begin to increase.1 The fact that most of our subjects where less than 40 years at the time of infection, might have acted as a "protective" factor rendering the other factors, i.e., sex and duration of infection unable to reach a statistically significant association with more rapid fibrosis. Also, another study of the same group showed that fibrosis progression begins to accelerate at 50 years of age independently of duration of infection;29 in our series median present age was 52.5 years. Hence, the low fibrosis progression in our study may have several explanations: young age at infection and young present age, along with the fact that patients derived from an asymptomatic "healthy" population like subjects included in nonclinical/ community settings.
As shown in previous reports HCV genotype is not associated with fibrosis.1,29 Although an association with genotype 3 which was recently reported,30 this could not be analyzed in our study because the small sample size. In line with the demonstration of an inconsistent association between a particular HLA DRB1 allele and fibrosis,31,32 presence of fibrosis was not related with a distinct frequency of some HLA DRB1 gene.
In parallel, increased BMI was not associated to fibrosis. Nevertheless, the relative contribution of obesity cannot be fully discarded in light of its well-known coexistence with insulin resistance, steatosis, and steatohepatitis, factors that were not assessed in the present study.33
Cumulated evidence has established that alcohol consumption has a deleterious effect on hepatitis C severity and our results are in line with this evidence as significant alcohol consumption (> 20 g/d$ - > 40 g/dö") was strongly associated with fibrosis progression. Several studies have shown that alcohol intake with levels of consumption greater than 50 g/day is associated with an increased rate of fibrosis progression.34,35,37 Nevertheless, no agreement exists about the potentially dangerous threshold, for which the impact of lower levels of alcohol consumption remains unclear.34,37-40
In the United States, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) has established age-and sex-specific recommended consumption thresholds.41 We consider that a different range for men and women was appropriate and a cut off of > 20 g/d for women and > 40g/d for men was simply, reliable and near the NIAAA recommendations. In our study, abstainers (n: 17) and mild drinkers (n: 10) where analyzed together. More stratification into different grades of alcohol consumption could not be done due to the small sample size. In larger studies it would be very useful to assess differences among abstainers, mild, moderate and heavy drinkers. Our data seem to support the hypothesis that mild consumption would not be associated with more rapid fibrosis in HCV infected patients.36,40
Features of present study provide a different and valuable look within the quite variable scenario on natural history of hepatitis C. On one hand, cohort studies in post-transfusional cases included patients with a history of post-transfusion hepatitis, most of them after cardiac surgery or haematological disorders.10,42-45 In our study, instead, patients were apparently healthy, asymptomatic subjects who attended a screening campaign. On the other hand our patients were not individuals referred for further assessment of their HCV infection status, conversely, they attended to a referral centre without evidence, until then, of their liver disease. These facts partly explain that the percentage of progression to cirrhosis of 2.5% in 21 years resembles the figures reported, in community-based and blood donor studies;5 and in retrospective-prospective as well as nonclinical studies.7
In line with other reports, our study points out that the natural history of many patients with HCV infections seems to be benign and non progressive for a long time. As above stated many studies suggest that fibrosis begins to accelerate with patient ageing, hence, a longer follow up might demonstrate changes in this otherwise benign course. This is supported by the fact that while the occurrence of cirrhosis in our series was low, presence of severe fibrosis was noticeable (20%).
Given the low rate of progression observed in population studies, young patients detected in this setting, with minimal or no alcohol consumption could be assessed with non-invasive techniques (e.g. transient elastography). Treatment could be delayed waiting for better options, in those without significant fibrosis according with predictors of treatment response like viral genotype and IL28B haplotype.
Although large studies are needed, it seems that occasional or mild alcohol consumers behave similar to abstainers in terms of fibrosis progression. A "safe" threshold for alcohol consumption in HCV infected patients is quite difficult to define, however, as others authors have suggested mild alcohol consumption is not associated with increased fibrosis36,40 and physicians must keep these data in mind when counselling these patients.
Acknowledgements and DisclosuresThe authors declared that they not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Abbreviations- •
HCV: hepatitis C virus.
- •
HLA: human leukocyte antigen.
- •
Anti-HCV: hepatitis C virus antibodies.
- •
RFLP: restriction fragment length polymorphism.
- •
HBsAg: hepatitis B surface antigen.
- •
anti-HBc: hepatitis B core antigen antibodies.
- •
HIV: human immunodeficiency virus.
- •
PCR: polymerase chain reaction.
- •
H&E: Hematoxylin and eosin.
- •
BMI: body mass index.
- •
OR: odds ratio.