Hepatic cystic tumours are a heterogeneous group of diseases with different aetiology and incidence, and with similar clinical signs and symptoms. They are classified as congenital, traumatic, parasitic, or neoplastic cysts. The congenital cystic tumours are the most prevalent, and include the simple cyst and polycystic hepatic disease. Other less common lesions are, hepatic cystadenoma, ciliated embryonic cyst, and a miscellaneous group. We have carried out a review of all benign non-parasitic hepatic cystic tumours, placing special emphasis on therapeutic strategies.
Los tumores quísticos hepáticos son un grupo heterogéneo de enfermedades con distinta etiología e incidencia, y con manifestaciones clínicas similares. Se clasifican en quistes congénitos, traumáticos, parasitarios o neoplásicos. Los tumores quísticos congénitos son los más prevalentes e incluyen al quiste simple y a la enfermedad poliquística hepática. Otras lesiones infrecuentes son el cistoadenoma hepático, el quiste hepático ciliado embrionario, y un grupo miscelánea. Hemos efectuado una revisión de todas lesiones quísticas hepáticas benignas no parasitarias, haciendo hincapié en las estrategias terapéuticas.
Hepatic cysts (HC) are a heterogeneous group of diseases with a different aetiology and prevalence, but are clinically similar.1–4 They can be classified as congenital, traumatic, parasitic and neoplastic.5–10 The congenital type is the most important one and includes simple cyst (SC) and polycystic liver disease (PLD).8,9,11–13 HC are diagnosed incidentally, as they are usually asymptomatic, benign and more frequent in women.2–4,6,14–17 Their incidence is unknown but it is estimated that 5% of the population have non-parasitic HC.7,8,15,17–22
Simple CystThe SC is the most common liver injury, with a prevalence in the adult population of between 0.1% and 7%.5,6,9–12,20,22–31 It is filled with serous fluid and has no communication with the biliary tree.10,12,16,23,24 It is more common in women, with a ratio of 2:1 for asymptomatic and 9:1 for symptomatic SC.2,5,8,17,19,22,24,27,29,31 It is probably due to an aberrant bile duct losing communication with the biliary tree,1,5,8,10,12,14,18,22,24,27–30,32 and cases of spontaneous disappearance have been reported.30
Macroscopically, it has a spherical or ovoid shape, unilocular and without septa. Its size ranges from a few millimetres to more than 20cm, and 60% of patients have a single lesion.6,23,24 Unlike PLD, multiple SC occupy less than 50% of a patient's liver.6,17 Microscopically, the SC has a column monolayer epithelium, similar to the biliary one, that may become necrotic if the intracystic pressure is high.5,6,23,24,27,29,30 There is no surrounding stroma in small SC and only a thin layer of connective tissue in large ones.6,24
SC is usually asymptomatic and diagnosed incidentally.9–12,17,22–25,28,29,31–33 When symptomatic (10%–15%), the most common symptom is abdominal pain.5,14,17,18,22,24,27,28,30,32 Other symptoms include nausea and vomiting, postprandial fullness, shoulder pain, dyspnoea and palpable abdominal tumour, etc.5,11,12,16–18,20,22,24,28,31,32 Analytical studies provide normal results unless there is compression of the biliary tree. Some SC present with high levels of intracystic CA19-9.9,18,34 SC complications occur in 5% of patients.11,20,23 The two most common complications are infection, often monomicrobial by E. coli, and bleeding.2,11,16,20,23,26,32 Other less common ones are as follows: traumatic or spontaneous rupture, torsion, compression of surrounding structures such as the inferior vena cava; portal vein, causing portal hypertension; the bile duct, producing cholestasis, cholangitis and jaundice; fistulisation to the duodenum or biliary tree; and exceptionally, malignisation.2,7–9,11,16,20,22–24,26,28,31,32,35–41
Intracystic haemorrhage usually occurs in elderly patients with large SC.13,33 The most common symptoms are rapid growth and abdominal pain.2,12,31,33 If bleeding is significant, it may lead to compressive thrombosis of the inferior vena cava.13,20,36 The intracystic haematic content may mimic a hepatic cystadenoma (HCy).13,33,38 Contrast ultrasound and magnetic resonance imaging (MRI) are useful for the diagnosis of intracystic haemorrhage.2,12,42 A bleeding SC must be treated surgically to prevent future complications,13,23 although percutaneous drainage, embolisation and observation, if it is not infected, have been used in patients with severe comorbidities.13
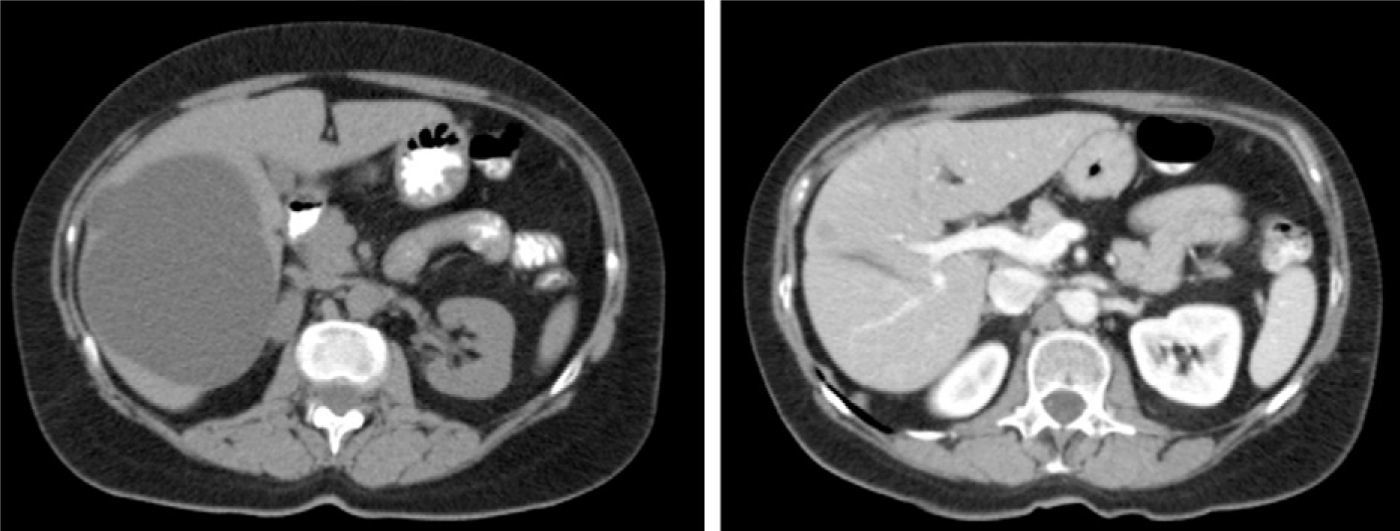
The most cost-effective imaging method for SC is abdominal ultrasound. The radiographic features are typical3,32,33: a circular or oval anechoic lesion, non-septated, with posterior enhancement caused by dense echoes behind the SC due to intracystic fluid.5,11,23,24,42 A CT scan confirms the presence of avascular cystic lesions with water density without contrast enhancement (Fig. 1).11 MRI is useful for the diagnosis and detection of intracystic complications: the SC is observed as a T2 homogeneous hyperintense lesion without contrast enhancement and low intensity on T1.9,11,23 Differential diagnosis arises with the hydatid cyst, especially in endemic areas, HCy and cystic metastases.1,10,14,18,24,43
Treatment is needed only if there are complications, and periodic monitoring suffices for asymptomatic patients.1,5,12,14,15,17,22,24,25,27–29,31,43 The size of the cyst itself is not an indication for surgery, although large ones are usually symptomatic.2,3,14,18,31 Treatment options may be non-surgical (puncture aspiration with or without injection of sclerosing agents) or surgical,14,22,28–30,32,44 which are divided into conservative procedures: fenestration, and other techniques now practically abandoned (cystojejunostomy or marsupialisation); or radical (cystectomy or hepatic resection).3,12,17,25,26,29,32,44
Aspiration under ultrasound/CT control may relieve the symptoms, but relapse is the norm. It makes sense to apply it only for patients at high surgical risk, as a diagnostic technique, in infected cysts or to find out whether the cyst causes the symptoms.1–3,5,6,11,12,15,17,18,22,23,28,29,32 The instillation of sclerosing agents (e.g., alcohol, minocycline, monoethanolamine oleate or pantopaque) improves the results obtained with aspiration but has a high recurrence rate (20%–90%) and complications: pain during the procedure, alcohol poisoning, cholangitis or eosinophilia.2,9,11,12,14,16,18,22,28,29,31 The presence of a coagulation disorder, intracystic bleeding or SC communication with the biliary tree contraindicates the use of puncturing.1,11,12,14,16,29
Fenestration is a technique that can be performed by laparotomy or laparoscopy.12,22,26,28,32,45,46 Its surgical risk is low, but long-term results are short-lived since late relapse is common. Laparoscopic fenestration was first described by Z’graggen in 1991 and has become the most commonly performed procedure in solitary symptomatic SC.3,6,17,18,22,27–29,43,47,48
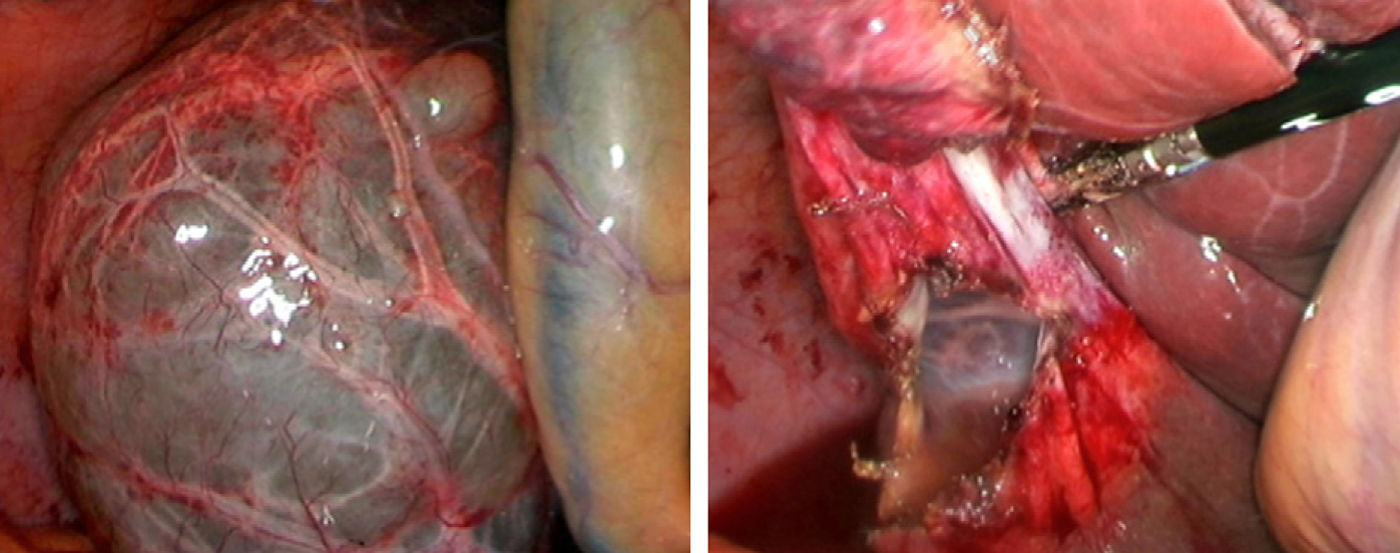
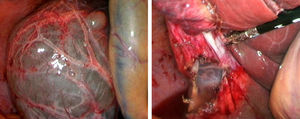
Laparoscopic fenestration (LF) starts with the puncture of the cyst for an analytical study (tumour markers, amylase, bilirubin and LDH), as well as a microbiological and cytological study of the intracystic fluid. The visible part of the SC is then dried with a hook, dissecting sealer, cutting stapler and is extracted into a sample bag for later histological analysis (Fig. 2).2,5,27,28,31,32 If there are multiple connected cysts, fenestration of the deep cysts can be performed via the external cysts.11,45 The windows made must be large enough to ensure permeability of the communication and prevent the SC from filling.6,11,15,28 A biopsy must be performed on any suspicious intracystic node.12,31 Fulguration of the unresected cystic epithelium has been performed with argon, electrocautery, and other similar tools.12,14,25,26,28,29,31,49 Currently, laparotomic fenestration is rarely indicated.31
The percentage of complications after LF of SC is between 0% and 18%.5,12,17,18,31 These include the following: abdominal hydatid cyst spread if there is an incorrect diagnosis, post-fenestration ascites, air embolism, pleural effusion, dyspnoea, biloma and haemorrhage.12,29,43,47 The rate of recurrence of symptoms is between 4% and 44%.5,14,15,17,18,26,28,29,31,43,48 Factors favouring recurrence after LF are: cysts centrally located or on the posterior right part, limited resection of the visible cyst wall, previously operated cysts, and the use of a hook instead of a dissecting sealer for resection.14,15,18,25,28 The very frequent radiological recurrence after LF is not always accompanied by the appearance of symptoms.17
Total cystectomy and hepatic resection are techniques performed occasionally.2,5,11,12,14,15,22,29,31,44 There is a publication on radiofrequency treatment of SC.50
The treatment for patients with infected SC is percutaneous puncture and antibiotics to improve the symptoms and provide a reliable diagnosis, deciding the most appropriate treatment later.7
Polycystic Liver DiseasePolycystic liver disease (PLD) has a prevalence of between 0.05% and 0.6%. It consists of multiple hepatic cysts occupying more than 50% of the liver parenchyma. It is as an inherited autosomal dominant disease, and usually occurs in combination with polycystic kidney disease or cysts in other organs (pancreas, spleen, ovaries and lung, although rarely in the latter).1,2,6,11,31,34,45,46,51–64 One-third of patients with PLD never develop kidney cysts, and these patients have genetic alterations on chromosome 19p13.2-13.1 (PRKSCH) and 6q (SEC63). This is different to patients with hepatorenal polycystic disease, with alterations on chromosomes 4 and 16 (PKD1 and 2).52,55,60,61,65–72 Patients with only PLD have larger cysts but with fewer complications than patients with hepatorenal polycystic disease.52,66
PLD is more common in women, who also tend to have more and larger cysts.46,60,64,66,73 Patients with PLD also have a higher incidence of intracranial aneurysms.11,31,51,62,64,71,72
Liver injury in PLD can be microcystic, macrocystic or biliary hamartomas (von Meyenburg complexes) distributed evenly over the liver and peribiliary areas.52,58,61,69 The normal parenchyma shows fibrosis and vascular changes.63 Cysts usually contain clear fluid and histologically are lined by cuboidal epithelium.58,61,69 The cysts grow in size, number and volume with age.65
PLD cysts are usually asymptomatic.45,51,52,57,58,60,61,72 When symptoms occur, due to increased volume or compression of adjacent structures, they are similar to those of SC.31,46,52,54,55,58,60–65,67,72,74,75 Complications are more common in symptomatic patients: for example, cyst infection, rupture, torsion, intracystic bleeding, portal hypertension causing ascites and/or oesophageal varices, compression of the vena cava, biliary obstruction or cystocutaneous fistula.45,51,52,55,57,58,60,61,65,67,68,72,76 Cystic infection or rupture is more common in patients with a kidney transplant, possibly due to immunosuppression.62 Most symptomatic patients (75%) have palpable hepatomegaly.54,61,62 Stigmata of liver disease and acute liver failure are rare.60,77
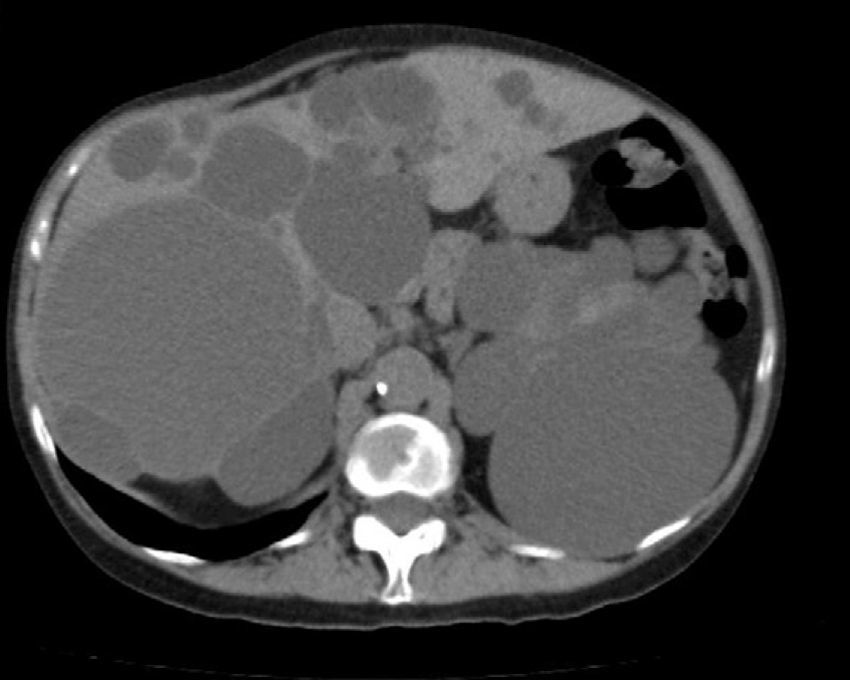
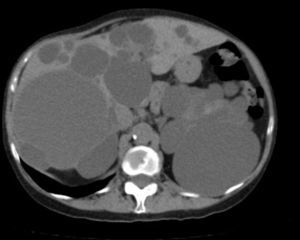
Diagnosis of PLD can be made by ultrasound, helical CT and MRI.55,58,60,61,67,72 CT is better at defining the extent of the liver disease and the involvement of adjacent organs.31 It shows multiple hypoattenuating liver lesions of various sizes with well-defined margins and no contrast enhancement (Fig. 3).60,72 In MRI, the lesions are of low intensity on T1 and hyperintense on T2,61 and this is very useful for determining intracystic bleeding.63 The liver biochemical profile is usually normal or slightly altered.2,52,55,60,61,64,72 The serum CA19-9 may be increased, and the intracystic one is usually very high, especially in large cysts.59
Patients with asymptomatic PLD require no treatment.2,52,53,55,57,58,62,74 There is no consensus on how to treat symptomatic patients.2,27,31,45,53,55,57,60,61,63,65,72 The goal of treatment is to reduce the size of the cysts, without compromising liver function, and achieve the longest possible period without symptoms.51
The choice of treatment must be based on the size and extent of the cysts and the presence or not of complications. Gigot devised a classification that defines a therapeutic strategy and divides the cysts into the following: type I, a few large cysts between 7 and 10cm; type II, multiple medium-sized cysts (5–7cm); and type III, multiple medium and small cysts (less than 5cm).26,31,51,72,78 Li and Schnelldorf proposed new classifications which have not been used as much.51,74
Type I patients can opt for LF of the accessible dominant cyst(s) or puncture.1 LF results in a reduction of 12.5% in cystic volume (range: 9.4%–24.7%) which produces a clinical improvement (less bloating and postprandial fullness), and a recurrence of 4.5%–16%.1,14,25,26,45,60,61,67,74,78 In some cases (e.g., infected cyst or high surgical risk), a percutaneous puncture can be performed to temporarily improve the symptoms.1,2,26,61,78 Its advantage is minimum morbidity and mortality, but the disadvantages are the high recurrence rate and low reduction in mass.46,53,58,61,67,72,79
There are 2 procedures for type II patients: fenestration and liver resection, or a combination of both. Both have significant morbidity, and long-term palliation of the symptoms is not achieved.3,7,15
Fenestration was classically performed by laparotomy by draining the internal cysts into the most superficial ones.45,51,61,72 Fenestration does not achieve the results expected due to the growth of other small or inaccessible cysts, and for not managing to collapse the liver easily due to its rigidity.11,31,45,46,61 In recent years there have been small series of LF in patients with PLD.26,31,45,60,67,78 Comparing both approaches, laparotomic fenestration results in a reduction in volume of 43%, a mean recurrence rate of 17% (11%–26%), a morbidity between 0% and 56% and a mortality between 0% and 11%.6,25,26,72 LF obtains an initial improvement in symptoms for over 70% of patients, recurrence is between 0% and 54%,26,31,45,60,61 mortality is practically nil and morbidity between 0% and 67% (including ascites, bleeding, biliary fistula and pleural effusion).26,45,48,67,72 The technical problems of LF are mistaking a cyst for a large venous trunk, leading to cataclysmic bleeding; and difficult access to the intrahepatic cysts or posterior segments.25,45,51,53,61,75 The published results do not give a clear preference for laparotomy or laparoscopic fenestration.51
Hepatic resection is occasionally used, but is the method of choice when the patient has portal hypertension and/or cysts situated in an anatomical region of the liver.
The combination of resection and fenestration of peripheral cysts and communication with the innermost ones is the technique with the best results, with a reduction in volume of 75% and a recurrence rate of 15%.1,26,46,51,53,62,63,67,69 The advantages are: reduction in volume, complex cyst elimination, and regeneration of healthy liver parts.63 Morbidity ranges between 20% and 100%, including vascular lesions, biliary lesions, postoperative ascites, which is very difficult to resolve as it exceeds the peritoneal absorption capacity (900ml/day), insufficient postoperative liver volume and kinking of the liver.45,46,51,61–63,67,72,74 Mortality is between 0% and 11%.46,51,53,63,67,72,74 The main disadvantages of this technique are high morbidity and mortality; it is not applicable to patients with a severe preoperative hepatorenal situation and, when it fails, there is little choice for the patient other than a liver transplant. It is always recommended to perform a cholecystectomy with cholangiography to prevent biliary leakage.58,63 Fenestration of the cysts near the hepatic veins and vena cava is important to prevent future compression complications.63,74
Type III can be treated by a combination of fenestration and/or resection or liver transplant.1,26,63,74 If the PLD-affected areas are close and leave enough viable parenchyma, resection with fenestration is feasible. However, when it is very diffuse, or the remaining volume is less than 30%, or there are histological changes in the liver parenchyma or there is renal failure, a transplant is indicated before any complications appear.31,63,64,68,78In 1988, Kwok performed the first PLD-induced liver transplant. Since then there have been 600 cases in Europe and PLD consists of 0.5% of indications for liver transplantation.51,56,64,68,73,77,80,81 The most common indication is the lethal exhaustion syndrome (cachexia, fatigue and pain).72 Women comprise 90% of transplant recipients, hepatorenal affectation is 90%, but only 53% are kidney transplants.56,63,64,68,73,74,77,80–83 Morbidity is 85% and perioperative mortality is 12.5%, due mainly to the poor pre-operative condition. Survival at 5 years is 85%.56,63,64,67,73,74,77,80–83 Palliation of the symptoms is excellent.56,63,64,67,73,74,77,80–83
These transplants are often complex due to previous surgery and the large size of the liver.63,67,77 Traditionally, it was recommended to start surgery via the hepatic hilum, use the veno-venous bypass and the classical technique with resection of the inferior vena cava,65 but the piggyback option is feasible in a large percentage of patients.63,81
There are several arguments against performing a liver transplant in PLD: it is a benign disease, there is usually hepatocellular failure, indefinite immunosuppressive therapy, surgery with associated morbidity and mortality and lack of donations.1,63,64,77,81 The arguments in favour are: good results and improved quality of life after transplantation.61,64,67 The right time to perform liver transplantation and the convenience of a hepatorenal transplant is a complex decision.63,64,67,77
Other therapeutic measures include treatment with octreotide and analogues but these have not been successful.34,74 A multicentre study of lanreotide obtained a reduction in cystic volume of 2.9%.65 Placing a stent in the vena cava in patients with intractable ascites caused by hepatomegaly may be effective.54
Hepatobiliary CystadenomasHepatobiliary cystadenomas (HCy) are rare liver tumours,6,84–93 comprising 5% of total hepatic cysts.4,84,85,87–97 Middle-aged women account for 85% of them.3,4,84–87,89–94,96,97 Its histogenesis is unknown.90,92–94,97
In 1985, 2 subtypes of HCy were defined, according to the presence or not of ovarian-like mesenchymal stroma.4,87,88,90,91,98 The HCy without stroma are more common in men, and become malignant more often.87,94,96,99 There is a sub-type of HCy without stroma with acidophilic cells that only occurs in men and is considered a semi-malignant variant.91 It is not known if the malignant variant (cystadenocarcinoma) is an evolution from a HCy or an initially malignant tumour.87,90,91 The rate of malignancy may reach 30%.84
HCy are multiloculated, well-defined, solitary lesions, usually with internal septations, papillary projections or nodules on the wall of the HCy or septa. At the microscopic level, they have the following layers: simple cuboidal or columnar monolayer epithelium, mesenchymal stroma (absent in the variant without stroma) and an outer layer of dense connective tissue.4,6,84,86,87,90–94,96–98,100,101 The intracystic fluid may be cloudy or clear with a gelatinous or mucinous appearance.87,94 HCy is variable in size (1.5–30cm).85,87,88,93,95 There may occasionally be communication between the HCy and the biliary tree. It is associated with mucinous cystic tumours of the pancreas.86,90,96
Most HCy are asymptomatic and are found incidentally.85,87,90–94 The symptoms it causes are similar to those of SC.4,21,85–91,93,94,97,100 Possible complications include intracystic haemorrhage, compression or obstruction of the bile duct, ascites, bacterial overinfection, rupture, recurrence and malignisation.85,89–91,94,97
Analytical studies are usually normal, with elevated liver function results if there is compression of the biliary tree.21,93,94,96 Markers in the intracystic fluid are high, especially for CA19-9.84,93,94,102 The presence of CA19-9 in the epithelium of HCy has been demonstrated immunohistochemically.4,13,86–88,91,92,97,102,103The ultrasound image shows an ovoid anechoic cystic mass with multiple hyperechoic internal septa and papillary projections in the septa or cyst wall.4,86,87,91,92 The CT image shows a multilobulated, well-defined, thick-walled cystic mass of low density, and usually has internal septa, mural nodules, and/or papillary projections with contrast enhancement.4,6,87,91,92,97 MRI is useful for evaluating the intracystic content features.94 Puncturing is not recommended due to the low diagnostic sensitivity and risk of spread.4,13,91 Correct preoperative diagnosis is between 30% and 95%.84 The presence of a significant solid component (nodular solid masses or marked parietal thickening), intracystic haemorrhage, wall calcifications, and the combination of nodules and septa are suggestive of malignancy.87,91,95–97
HCy treatment is complete surgical resection, as it is a potentially malignant lesion, which commonly relapses after partial surgery due to the inability to preoperatively distinguish between HCy and cystadenocarcinoma.3,4,6,10,21,84–86,88–90,94,96,97,99,100 The decision to perform complete enucleation or anatomic resection depends on the location of the HCy.4,89,91,92,94,99 HCy with mesenchymal stroma is more easily enucleated.91
Patients diagnosed preoperatively for SC who undergo a LF with a final diagnosis of HCy must be operated on for complete excision.91,93
Ciliated Hepatic Foregut CystCiliated hepatic foregut cyst (CHFC) is an uncommon liver cystic lesion, with only 100 cases being published.104–108 It is postulated that CHFC derives from intrahepatic embryonic remnants that differentiate towards respiratory tissue rather than biliary.6,105–107,109
It is usually benign, solitary, single, subcapsular and unilocular, and is often located in segment IV. It is of variable size, but usually under 4cm.104–111 Its content is viscous or mucoid.104,106,108,109 The mean age of onset is 48 years with a slight predominance towards men.104,105,110 Histologically, there are 4 layers: mucin-producing pseudostratified ciliated columnar epithelium, subepithelial connective tissue, stroma, and fibrous capsule.6,105,107–109,111 Three cases of squamous cell carcinoma in CHFC that developed in previous squamous metaplasia foci have been described, suggesting a progression from dysplasia to carcinoma.104,105,111–113
Its diagnosis is usually incidental because they are asymptomatic in 80% of cases.104–106,108,110 The most typical symptom is abdominal pain, although it can also cause jaundice or portal hypertension.104–108,111,112
On the ultrasound image, it appears as an anechoic or hypoechoic, well-defined, round lesion, with a visible intracystic echogenic mass.104,110 The CT image shows hypodense lesions without contrast enhancement. On MRI, the T2 hyperintensity is characteristic, with a variable T1 intensity depending on the content.104,106–109,111 Puncturing has a positive predictive value of 76%.104
The traditional treatment was puncturing with aspiration or injection of sclerosing agents, with surgical treatment recommended only in symptomatic lesions with uncertain diagnosis.106,107 Publication of 3 cases of malignisation (3% of the total) raises doubts about the most appropriate treatment.106,111,112 CHFC must always be resected if larger than 4cm (the 3 patients with large cysts had malignant CHFC), symptomatic, with progressive growth or when there is a mass on the cyst wall.104,106,111 Complete laparoscopic resection of CHFC is an interesting option.111
Other Rare Cystic LesionsOther rare cystic lesions have been described:
- -
Intrahepatic pancreatic pseudocyst: only 27 cases were described up to 2006. They are usually located in the left hepatic lobe, are usually asymptomatic, and diagnosis is incidental. Diagnostic confirmation comes after puncture when there is a fluid rich in amylase.114 Treatment ranges from monitoring and percutaneous drainage to surgery114;
- -
Post-traumatic cysts: the conservative management of liver injuries has increased the number of post-traumatic cysts. Only those symptomatic ones or those with diagnostic uncertainty should be operated on1,5,7,115;
- -
Hamartomas: also called von Meyenburg complexes. They are caused by a failure of involution of the embryonic bile ducts.116 They are multiple lesions of 0.1–1.5cm and are not communicated with the bile duct.116 Autopsy incidence varies between 0% and 2.8%. They are usually incidental findings, as they are usually asymptomatic but may exceptionally cause microabscesses or degenerate into cholangiocarcinoma.116,117 A CT image may be confused with multiple liver metastases but MRI provides an accurate diagnosis116;
- -
Epidermoid cyst;
- -
Lymphangiomas;
- -
Biloma: These are caused by spontaneous, traumatic or iatrogenic rupture of the biliary system;
- -
Biliary intraductal papillary mucinous tumour: There are 2 types, cystic and non-cystic.101
The authors have no conflicts of interest to declare.
Please, cite this article as: Ramia JM, et al. Tumores hepáticos quísticos benignos no parasitarios. Cir Esp. 2011;89:565–73.